The Most Important factor in making liver transplantation practical was the introduction of new immunosuppressant agents of which cyclosporine (CyA) has had the greatest impact.1 The most common cause of hepatic allograft failure still is rejection, however, OKT3 (Ortho Pharmaceutical, Raritan, NJ) is a mouse anti-human T cell antibody that in conjunction with azathioprine and steroids has been proven effective in the reversal of acute rejection in renal transplant recipients.2,3 Recently, two separate clinical trials have shown the efficacy of OKT3 in treating acute hepatic allograft rejection. An interesting observation was that the high recurrence rate of rejection previously seen in other studies after stopping OKT3 was not observed with CyA and steroids for maintenance.4,5 The purpose of this study was to analyze the impact of OKT3 on reversal of acute hepatic rejection and on the overall graft and patient survival.
PATIENTS AND METHODS
From November 1984 to December 1985, 157 liver transplant recipients received a course of OKT3, with at least 2 months of subsequent analysis. From August 1983 to December 1985, 237 other patients underwent hepatic transplantation but did not receive OKT3; they served as the control group. The following parameters were compared for age, sex, degree of sensitization, degree of HLA matching, and graft and patient survival.
The 157 OKT3-treated patients were stratified in three different groups according to the period between transplantation and the initiation of OKT3 therapy.
Patient Groups
Group I
The OKT3 treatment was started <10 days postoperatively. Sixty-eight patients fell into this category and received OKT3 with a median of 6 days. Histologic evidence of rejection was noted in 48 (71%) patients; in the remaining 20 patients (29%), however, hepatic biopsies showed findings consistent with ischemic (harvesting) injury. Twenty-two of these patients (32%) had postoperative renal impairment that precluded the use of CyA. Thus, the OKT3 was being used not only to treat rejection but also as a CyA-sparing device.
Group II
OKT3 was administered for 10 to 90 days postoperatively in 73 patients with a median of 19 days. Sixty-four (88%) had histologic evidence of rejection. The causes of graft dysfunction in the remaining 9 patients were cytomegalovirus hepatitis in 4 (5%), ischemic injury in 4 (5%), and biliary obstruction in 1 (2%).
Group III
OKT3 therapy was started later than three months in 16 patients, after a median of 420 days. All patients had histologic evidence of cell-mediated rejection, although some had findings consistent with chronic rejection. These patients had no evidence of ischemic liver damage or renal failure.
OKT3 was administered following the precautions previously described.4 CyA and steroids were continued during the OKT3 therapy, and during this period the CyA dose was adjusted in order to achieve therapeutic levels.
Therapeutic Response
Liver biopsies were performed before or shortly after the onset of OKT3 therapy in 140 (89%) of the patients treated with OKT3 (Table 1). The biopsy specimens were processed and analyzed according to the criteria previously described.6 Biopsies were repeated at the end of the OKT3 therapy in 85 (of the 140) patients who had biopsies before therapy was initiated.
Table 1.
Results of Hepatic Biopsies in Liver Transplant Recipients at the Beginning of OKT3 Therapy
| Group | Rejection (%) | Ischemia (%) | Other (%) |
|---|---|---|---|
| I | 71 | 29 | 0 |
| II | 88 | 5 | 7 |
| III | 100* | 0 | 0 |
Many patients had signs of chronic rejection.
The response to therapy was classified as “full” response if liver chemistries returned to normal or near normal within the first 2 weeks following therapy, as partial response if biochemical parameters improved with or without histologic improvement, and as no response if liver chemistries either did not improve or worsened.
Statistical Analysis
Fischer’s exact test was used to determine statistical significance between the treated and control groups. Life-table analysis was performed to determine allograft as well as patient survival curves. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Fifty-seven of the 157 liver recipients were children with an average age of 6.8 ± 5 (SD) years, ranging from 6 months to 18 years. The average age for the 100 adults was 41 ± 11 (SD) years, range 19 to 63 years. The overall average age for the OKT3 group was 28.6 years v 23.4 years for the control group. Primary transplantation preceeded OKT3 therapy in 135 (86%) of the patients, and 22 (14%) underwent retransplantation before OKT3 therapy.
All grafts used for hepatic recipients were selected without knowledge of the HLA types prior to transplantation. At the HLA A, B, and DR loci, the antigens matched averaged 1.28 ± 0.99 (range 0 to 4, maximum 6) v 1.10 ± 0.98 for the control group. Neither was the degree of presensitization, ie, (panel-reactive antibody, PRA) against a lymphocyte panel (PRA), significantly different. The mean PRA for the treated group was 11.1% v 10.4% for the control group. The incidence of hepatic transplantation despite a positive T cell cross-match was 13% in the OKT3 treatment groups as compared with 17% in the control group.
The overall response rate of the 157 liver transplant recipients treated with OKT3 was 79%; 21% showed no improvement. When these data were stratified to the different groups, the results shown in Table 2 were obtained.
Table 2.
Response to OKT3 Therapy and Incidence of Retransplantation in Liver Transplant Recipients
| Overall | Full | Partial | None | Retrans- plantation |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) |
| I (n = 68) | 48 | 71 | 26 | 38 | 22 | 33 | 20 | 29 | 22 | 32 |
| II (n = 73) | 64 | 88 | 50 | 69 | 14 | 19 | 9 | 12 | 9 | 12 |
| III (n = 16) | 11 | 69 | 8 | 50 | 3 | 19 | 5 | 31 | 6 | 37 |
Patient Groups
Group I
Forty-eight (71%) of the 68 patients showed improvement with OKT3 therapy. In 20 cases, the causes of graft dysfunction were ascribed to causes other than cell-mediated rejection, namely harvesting injury in 15 and primary graft nonfunction in 5. When these 20 patients were eliminated, the response rate in this group was only slightly better, with 36 patients (76%) of 48 showing improvement. This observation suggested that OKT3 was a valuable adjunct in management by providing immunosuppression at a crucial time when optimal doses of CyA could not be given safely.
Group II
A therapeutic response to OKT3 therapy was noted in 64 (88%) of the 73 patients. In 9 of the 73 patients causes of graft dysfunction other than rejection were identified: cytomegalovirus hepatitis (4 patients), ischemic injury (4 patients) and biliary obstruction (1 patient). Omission of these nine patients improved the response rate to 94%, with 72% having a complete response. There were only four (6%) failures in this cleaned-up group of 64.
Group III
Eleven (69%) of the 16 patients showed improvement of the biochemical parameters and 8 had full reversal. The failure rate was 31%.
Recurrence of Rejection and Impact on Retransplantation
During the period of follow-up to February 1986, only 25 (20%) of the 125 patients demonstrating a response to OKT3 therapy experienced further rejection episodes. The recurrent rejection episodes occurred a mean of 3.3 months following completion of OKT3 (range 0.5 to 11.0 months). Sixteen of the recurrent rejections were successfully treated with high-dose steroids, with return of normal hepatic function. The other nine patients subsequently lost their grafts and required retransplantation.
The nature of the case selection biased all statistics against the OKT3 groups in that the drug was used for the sickest patients and for those with refractory rejections. Among all 157 patients treated with OKT3 for graft dysfunction, the incidence of retransplantation was 23.6%, almost identical to the favored control group (22.2%). The impact of OKT3 on retransplantation was most evident in the patients of group II who almost always had cell-mediated rejection that was resistant to steroid therapy. In these patients, the incidence of retransplantation was only 12%. Patients of group III had an ultimate retransplant rate of 37%, whereas the rate in group I was 32%.
IMPACT OF OKT3 ON ALLOGRAFT AND PATIENT SURVIVAL
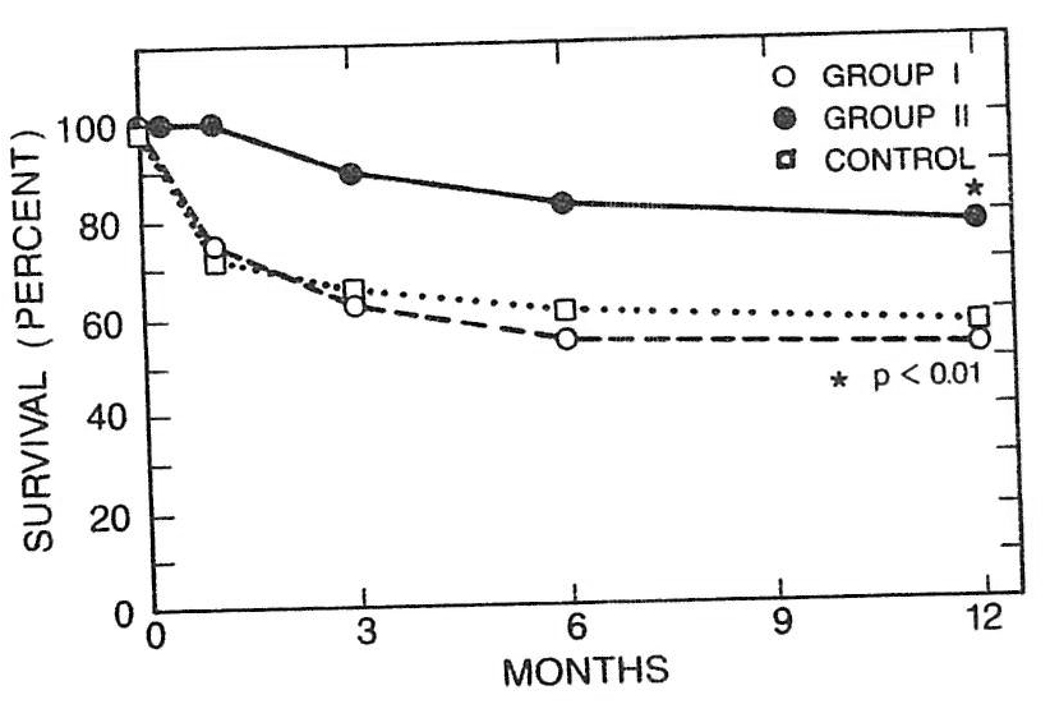
The graft survival in the control group at 1 year was 54.9%, whereas in the OKT3-treated group it was 64.4%. This difference was statistically significant (P < .01). The 1-year graft survival in group I and group III was 64.4% and 68.8%, respectively, and the difference was not statistically different from that of the control group. In contrast, the 1-year graft survival in group II was 76.7%, and this difference was very significant (P < .001). The results are summarized in Fig 1.
Fig 1.
Life-table analysis of graft survival in groups I and II (described in text) v control.
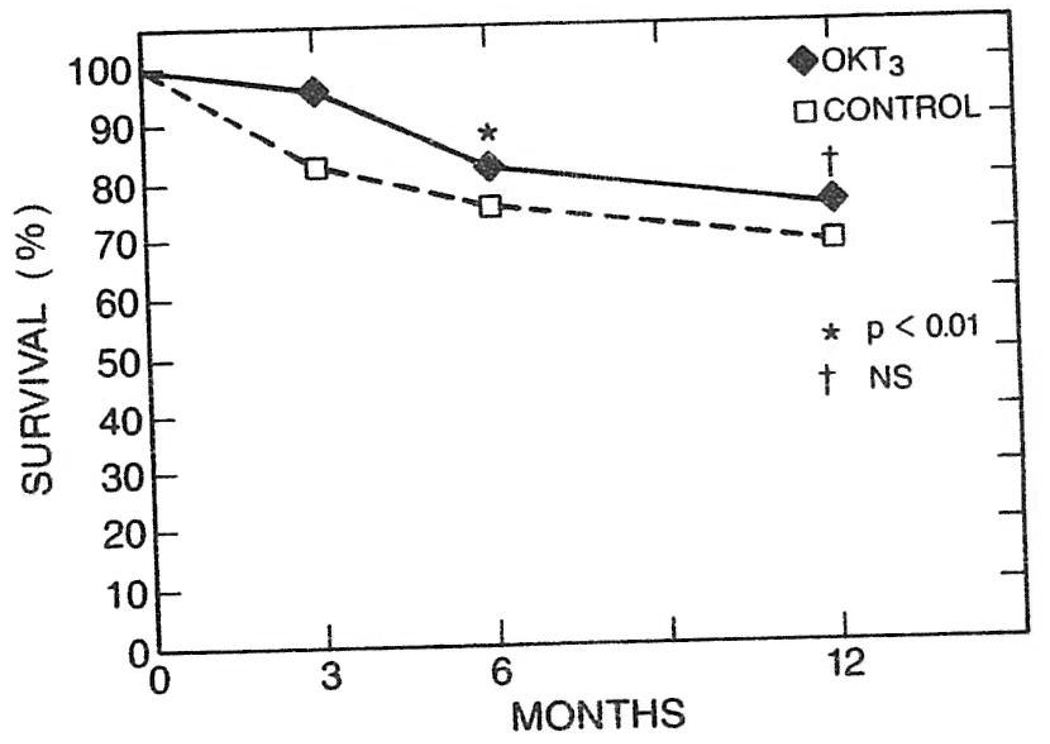
The patient survival in the control group at 6 months was 73.6% as compared with 82.9% of the OKT3-treated group (P < .01). The 6-month recipient survival in group II was 86.7% (P < .005). Survival was still better in the OKT3 group at 1 year (75.1% v 71.6%), but this difference was not statistically significant (Fig 2).
Fig 2.
Life-table analysis of patient survival of the OKT3-treated group as compared with the control group.
DISCUSSION
The purest conditions for assessment of OKT3 were in patients treated between 10 and 90 days after OLT (group II). In these recipients, rejection was the cause of graft dysfunction in almost 90% of cases.
In contrast, patients who required OKT3 within 10 days of OLT frequently had other causes of graft dysfunction. In this difficult group of patients, the diagnosis of rejection was difficult to make. The harvesting injury often was dominant on histologic examination and could mask the findings of rejection. Nevertheless, a significant number of patients without an unequivocal diagnosis of rejection benefited from OKT3 therapy. In these recipients who also had a high incidence of renal impairment, the dose of CyA could be reduced to allow recovery of the kidneys while effective immunosuppression was maintained with OKT3. OKT3 was also effective in a surprising number of patients treated ≥3 months after transplantation (group III), even though many had signs of chronic rejection on histologic examination in addition to acute rejection. OKT3 is probably not effective in patients with irreversible hepatic damage from the kind of chronic rejection that destroys small bile ducts and obliterates the arterial supply.
The final analysis of a new immunosuppressive agent is the impact of that drug on overall graft and patient survival. The present investigation showed that OKT3 improved graft survival and 6-month patient survival even though the OKT3-treated recipients were those with the greatest rejection and other difficulties. The patient survival of the OKT3-treated group at 1 year was not different from that of the control group.
SUMMARY
OKT3 was an effective immunosuppressant agent in patients with acute cell-mediated allograft rejection that had not responded to initial steroid therapy. OKT3 was also valuable for treating patients with early hepatic graft dysfunction caused by other factors than rejection. In such recipients, the doses of CyA can be greatly reduced, allowing recovery of frequently damaged kidneys while maintaining effective immunosuppression.
Acknowledgments
Supported by research grants from the Veterans Administration and National Institutes of Health project Grant No. AM-29961.
REFERENCES
- 1.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosmi AB, Colvin R, Burton R, et al. N Engl J Med. 1981;305:308. doi: 10.1056/NEJM198108063050603. [DOI] [PubMed] [Google Scholar]
- 3.Cosmi AB, Burton R, Colvin R, et al. Transplantation. 1981;32:535. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Fung JJ, Demetris AJ, Porter KA, et al. Nephron. doi: 10.1159/000184431. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Fung JJ. Transplant Proc. 1986;18:937. [PMC free article] [PubMed] [Google Scholar]
- 6.Demetris JA, Lasky S, VanThiel DH, et al. Am J Pathol. 1985;118:151. [PMC free article] [PubMed] [Google Scholar]