Abstract
Malaria results in >1,000,000 deaths per year worldwide. Although no licensed vaccine yet exists, much effort is currently focused on subunit vaccines that elicit CD8 T cell responses directed against Plasmodium parasite liver stage antigens. Multiple immune-effector molecules play a role in anti-microbial immunity mediated by memory CD8 T cells including IFN-γ, perforin, TRAIL, FasL and TNF-α. However, it is not known which pathways are required for memory CD8 T cell-mediated immunity against liver stage Plasmodium infection. Here, we used a novel immunization strategy to generate memory CD8 T cells in the BALB/c mouse model of P. berghei or P. yoelii sporozoite infection to examine the role of immune-effector molecules in resistance to the liver stage infection. Our studies reveal that endogenous memory CD8 T cell-mediated protection against both parasite species is in part IFN-γ-dependent, while perforin was only involved in protection against P. yoelii. We further show that neutralization of TNF-α in immunized mice markedly reduces memory CD8 T cell-mediated protection against both parasite species. Thus, our studies identify both IFN-γ and TNF-α as important components of the non-cytolytic pathways that underlie memory CD8 T cell-mediated immunity against liver stage Plasmodium infection. Our studies also show that the effector pathways memory CD8 T cells utilize to eliminate liver stage infection are in part Plasmodium species-specific. This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Introduction
Infection of humans with Plasmodia, the causative agents of malaria, exacts a significant toll on public health, causing pathologies ranging from fever to coma and death (1, 2). More than one million people die annually from Plasmodium infections and approximately forty percent of the global population is at risk for exposure (1, 3). Thus, Plasmodium infection remains an enormous public health problem worldwide and effective tools to combat infections, including efficacious vaccines, are desperately needed.
Plasmodium species are transmitted through the bite of female Anopheles mosquitoes (4–6). Upon inoculation into the naïve host, Plasmodium sporozoites enter the blood stream and passively transit to the liver where they ultimately initiate replication and differentiation in hepatocytes (7, 8). The liver stage (i.e. pre-erythrocytic) infection lasts approximately 6–8 days in humans and is wholly asymptomatic, whereas subsequent release of blood stage merozoites from infected hepatocytes underlies clinical malaria. Thus, stopping Plasmodium infection during the asymptomatic liver stage represents an attractive goal of vaccination. By extension, an ideal pre-erythrocytic vaccine to combat human Plasmodium infection would be formulated such that it affords protection against both clinically relevant species of Plasmodium, P. falciparum and P. vivax. However, it is not known whether protective immune responses targeting liver stage infection of multiple Plasmodium species would involve overlapping immune-associated effector molecules or pathways.
Rodent-specific species of Plasmodium, such as P. berghei and P. yoelii, have been widely used to characterize host-parasite interactions, pathogenesis and protective immunity following inoculation of sporozoites into laboratory mice. Indeed, sporozoite infection of mice exquisitely mimics the early stages of hepatocyte entry and liver stage replication/differentiation that occurs in humans, although with an accelerated time-course (7–9). For these reasons, P. berghei- or P. yoelii-infected laboratory mice are widely used as models of pre-erythrocytic, anti-Plasmodial vaccination. To date, the most efficacious vaccination strategies targeting liver stage Plasmodium infection employ the use of attenuated whole-parasite vaccines, including radiation-attenuated sporozoites (RAS)3 (10–14) and genetically attenuated parasites (GAP) (15–18). In rodent models, RAS- and GAP-induced protective immunity to liver stage infection requires the induction of CD8 T cell responses (19–21). However, the attenuated whole-parasite immunizations also elicit cell-mediated responses that include the participation of CD4 T cells, NK cells and B cells (21–25). Consistent with this multifactorial immune response elicited by whole-parasite immunizations, the immune-associated effector molecules required for protection are complex and studies of their role in protection are oftentimes conflicting (21, 25–29).
Despite decades of research using rodent models of Plasmodium infection to help guide vaccine development, and the identification of CD8 T cells as critical mediators of protective immunity against liver stage infection, this work has not yet led to the development of an easily translatable vaccine. Perhaps one reason for this relates to our limited understanding of the precise numerical, phenotypic and functional requirements for protective memory CD8 T cell responses targeting liver stage Plasmodium infection. In addition, despite the significant biological differences shown to exist between effector and memory CD8 T cell populations, in terms of transcriptional profile, cell surface phenotype and functional properties (30), little consideration has been given to the notion that long-lived, vaccine-induced protection against liver stage Plasmodium infection would be mediated by memory CD8 T cells, not recently stimulated effector CD8 T cells. Indeed, mice multiply immunized with RAS are often challenged 1–2 weeks following the last boost, thus evaluating protection by effector rather than true memory CD8 T cells. Similarly, T cell receptor transgenic (TCR-Tg) CD8 T cells have been used to examine the functional requirements for immunity against liver stage P. yoelii infection, but only during the effector stage of the T cell response (31–33). Another point relevant to these previous studies is the assumption that immune-effector pathways are shared between protective CD8 T cells targeting either P. berghei or P. yoelii liver stage infection. Thus, the precise characteristics and requirements for memory CD8 T cell-mediated protection against liver stage Plasmodium infection remain poorly characterized. Moreover, whether protective immune-effector pathways overlap for multiple Plasmodium species has never been directly examined.
Using a novel immunization strategy, we recently reported the numerical requirements for immunity mediated solely by memory CD8 T cells against P. berghei liver stage infection (34). Herein, we employ the same immunization approach, where the sole immune cells mediating protection are bona fide endogenous, polyclonal populations of memory CD8 T cells, to dissect the phenotypic and functional requirements for memory CD8 T cell mediated protection against liver stage P. berghei and P. yoelii infection. We generated P. berghei- or P. yoelii-specific, memory CD8 T cell responses in WT and BALB/c mice lacking expression of key immune-effector molecules to test the hypothesis that multiple CD8 T cell effector mechanisms are required for memory CD8 T cell-mediated protection of mice challenged with Plasmodium sporozoites. Our results reveal that IFN-γ and TNF-α contribute to memory CD8 T cell-mediated protection against both P. berghei and P. yoelii liver stage infection, but perforin only plays a role in resolving P. yoelii infection. Thus, key immune-effector molecules differentially regulate memory CD8 T cell-mediated immunity for two species of rodent Plasmodium.
Materials and Methods
Mice and immunizations
Specific pathogen-free wild type BALB/c mice were purchased from the National Cancer Institute. Specific pathogen-free IFN-γ−/−, perforin−/− and gld BALB/c mice were maintained in our own breeding colonies. TRAIL−/− BALB/c mice were provided by Dr. Thomas Griffith, University of Iowa. Mice immunized as previously described (34). Briefly, mice were primed via tail vein injection of 1×106 splenic DC coated with peptide corresponding to CS252-260 or CS280-288 for P. berghei or P. yoelii, respectively. Seven days later, mice were boosted with 2×107 CFU LM-CS252-260 or LM-CS280-288. All animal studies were approvedby the University of Iowa Animal Care and Use Committee.
Mosquitoes and parasites
Female Anopheles stephensi (Liston) mosquitoes strain STE2 (MR4-128, MR4, ATCC) infected with Plasmodium berghei ANKA clone 234 were obtained from Iowa State University. Female A. stephensi mosquitoes infected with Plasmodium yoelii yoelii clone 17XNL were provided by the insectary at New York University. Infectious P. berghei and P. yoelii sporozoites were hand dissected from mosquito salivary glands, evaluated for viability using propidium iodide staining, counted and inoculated into mice via tail vein injection as previously described (34).
Enumeration of antigen-specific CD8 T cells
Total numbers of splenic CS252-260- or CS280-288-specific CD8 T cells were determined by ex vivo intracellular cytokine staining (ICS) for IFN-γ or TNF-α following a 5.5 hour incubation with brefeldin A in the presence or absence of CS252-260 or CS280-288 peptides. Total numbers of liver and frequency of circulating CS252-260- or CS280-288-specific CD8 T cells were determined by ICS for IFN-γ or TNF-α as described above, except that peptide-coated P815 cells were used as APC during the 5.5 hour incubation. In parallel, aliquots of spleen, liver and blood mononuclear cells from some mice were stained with MHC class I H-2Kd/CS252-260 or H-2Kd/CS280-288 tetramers.
Functional avidity and TCR Vβ chain utilization
For functional avidity analyses, splenocytes were harvested from immunized mice and stimulated ex vivo in the presence of tenfold dilutions of peptide corresponding to CS252-260. After 5.5 hours, cells were stained for intracellular TNF-α as described above. Data were normalized to the frequency of CS252-260-specific CD8 T cells detected using the highest titration of peptide (1μM). For TCR Vβ chain analyses, splenocytes were harvested from naïve or immunized mice and stained with H-2Kd/CS252-260 tetramer. Cells aliquots were subsequently stained for CD8 and twelve of the most common TCR Vβ chains (FITC-anti-Vβ2, 3, 4, 5.1/5.2, 6, 7, 8.2/8.2, 9, 10b, 11, 13 or 14). Data are expressed as the frequency of CS252-260-specific or total splenic CD8 T cells that express each TCR Vβ chain.
TLR agonist administration
Naïve WT BALB/c mice were infected with P. berghei or P. yoelii sporozoites as indicated. At various times, mice were administered 100 μg CpG i.p. (ODN1826, IDT, Coralville, IA), 1 μg LPS i.v. or 200 μg PolyI:C i.v. (both from Sigma, St. Louis, MO). Serum cytokine analyses following administration of TLR agonists were performed using BioPlex Assay (Bio-Rad, Hercules, CA).
In vivo cytokine neutralization
0.5 mg of anti-TNF-α (clone XT-22), 0.5 mg anti-IFN-γ (clone XMG1.2) or 0.5 mg normal rat IgG2a was administered by i.p. injection one day before and one day following sporozoite challenge, or as indicated.
Evaluation of parasitemia
Thin blood smears were performed on naïve and immunized mice 10 days following sporozoite challenge. Parasitized red blood cells were identified by Giemsa stain. Protection is defined as the absence of blood stage parasitemia following examination of at least 15 high-powered (100x) fields.
Statistics
Statistical significance was determined by Student’s t or chi-squared tests where indicated.
Results
P. berghei-specific memory CD8 T cells are generated and persist in WT and mutant BALB/c mice following DC-prime, LM-boost immunization
We recently reported a novel immunization strategy in BALB/c mice that elicits a population of memory CD8 T cells that affords life-long protection against multiple P. berghei sporozoite challenges. By design, memory CD8 T cells are only cell-type capable of providing sterile protection against liver stage infection in this system. Thus, this model permits the direct study of the immunological mechanisms that contribute to memory CD8 T cell-mediated protection against liver stage Plasmodium infection. Therefore, in order to test our central hypothesis, we generated Plasmodium-specific memory CD8 T cell populations in BALB/c WT, IFN-γ−/− (GKO), perforin−/− (PKO), TRAIL−/− (TRAIL-KO) and gld (lacking functional FasL) mice as previously described (34). Briefly, animals were immunized via tail vein with 1×106 WT splenic dendritic cells (DC) coated with peptides corresponding to the immunodominant, H-2Kd-restricted CD8 T cell determinant derived from the circumsporozoite (CS) protein of P. berghei (DC-CS252-260) or P. yoelii (DC-CS280-288). Seven days later, mice were boosted with 2×107 CFU of an attenuated recombinant strain of Listeria monocytogenes (LM) expressing the same minimal determinant as a secreted fusion protein (LM-CS252-260 or LM-CS280-288). We have previously shown that attenuated LM is cleared on day 2 in DC-primed WT mice (35), and that immune GKO and PKO mice clear virulent LM with the same kinetics as WT mice (36, 37). Sixty to 100 days post-immunization, when numerically and phenotypically stable memory CD8 T cell populations have been established, CS252-260-immune WT and mutant BALB/c mice were challenged with 1000 P. berghei sporozoites, a stringent challenge dose of ~5x infectious dose (ID)50 for naïve BALB/c mice (data not shown).
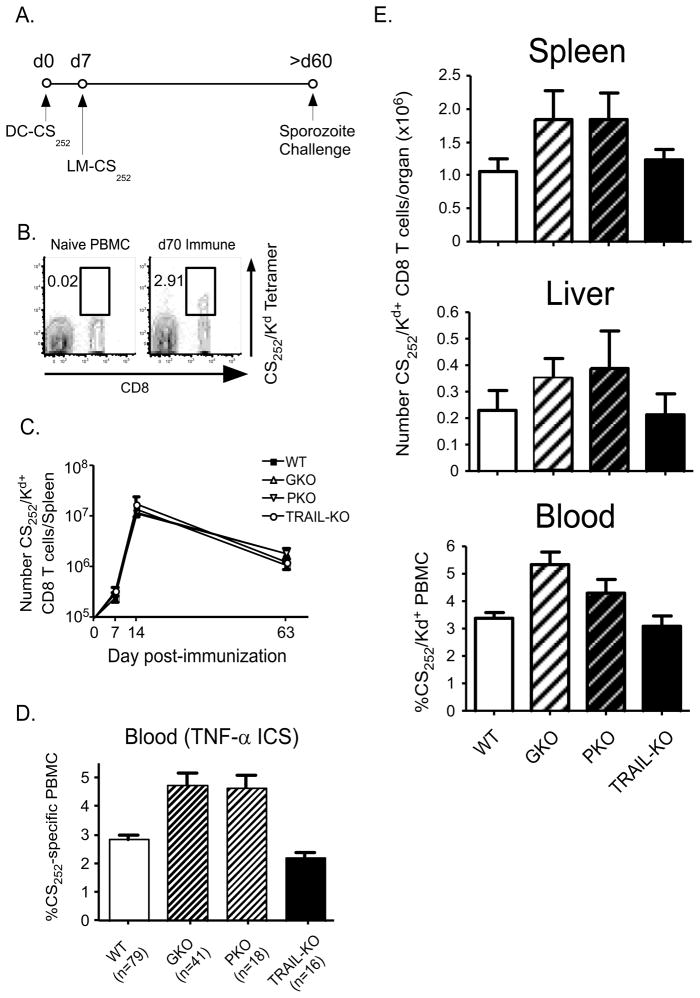
It is well known that the absence of IFN-γ and/or perforin influences the magnitude of antigen (Ag)–specific, primary memory CD8 T cell responses in BALB/c mice (38). For this reason we first determined whether the absence of IFN-γ, perforin, TRAIL or functional FasL influenced the kinetics, magnitude or distribution of Plasmodium-specific, memory CD8 T cells following DC-prime, LM-boost immunization of WT and mutant BALB/c mice (Figure 1A). Using MHC class I tetramer reagents (Figure 1B), we found that prime-boost immunization elicited CS252-260-specific memory CD8 T cell responses in mutant mice that were as large or larger than responses in WT BALB/c mice in the spleen, blood and liver (Figure 1C,E). In the case of mice lacking IFN-γ or perforin, the numbers of memory CD8 T cells were ~25–40% higher in each tissue compartment analyzed compared to WT BALB/c (Figure 1C,E). These data also reveal that the relationship between circulating and tissue-resident CS252-260-specific CD8 T cell numbers are maintained among WT and mutant BALB/c mice, independent of the absence of key immune-effector molecules (Figure 1E). The numerical relationships between memory CD8 T cell responses among WT and mutant mice were also preserved when we examined the ability of circulating (Figure 1D) and tissue-resident (data not shown) CS252-260-specific memory cells to produce TNF-α following ex vivo stimulation with peptide. In addition to these data examining P. berghei (CS252-260)-specific responses, we also observed similar memory CD8 T cell responses in WT and mutant BALB/c mice immunized against P. yoelii using the CS280-288 CD8 T cell determinant (data not shown). Collectively, these results show that the magnitude of the immunization-induced memory CD8 T cell response in mutant BALB/c mice is as large or larger than WT mice, independent of the lack of the relevant immune-effector molecules.
Figure 1. Similar kinetics and magnitude of memory CD8 T cell responses following DC-CS252-260-prime, LM-CS252-260-boost immunization of WT and mutant BALB/c mice.
(A) Experimental design. Parasite-specific memory CD8 T cell responses were generated in WT and mutant BALB/c mice as described in Materials and Methods. The magnitude, kinetics and phenotype of CS252-260-specific memory CD8 T responses in the blood, liver or spleen were characterized greater than 60 days post immunization. (B) Representative H2-Kd/CS252-260 tetramer staining used to detect memory CD8 T cell responses. (C) Kinetics of splenic CS252-260-specific memory CD8 T cell responses in WT and mutant BALB/c mice. Data represent Mean +/− SD for 3 independent experiments with 3–5 mice/group. (D) Magnitude of memory CD8 T cell responses in the blood of WT and mutant BALB/c mice detected following ex vivo stimulation of PBMC with CS252-260 peptide and ICS for TNF-α. (E) Magnitude of the CS252-260-specific memory CD8 T cell responses in the spleen, liver and peripheral blood of WT and mutant BALB/c mice as detected by tetramer staining. Analyses were performed >60 days post DC-prime, LM-boost immunization. Data represent Mean +/− SD for 3 independent experiments with 3 mice/group.
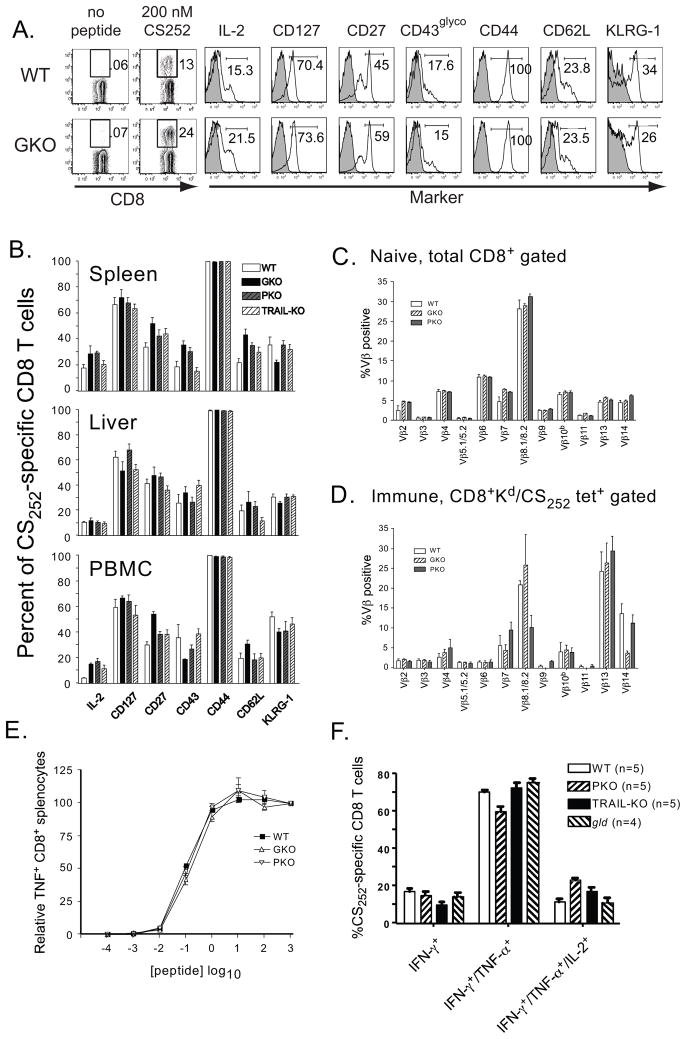
We next determined whether the phenotype, TCR repertoire or functional avidity differed among WT and mutant memory CD8 T cell responses following DC-prime, LM-boost immunization. When we examined CS252-260-specific memory CD8 T cells >80 days following DC-prime, LM-boost immunization, we found no striking differences in cell surface expression of CD127, CD27, CD43glyco (1B11), CD44, CD62L or KLRG-1 on CS252-260-specific memory CD8 T cells recovered from the spleen, blood or liver of WT and mutant mice (Figure 2A,B). Furthermore, we also observed no differences in TCR Vβ chain repertoire or functional avidity (Figure 2C-E; for simplicity, data not shown for TRAIL-KO and gld). Recent work has identified correlations between memory CD8 T cell polyfunctionality and their protective capacity in the context of Plasmodium sporozoite challenge (39). When we evaluated the ability of memory CD8 T cells to co-produce IFN-γ, TNF-α and IL-2, we found no marked differences among WT and mutant (IFN-γ-competent) memory CD8 T cells (Fig 2F). Collectively, the data in Figures 1 and 2 argue that any potential differences in memory CD8 T cell-mediated protection among WT and mutant BALB/c mice following sporozoite challenge would be due to the lack of the relevant immune-effector molecule, not as a consequence of reduced parasite-specific memory CD8 T cell numbers, or altered distribution or cell surface phenotype.
Figure 2. Similar phenotypic and functional properties of CS252-260-specific memory CD8 T cells from WT and mutant BALB/c mice following DC-prime, LM-boost immunization.
(A) Gating strategy and cell surface staining histograms for representative WT and IFN-γ−/− (GKO) mice are shown. (B) Summary of phenotypic analyses of CS252-260-specific memory CD8 T cells recovered from spleen, liver and blood of WT and mutant BALB/c mice >80 days post-immunization. Data in B represent analyses of 6–9 individual mice/group from multiple independent experiments. (C) TCR Vβ chain utilization of total splenic CD8 T cells recovered from the spleens of naïve WT and mutant BALB/c mice. Data represent the Mean +/− SD for 3 mice/group. (D) TCR Vβ chain utilization of Kd/CS252-260 tetramer-positive memory CD8 T cells recovered from the spleens of WT, GKO and PKO BALB/c mice 114 days following DC-prime, LM-boost immunization. Data represent the Mean +/− SD for 3 mice/group. (E) Functional avidity of CS252-260- specific memory CD8 T cells recovered from the spleens of the WT, GKO and PKO mice. The concentration of CS252-260 peptide required to elicit the ½ maximal response was determined as described in Materials and Methods. Data represent the Mean +/− SD for 3 mice/group. (F) Polyfunctionality of WT and mutant (IFN-γ-competent) CS252-260-specific memory CD8 T cells. PBMC from the indicated mouse strains were stimulated ex vivo in the presence of CS252-260 peptides. The proportion of single, double or triple positive cytokine-producing cells was determined by Boolean gating using FloJo software. Data represent Mean +/− SD for 7–10 individual mice/strain.
Memory CD8 T cell-mediated protection against P. berghei liver stage infection is associated with expression of IFN-γ, but not expression of perforin, TRAIL or functional FasL
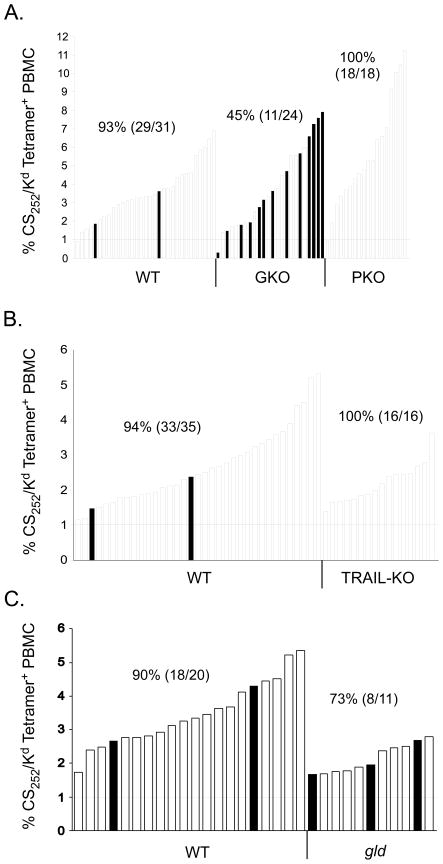
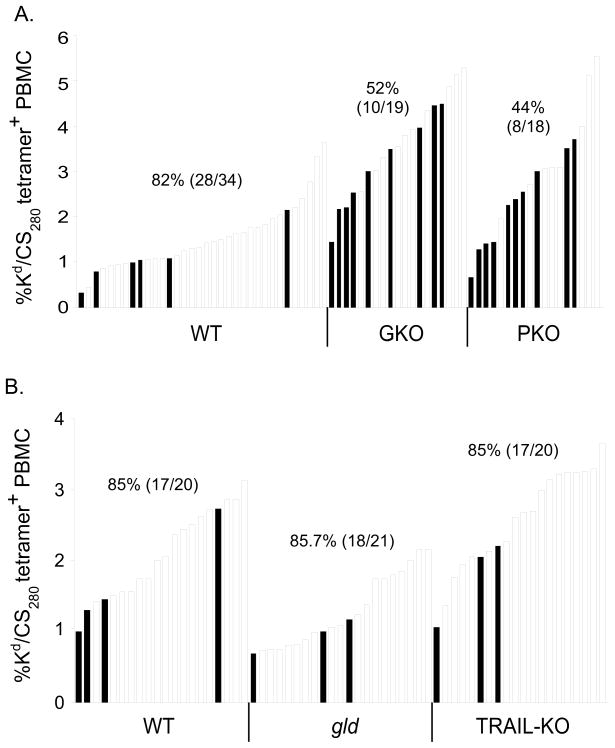
We recently reported that ~95% long-term protection against P. berghei sporozoite challenge could be achieved in WT BALB/c mice if the frequency of immunization-induced CS252-260-specific memory CD8 T cells exceed 1% of all peripheral blood mononuclear cells (34). In that scenario, protection was defined as the prevention of blood stage parasitemia following challenge with 1000 P. berghei sporozoites. Using this same approach, we first evaluated the ability of memory CD8 T cells in WT, GKO and PKO BALB/c mice to mediate resistance to P. berghei sporozoite challenge. For these studies, cohorts of WT and mutant mice were DC-primed, LM-boosted as described above. Greater than 60 days following boost, the frequency of circulating CS252-260-specific CD8 T cells was determined in individual mice, which were ranked by immune status to reveal potential numerical effects on protection (Figure 3A). Three to four days later, groups of mice were challenged with 1000 P. berghei sporozoites. In line with our previous work, >93% (29/31) of immunized WT mice resisted the sporozoite challenge and remained protected from the development of blood stage parasitemia. In the same experiments, we found that 100% (18/18) of PKO mice exhibiting >1% CS252-260-specific PBL resisted the sporozoite challenge (χ2=1.21, p=0.271 for WT vs PKO). In contrast, we found that only 45% of GKO mice resisted P. berghei sporozoite challenge (Figure 3A, χ2=11.62, p=0.00065 for WT vs GKO). Importantly, only 1/24 GKO animals displayed circulating T cell numbers below the 1% threshold required for 95% protection in WT BALB/c mice. Thus, the enhanced susceptibility of GKO mice is not due to reduced numbers of CS252-260-specific memory CD8 T cells. These data clearly demonstrate an important, but non-essential, role for IFN-γ in memory CD8 T cell-mediated protection against P. berghei liver stage infection. Moreover, these data show that the absence of direct perforin-mediated killing of infected hepatocytes by memory CD8 T cells does not influence protective immunity following P. berghei sporozoite challenge.
Figure 3. IFN-γ, but not perforin, TRAIL or FasL, contributes to memory CD8 T cell-mediated protection against liver stage P. berghei infection.
(A) WT, GKO and PKO BALB/c mice were immunized as described in Figure 1. Eighty to 120 days following immunization the frequency of circulating CS252-260-specific memory CD8 T cells in all mice was determined by Kd/CS252-260 tetramer staining. Each bar represents an individual mouse and the horizontal line denotes the 1% numerical threshold required for sterilizing immunity in >95% of WT BALB/c mice (34). Two to 3 days following T cell analyses, mice were challenged i.v. with 1000 P. berghei sporozoites. The presence of blood stage parasitemia was evaluated with Giemsa stain 10 days later. Filled bars represent parasitized mice of the indicated genotype. Numbers indicate % protection (no. protected/no. challenged). Data in A are pooled results from 2 independent experiments (χ2=1.21, p=0.271 for WT vs PKO; χ2=11.62, p=0.00065 for WT vs GKO). (B-C) WT and TRAIL-deficient BALB/c mice (B) or WT and gld BALB/c mice (C) were immunized and T cell numbers were evaluated in individual mice as described above. Mice were challenged with 1000 P. berghei sporozoites and blood stage parasitemia was evaluated with Giemsa stain of thin blood smears 10 days later. Numbers indicate % protection (no. protected/no. challenged). Data in B are pooled results from 2 independent experiments (χ2=0.952, p=0.329 for WT vs TRAIL-KO). Data in C are pooled results from 2 independent experiments (χ2=1.56, p=0.211 for WT vs gld).
Inflammatory or microbial insults to the liver can result in hepatocyte upregulation of the TRAIL receptor, DR5 (40). Furthermore, in models of influenza-infected mice, engagement of DR5 by TRAIL expressed on CD8 T cells can result in the enhanced elimination of virus-infected, DR5+ target cells (41), similar to the cell death pathway triggered following engagement of Fas by FasL (42). For these reasons, we next evaluated protection in TRAIL-deficient and gld BALB/c mice (lacking functional FasL). We found that immune mice deficient in TRAIL displayed no defect in resistance to sporozoite challenge, with 100% (16/16) animals resisting sporozoite challenge (Figure 3B, χ2=0.952, p=0.329 for WT vs TRAIL-KO). Furthermore, when we examined protection in gld BALB/c mice, we found similar resistance between gld and WT mice with >1% circulating CS252-260-specific memory CD8 T cells (Figure 3C, χ2=1.56, p=0.211). Thus, in a setting in which memory CD8 T cells are the only cell type capable of mediating protection, our data identify IFN-γ as an important component of the pathways of protection against liver stage P. berghei infection. Collectively, these data suggest that memory CD8 T cell-mediated protection against liver stage P. berghei infection does not strictly depend on direct memory CD8 T cell killing of infected hepatocytes through extrinsic cell death pathways involving perforin, TRAIL or FasL.
TNF-α contributes to memory CD8 T cell-mediated protective immunity against liver stage P. berghei infection
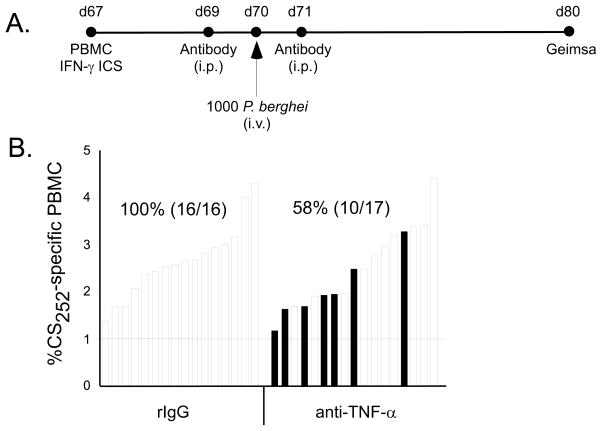
The observation that IFN-γ is a critical component of resistance raises the possibility that additional inflammatory cytokines may be involved in memory CD8 T cell-mediated protection against P. berghei liver stage infection. A significant proportion of memory CD8 T cells co-express IFN-γ and TNF-α upon antigen reencounter (43), and TNFR1 is rapidly induced on hepatocytes following liver injury or insult (44). It has also been shown that TNF-α, in conjunction with IL-6, can inhibit the in vitro differentiation of pre-erythrocytic forms of Plasmodia (45–47). Thus, we hypothesized that TNF-α may also play an important role in memory CD8 T cell-mediated protection against P. berghei sporozoite challenge. Because the tnfa gene lies within the MHC locus, tnfa−/− mice are not available on the BALB/c background. Therefore, we performed in vivo neutralization of this cytokine in immune BALB/c mice via two intraperitoneal injections of the anti-TNF-α mAb, clone XT-22 (Figure 4A). Strikingly, we found that in DC-prime, LM-boost immunized WT BALB/c mice with >1% circulating CS252-260-specific memory CD8 T cells, the in vivo neutralization of TNF-α sharply reduced memory CD8 T cell-mediated protection from 100% to less than 60% (Fig 4B, χ2=8.36, p=0.004). These results clearly identify an in vivo role for TNF-α in protection mediated by memory CD8 T cells. As a composite, our data identify two inflammatory cytokines, IFN-γ and TNF-α, as important regulators of protective immunity against liver stage P. berghei infection mediated by immunization-induced, memory CD8 T cells. Collectively, these data highlight the importance of indirect, anti-parasitic activities of IFN-γ and TNF-α on P. berghei-infected hepatocytes and deemphasize the contribution of direct target cell killing through extrinsic cell death pathways involving perforin, TRAIL and FasL.
Figure 4. TNF-α contributes to memory CD8 T cell-mediated protective immunity against liver stage P. berghei infection.
(A) Experimental design. Circulating CS252-260-specific memory CD8 T cell frequencies in individual WT mice were determined using tetramer staining 67 days following immunization. 500μg of anti-TNF-α or rat IgG control Abs were administered one day before and one day following challenge of mice with 1000 P. berghei sporozoites. (B) Diminished memory CD8 T cell-mediated protection following TNF-α neutralization in WT BALB/c mice bearing >1% circulating CS252-specific PBMC. Each bar represents an individual mouse. Filled bars indicate parasitized mice as determined by Giemsa stain of thin blood smears 10 days following challenge. Numbers indicate % protection (no. protected/no. challenged). Data are pooled results from 2 independent experiments (χ2=8.36, p=0.004 for rIgG vs anti-TNF-α).
Pathways of memory CD8 T cell-mediated protection are non-overlapping for P. yoelii and P. berghei liver stage infection
We next extended our studies to determine the relative contributions of these same key immune-effector molecules following P. yoelii sporozoite challenge of DC-prime, LM-boost BALB/c mice. When we immunized cohorts of WT, GKO and PKO mice against the CS280-288 determinant derived from P. yoelii, we found that >82% of WT mice resisted a challenge of 100 P. yoelii sporozoites (Figure 5A), a physiological challenge dose that is ~8x the ID50 for naïve BALB/c mice (data not shown). Similar to our results following P. berghei challenge, these experiments also revealed a profound defect in the ability of immunized GKO mice to resist P. yoelii sporozoite challenge, with only half of mice remaining free of blood stage infection (Figure 5A;χ2=5.31, p=0.021 for WT vs GKO). However, and in contrast to P. berghei, a substantial fraction (<55%) of PKO mice were also unable to resist P. yoelii sporozoite challenge (Figure 5A; χ2=7.94, p=0.005 for WT vs PKO). These results indicate that both IFN-γ and perforin play an important role in memory CD8 T cell-mediated protection against P. yoelii liver stage infection. Moreover, these data show that the pathways of memory CD8 T cell-mediated protection differ for two rodent species of Plasmodium. Of note, while the numerical threshold for memory CD8 T cell-mediated resistance of WT BALB/c mice to P. yoelii sporozoite challenge has not been formally investigated, these data hint that the threshold may be higher than that previously reported for P. berghei (34). Nevertheless, on average, greater numbers of CS280-288-specific memory CD8 T cells were detected in GKO and PKO mice evaluated in these experiments, ruling out that enhanced susceptibility of mice lacking IFN-γ or perforin is due to reduced numbers of memory CD8 T cells that develop following DC-prime, LM-boost immunization. Importantly, we also investigated whether the enhanced susceptibility of immunized PKO mice to P. yoelii challenge could be related to impaired cytokine expression in cell lacking perforin. However, an examination of the Mean Fluorescence Intensity (MFI) of TNF-α staining in WT versus PKO CS280-288-specific memory CD8 T cells following ex vivo stimulation revealed that PKO cells express ~40% more TNF-α on a per cell basis as compared to WT cells (TNF-α MFI of 5730+/−840 and 8972+/−322 in WT and PKO cell, respectively. P=0.0173). On the other hand, the per cell expression of IFN-γ was not significantly different between PKO and WT CS280-288-specific memory CD8 T cells (IFN-γ MFI of 1810+/−400 and 2298+/−550 in WT and PKO cell, respectively. P=0.151). Thus, impaired cytokine expression by PKO CS280-288-specific memory CD8 T cell is unlikely to explain the enhanced susceptibility of PKO mice relative to WT mice.
Figure 5. IFN-γ and perforin, but not TRAIL or FasL, contribute to memory CD8 T cell-mediated protective immunity following P. yoelii sporozoite challenge.
(A) WT and mutant BALB/c mice were primed with DC-CS280-288 and boosted 7 days later with LM-CS280-288. Greater than 70 days following immunization, the frequency of circulating CS280-288-specific memory CD8 T cells in individual mice was determined by H2- Kd/CS280-288 tetramer staining. Mice were subsequently challenged with 100 P. yoelii sporozoites and monitored for blood stage parasitemia on d10 p.i. Filled bars represent parasitized mice of the indicated genotype. Numbers indicate % protection (no. protected/no. challenged). Data in A are pooled results from 2 independent experiments (χ2=5.31, p=0.021 for WT vs GKO; χ2=7.94, p=0.005 for WT vs PKO). (B) WT, gld and TRAIL-deficient BALB/c mice were immunized as described in A. Greater than 60 days following immunization, mice were challenged with 100 P. yoelii sporozoites and protection was evaluated as described above. Data in B are pooled results from 2 independent experiments (χ2=0.004, p=0.949 for WT vs gld; χ2=0, p=1.0 for WT vs TRAIL-KO).
Based on the striking susceptibility phenotype of immunized PKO mice following P. yoelii sporozoite challenge, we further evaluated the potential contribution of other pathways involved in target cell elimination through direct killing by memory CD8 T cells, including FasL and TRAIL. However, when we evaluated cohorts of immunized WT, gld and TRAIL-deficient BALB/c mice, there was no evidence of enhanced susceptibility in the mice lacking functional FasL or TRAIL expression compared to WT BALB/c mice (Figure 5B;χ2=0.004, p=0.949 for WT vs gld; χ2=0, p=1.0 for WT vs TRAIL-KO). Together, these data suggest that IFN-γ and perforin, but not FasL or TRAIL, play important roles in the protection mediated by memory CD8 T cells against liver stage P. yoelii infection.
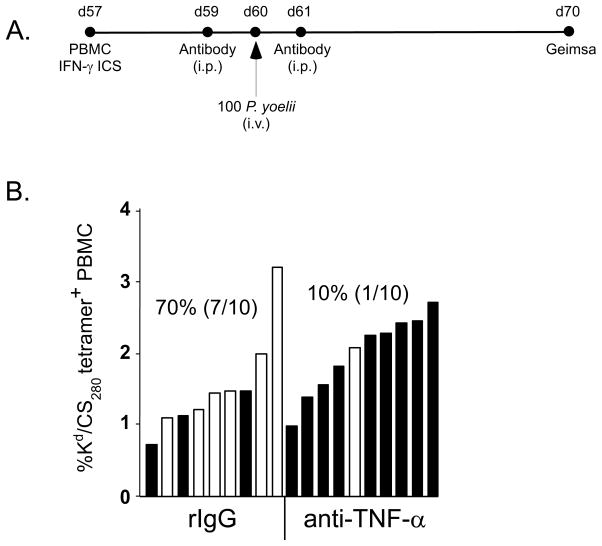
Having identified TNF-α as a novel component of memory CD8 T cell-mediated protection against P. berghei liver stage infection, we also evaluated the role of this cytokine in the setting of P. yoelii sporozoite challenge of immunized mice. WT BALB/c mice were immunized against the CS280-288 determinant, T cell responses were assayed in the peripheral blood, and mice were subsequently challenged with 100 P. yoelii sporozoites. The monoclonal antibody XT-22 or rat IgG was administered one day before and one day after challenge (Figure 6A). When we assayed for blood stage parasitemia 10 days after sporozoite challenge, we found that protection was reduced from 70% in rat IgG-treated mice to 10% following in vivo neutralization of TNF-α (Figure 6B; χ2=7.50, p=0.006). Thus, as we observed for the P. berghei model, TNF-α also plays an important role in memory CD8 T cell-mediated protection against liver stage P. yoelii infection.
Figure 6. Diminished memory CD8 T cell-mediated protection against P. yoelii sporozoite challenge following TNF-α neutralization in WT BALB/c mice.
(A) Experimental design. T cell analyses were performed 57 days following DC-CS280-288- prime, LM-CS280-288-boost immunization of WT BALB/c mice. 500μg of anti-TNF-α or rat IgG control Abs were administered one day before and one day following challenge of mice with 100 P. yoelii sporozoites. Protection was evaluated as described above. Numbers indicate % protection (no. protected/no. challenged). Data are pooled results from 2 independent experiments (χ2=7.50, p=0.006 for rIgG vs anti-TNF-a).
Systemic inflammation triggered 24 hrs after sporozoite challenge prevents P. berghei, but not P. yoelii, liver stage development and subsequent blood stage infection
It has been reported that direct recognition of P. yoelii-infected hepatocytes by CS280-288-specific TCR-Tg effector CD8 T cells is required for protective immunity (48). However, our data raise the possibility that for both P. yoelii and P. berghei, recognition of other, non-infected, CS-antigen-bearing cells (i.e. Kupffer cells, dendritic cells, parasite-traversed hepatocytes) by memory CD8 T cells could also initiate or potentiate the release of inflammatory cytokines. Such cytokines could therefore collaterally influence Plasmodium survival or differentiation within infected cells “at a distance” from the APC:memory T cell conjugate. Thus, given a clear role for both IFN-γ and TNF-α proinflammatory cytokines in halting liver stage P. berghei and P. yoelii infection of immunized mice, we reasoned that systemic, TLR agonist-induced inflammation might be sufficient to protect mice following challenge with either species of parasite.
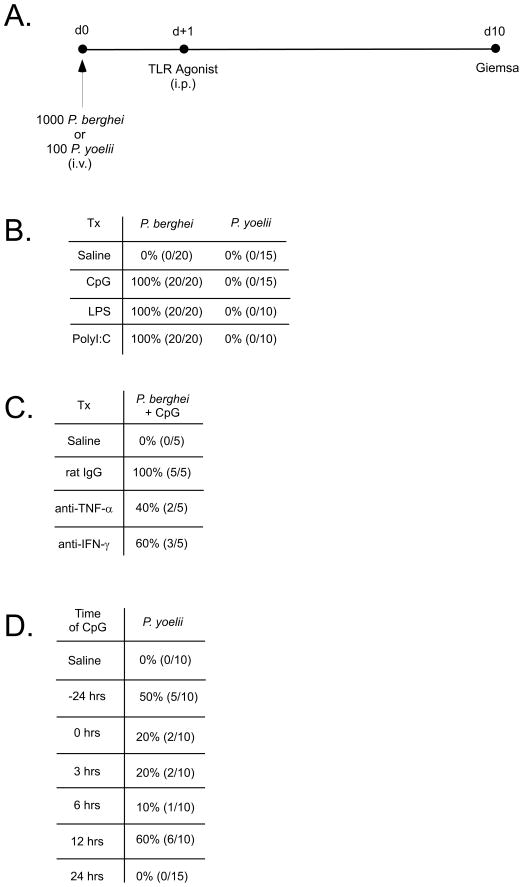
Previous work has determined that TLR9 agonist (CpG)-induced, generalized inflammation triggered prior to P. yoelii sporozoite challenge was sufficient to protect mice from the development of blood stage infection (49). However, we wished to ensure that we were evaluating effects on liver stage development and not hepatocyte entry or initiation of infection. Thus, we administered TLR agonists 24 hrs post-challenge, giving the parasites time to establish liver infection (Figure 7A). This time frame is particularly reasonable given that the liver stage developmental program for rodent Plasmodium strains is generally completed by 48–60 hrs (9). Strikingly, for groups of mice infected with 1000 P. berghei sporozoites and subsequently treated with TLR9, TLR4 or TLR3 agonists (CpG, LPS or PolyI:C, respectively), we observed that all treatments blocked liver stage differentiation and protected 100% of mice from the development of blood stage infection (Figure 7B). In contrast, none of the TLR agonists proved sufficient to block liver stage differentiation following infection with 100 P. yoelii sporozoites when administered 24 hrs post-challenge (Figure 7B). To determine whether IFN-γ and/or TNF-α contributed to the results observed with P. berghei-infected CpG-treated mice, we neutralized these cytokines in parallel experiments. We found that we could partially reverse the protective effect of CpG treatment on P. berghei liver stage development following neutralization of either TNF-α or IFN-γ (Figure 7C), demonstrating that the protective effect of the TLR9 agonist is partially mediated by these two cytokines. Previous reports suggest that P. yoelii replicates faster in the rodent liver compared to P. berghei, based on the kinetics of parasite rRNA accumulation (50). Therefore, we attempted to determine whether a window of susceptibility to inflammation exists for P. yoelii. To do this, we administered CpG one day prior, during, or at regular intervals after sporozoite challenge. In line with previous studies (49), we found that pre-treatment of mice with CpG partially prevented the development of blood stage P. yoelii parasitemia (Figure 7D). We also determined that P. yoelii is only partially susceptible to the effects of CpG following sporozoite challenge, and only if CpG is administered by 12 hrs post-challenge (Figure 7D). Collectively, these data show that while there is partial overlap, there are significant and inherent differences between two rodent species of Plasmodia with respect to the immune-effector molecules that act to prevent or block liver stage differentiation.
Figure 7. Generalized inflammation protects against P. berghei, but not P. yoelii, liver stage infection.
Experimental overview (A) and tabulated results (B) of P. berghei and P. yoelii sporozoite challenge studies conducted with the TLR agonists, CpG, LPS and PolyI:C. Data in B are pooled results from 3 independent experiments. Numbers indicate % protection (no. protected/no. challenged). (C) Neutralization of TNF-α or IFN-γ abrogates CpG-induced protection against P. berghei sporozoite challenge. Neutralizing antibodies were administered to mice at the time of sporozoite challenge. Data in C are results from a single experiment. (D) P. yoelii is less susceptible to generalized inflammation induced by administration of CpG to naïve WT BALB/c mice. CpG was administered at the indicated time surrounding P. yoelii sporozoite challenge. Data in D are pooled results from 2 independent experiments.
Discussion
In this study we demonstrate that there are both overlapping immune-effector pathways, such as those involving IFN-γ or TNF-α, and non-overlapping pathways, such as perforin-associated direct killing, used by CS-specific memory CD8 T cells to prevent P. berghei and P. yoelii liver stage infection. We also demonstrate the generalized inflammation is sufficient to halt liver stage P. berghei, but not P. yoelii, liver stage survival or differentiation. Collectively our data show that certain key immune-effector molecules differentially impact CS-specific memory CD8 T cell-mediated protective immunity to liver stage Plasmodium infection, and that the pathways that limit or prevent liver stage infection are in part Plasmodium species-specific. Whether the immune-effector pathways are similar for protective memory CD8 T cells targeting non-CS antigens remains to be determined. However, our data clearly show that resistance to the pre-erythyocytic cycle of Plasmodia infections mediated by bona fide CS-specific memory CD8 T cells is multifactorial, with no single immune-effector molecule required for sterile protection.
While a role for IFN-γ in preventing liver stage Plasmodia infection has long been described in the context of whole-parasite immunization models, our results are the first to show that IFN-γ is central to protection mediated by bona fide CS-specific memory CD8 T cells. Indeed, for several studies utilizing attenuated whole-parasite immunization, the contribution of IFN-γ has been controversial (21, 25–28). While the reasons for these disparate results are not clear, they are likely related to; 1) unequal numbers, phenotype or distribution of parasite-specific CD8 T cells that arise following RAS immunization of WT, IFN-γ−/− or IFN-γR−/− mice; 2) the participation of additional arms of the adaptive immune response following multiple whole-parasite immunizations, including CD4 T cells and antibody-secreting B cells; or 3) the timing of the sporozoite challenge: RAS-immune mice and recipients of WT or IFN-γ−/− TCR-Tg T cells are generally challenged very early following the last booster immunization (1–2 weeks). For RAS models, this early challenge is performed because RAS-induced immunity often wanes (12, 14, 51, 52). Thus, most previous work has commonly examined protection during the effector phase of the CD8 T cell response, in systems wherein parasite-specific CD4 T cells and antibodies also contribute to protection. Recent work in our lab has ruled out any protective contribution of CD4 T cells and antibodies in DC-prime, LM-boost immunized mice (53). Indeed, the experiments described in our study directly examine protection mediated by late-memory (>60 days post immunization) CS-specific CD8 T cells and clearly demonstrate a critical role for IFN-γ in protection against Plasmodium liver stage infection. Given the exceedingly high numerical requirements for sterilizing immunity following sporozoite challenge (34), we found it infeasible to evaluate sterilizing anti-sporozoite immunity following adoptive transfer of WT and mutant CS-specific memory CD8 T cells. While it may be possible to examine reductions in parasite rRNA burden in such adoptive transfer experiments, this requires challenging mice with supraphysiological doses of sporozoites (~10,000), a scenario that we wished to avoid. Thus, at present we have been unable to address a memory CD8 T cell-intrinsic role for IFN-γ. On the other hand, the use of sterilizing immunity as a read-out for protection also has limitations, as the elimination of all but a single infected hepatocyte can still result in blood stage parasitemia. Indeed, we cannot rule out that vaccinated GKO mice that succumbed to blood stage infection following P. berghei sporozoite challenge (Figure 3A) may well have eliminated 99% of infected hepatocytes. Nevertheless, our studies convincingly demonstrate the contribution of IFN-γ in the most stringent of settings (the requisite targeting of 100% of infected hepatocytes) where CS-specific memory CD8 T cells are the only adaptive immune cell type capable of responding to the early liver stage of the parasite infection.
How IFN-γ contributes to protection in vivo is not definitively established, but several studies have implicated IFN-γ-induced hepatocyte upregulation of iNOS as a critical component of this cytokine’s protective effect. Indeed, the administration of inhibitors of iNOS to sporozoite-challenged, IR-S vaccinated mice, or hepatocyte cultures, has suggested an important role for hepatocyte-derived nitric oxide in limiting parasite survival and/or development within infected cells (19) (28, 54). We attempted to evaluate the contribution of iNOS to our CS-specific memory CD8 T cell-mediated protective model, but we were unable to find any effect following in vivo administration of the iNOS inhibitors L-NIL or aminoguanidine to DC-primed, LM-boosted WT BALB/c mice (data not shown). However, we did find a very modest effect following in vivo administration of another inhibitor of iNOS, s-methylisothiourea (data not shown), suggesting that in our model the IFN-γ/TNF-α-iNOS axis contributes to protection. It has also been suggested that CD8 T cell-derived IFN-γ functions through a positive feedback loop involving the induction of IL-12 from liver macrophages or DC, which subsequently triggers the release of additional IFN-γ from liver-resident NK cells (23, 55). When we examined the potential contribution of this pathway in our immunization model, we found no difference in the ability of DC-prime, LM-boost immunized IL-12p40−/−BALB/c mice to resist sporozoite challenge when compared to WT mice (data not shown). Lastly, in vitro administration of IFN-γ has been shown to induce the upregulation of hepatocyte MHC class I. Thus, in our model it is possible that IFN-γ induces MHC class I expression on infected target cells, which may enhance recognition by memory CD8 T cells as they mediate protection thorough direct cytotoxicity or through the elaboration of anti-parasitic cytokines. Indeed, whether IL-12, iNOS or the upregulation of hepatocyte MHC class I are central to the protective pathways mediated by IFN-γ-competent, CS-specific memory CD8 T cells remains to be determined.
The novel observation of a protective role for TNF-α in the context of memory CD8 T cell-mediated protective immunity is also of interest. Similar to IFN-γ, this proinflammatory cytokine has been shown to participate in the induction of iNOS when administered in combination with IL-6 to cultured hepatocytes (45–47). Interestingly, when we monitored serum cytokine changes following sporozoite challenge of naïve and immunized WT BALB/c mice, we found only IL-6 and IFN-γ among 15 other cytokines and chemokines), were significantly upregulated (data not shown). Thus, in the context of P. berghei sporozoite challenge, where the parasite is sensitive to non-specific, generalized inflammation (Figure 4), TNF-α may act in concert with IL-6 (and possibly IFN-γ) to limit parasite survival or block differentiation within hepatocytes. Whether IL-6 and TNF-α, or IFN-γ and TNF-α, act synergistically in our model of CS-specific memory CD8 T cell-mediated protection is currently under investigation.
The notion that memory CD8 T cells afford protection against P. berghei and P. yoelii via at least one non-overlapping effector pathway was surprising. Our data suggest that for P. berghei, it may be sufficient for CS-specific memory CD8 T cells to initiate or potentiate an inflammatory cascade that culminates in the developmental inhibition or killing of parasites within host hepatocytes, via TNF-α and/or IFN-γ. Thus, although direct killing infected hepatocytes by CS-specific memory CD8 T cells may contribute to protection against P. berghei liver infection, our data show that this mode is non-essential, as removal of the classical extrinsic cell death pathways mediated by cytotoxic CD8 T cells (i.e. perforin, FasL, TRAIL) does not influence protection. On the other hand, that we observe significantly reduced protection in PKO mice following P. yoelii sporozoite challenge suggests greater emphasis on pathways involving direct killing of infected cells in the context of P. yoelii infection, independent of the FasL- and TRAIL-mediated pathways. This surprising observation suggests the possibility that for other species of Plasmodia, including the two clinically relevant human species, P. faciparum and P. vivax, the pathways of CD8 T cell-mediated protective immunity may also differ.
Although efforts thus far have met with limited success, the development of an efficacious pre-erythrocytic vaccine remains an important goal for combating human Plasmodium infection. Our work identifies critical similarities and differences between the pathways that CS-specific memory CD8 T cells utilize to protect against liver stage infection for two species of rodent Plasmodia. Our work also implicates TNF-α as an additional critical in vivo component of protective, anti-sporozoite immunity. Whether similar discordance in protective memory CD8 T cell effector molecules and pathways exists for the two clinically relevant species of human Plasmodia, P. falciparum and P. vivax, remains to be determined. However, our data highlight that key differences may exist and that such information should be considered in the rational design of vaccines targeting liver stage Plasmodium infection of humans.
Acknowledgments
We thank members of the J.T.H. laboratory for helpful discussion, Dr. Vladimir Badovinac for critical comments on the manuscript and Lecia Pewe and Jemmie Hoang for technical assistance. We thank Brendan Dunphy, Brad Tucker and Lyric Bartholomay from Iowa State University for providing P. berghei-infected mosquitoes. We also thank Ana Rodriguez and the Insectary Staff at New York University for providing P. yoelii-infected mosquitoes.
Footnotes
This work was supported by grants from the National Institutes of Health, the Department of Microbiology and Carver College of Medicine, University of Iowa, and a National Institutes of Health Postdoctoral Training Grant (T32 AI0726024 to N.S.B).
Abbreviations used in this paper: RAS, radiation-attenuated sporozoite; GAP, genetically-attenuated parasite; IFN-γ, GKO; perforin−/−, PKO; DC, dendritic cell; LM; Listeria monocytogenes
References
- 1.Todryk SM, Hill AV. Malaria vaccines: the stage we are at. Nature reviews. 2007;5:487–489. doi: 10.1038/nrmicro1712. [DOI] [PubMed] [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Hay SI, Snow RW. The Malaria Atlas Project: Developing Global Maps of Malaria Risk. PLoS Med. 2006;3:e473. doi: 10.1371/journal.pmed.0030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidjanski S, Vanderberg JP. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. The American journal of tropical medicine and hygiene. 1997;57:426–429. doi: 10.4269/ajtmh.1997.57.426. [DOI] [PubMed] [Google Scholar]
- 5.Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nature medicine. 2006;12:220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- 6.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. International journal for parasitology. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Baer K, Klotz C, Kappe SH, Schnieder T, Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS pathogens. 2007;3:e171. doi: 10.1371/journal.ppat.0030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science (New York, NY. 2006;313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 9.Meis JF, Verhave JP, Jap PH, Sinden RE, Meuwissen JH. Malaria parasites--discovery of the early liver form. Nature. 1983;302:424–426. doi: 10.1038/302424a0. [DOI] [PubMed] [Google Scholar]
- 10.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 11.Nussenzweig RS, Vanderberg JP, Most H, Orton C. Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites. Nature. 1969;222:488–489. doi: 10.1038/222488a0. [DOI] [PubMed] [Google Scholar]
- 12.Pacheco ND, McConnell E, Beaudoin RL. Duration of immunity following a single vaccination with irradiated sporozoites of Plasmodium berghei. Bulletin of the World Health Organization. 1979;57(Suppl 1):159–163. [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffe RI, Lowell GH, Gordon DM. Differences in susceptibility among mouse strains to infection with Plasmodium berghei (ANKA clone) sporozoites and its relationship to protection by gamma-irradiated sporozoites. The American journal of tropical medicine and hygiene. 1990;42:309–313. doi: 10.4269/ajtmh.1990.42.309. [DOI] [PubMed] [Google Scholar]
- 14.Winger LA, Sinden RE. Immunoprotection in mice susceptible to waning memory against the pre-erythrocytic stages of malaria after validated immunisation with irradiated sporozoites of Plasmodium berghei. Parasitology research. 1992;78:427–432. doi: 10.1007/BF00931700. [DOI] [PubMed] [Google Scholar]
- 15.Mueller AK, Deckert M, Heiss K, Goetz K, Matuschewski K, Schluter D. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. The American journal of pathology. 2007;171:107–115. doi: 10.2353/ajpath.2007.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarun AS, Dumpit RF, Camargo N, Labaied M, Liu P, Takagi A, Wang R, Kappe SH. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. The Journal of infectious diseases. 2007;196:608–616. doi: 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- 17.Douradinha B, van Dijk MR, Ataide R, van Gemert GJ, Thompson J, Franetich JF, Mazier D, Luty AJ, Sauerwein R, Janse CJ, Waters AP, Mota MM. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. International journal for parasitology. 2007;37:1511–1519. doi: 10.1016/j.ijpara.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Jobe O, Lumsden J, Mueller AK, Williams J, Silva-Rivera H, Kappe SH, Schwenk RJ, Matuschewski K, Krzych U. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex Class I-dependent interferon-gamma-producing CD8+ T cells. The Journal of infectious diseases. 2007;196:599–607. doi: 10.1086/519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seguin MC, Klotz FW, Schneider I, Weir JP, Goodbary M, Slayter M, Raney JJ, Aniagolu JU, Green SJ. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. The Journal of experimental medicine. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White KL, Snyder HL, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol. 1996;156:3374–3381. [PubMed] [Google Scholar]
- 21.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 22.Weiss WR, Sedegah M, Berzofsky JA, Hoffman SL. The role of CD4+ T cells in immunity to malaria sporozoites. J Immunol. 1993;151:2690–2698. [PubMed] [Google Scholar]
- 23.Doolan DL, Hoffman SL. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 24.Oliveira GA, Kumar KA, Calvo-Calle JM, Othoro C, Altszuler D, Nussenzweig V, Nardin EH. Class II-restricted protective immunity induced by malaria sporozoites. Infect Immun. 2008;76:1200–1206. doi: 10.1128/IAI.00566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 26.Zavala F. The multifactorial character of the protective immunity induced by immunization with sporozoites. Res Immunol. 1991;142:654–658. doi: 10.1016/0923-2494(91)90144-8. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues EG, Claassen J, Lee S, Wilson JM, Nussenzweig RS, Tsuji M. Interferon-gamma-independent CD8+ T cell-mediated protective anti-malaria immunity elicited by recombinant adenovirus. Parasite immunology. 2000;22:157–160. doi: 10.1046/j.1365-3024.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji M, Miyahira Y, Nussenzweig RS, Aguet M, Reichel M, Zavala F. Development of antimalaria immunity in mice lacking IFN-gamma receptor. J Immunol. 1995;154:5338–5344. [PubMed] [Google Scholar]
- 29.Renggli J, Hahne M, Matile H, Betschart B, Tschopp J, Corradin G. Elimination of P. berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite immunology. 1997;19:145–148. doi: 10.1046/j.1365-3024.1997.d01-190.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarty S, Baldeviano GC, Overstreet MG, Zavala F. Effector CD8+ T lymphocytes against liver stages of Plasmodium yoelii do not require gamma interferon for antiparasite activity. Infect Immun. 2008;76:3628–3631. doi: 10.1128/IAI.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockburn IA, Zavala F. T cell memory in malaria. Curr Opin Immunol. 2007;19:424–429. doi: 10.1016/j.coi.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Morrot A, Zavala F. Effector and memory CD8+ T cells as seen in immunity to malaria. Immunological reviews. 2004;201:291–303. doi: 10.1111/j.0105-2896.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 36.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 37.White DW, Harty JT. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J Immunol. 1998;160:898–905. [PubMed] [Google Scholar]
- 38.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science (New York, NY. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 39.Reyes-Sandoval A, Sridhar S, Berthoud T, Moore AC, Harty JT, Gilbert SC, Gao G, Ertl HC, Wilson JC, Hill AV. Single-dose immunogenicity and protective efficacy of simian adenoviral vectors against Plasmodium berghei. Eur J Immunol. 2008;38:732–741. doi: 10.1002/eji.200737672. [DOI] [PubMed] [Google Scholar]
- 40.Mundt B, Wirth T, Zender L, Waltemathe M, Trautwein C, Manns MP, Kuhnel F, Kubicka S. Tumour necrosis factor related apoptosis inducing ligand (TRAIL) induces hepatic steatosis in viral hepatitis and after alcohol intake. Gut. 2005;54:1590–1596. doi: 10.1136/gut.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 43.Badovinac VP, Harty JT. Intracellular staining for TNF and IFN-gamma detects different frequencies of antigen-specific CD8(+) T cells. Journal of immunological methods. 2000;238:107–117. doi: 10.1016/s0022-1759(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 44.Rudiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 45.Nussler A, Pied S, Goma J, Renia L, Miltgen F, Grau GE, Mazier D. TNF inhibits malaria hepatic stages in vitro via synthesis of IL-6. International immunology. 1991;3:317–321. doi: 10.1093/intimm/3.4.317. [DOI] [PubMed] [Google Scholar]
- 46.Pied S, Renia L, Nussler A, Miltgen F, Mazier D. Inhibitory activity of IL-6 on malaria hepatic stages. Parasite immunology. 1991;13:211–217. doi: 10.1111/j.1365-3024.1991.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 47.Nussler A, Drapier JC, Renia L, Pied S, Miltgen F, Gentilini M, Mazier D. L-arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991;21:227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- 48.Chakravarty S, I, Cockburn A, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nature medicine. 2007;13:1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 49.Gramzinski RA, Doolan DL, Sedegah M, Davis HL, Krieg AM, Hoffman SL. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect Immun. 2001;69:1643–1649. doi: 10.1128/IAI.69.3.1643-1649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briones MR, Tsuji M, Nussenzweig V. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Molecular and biochemical parasitology. 1996;77:7–17. doi: 10.1016/0166-6851(96)02574-1. [DOI] [PubMed] [Google Scholar]
- 51.Nussenzweig R, Vanderberg J, Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity. Military medicine. 1969;134:1176–1182. [PubMed] [Google Scholar]
- 52.Sedegah M, Brice GT, Rogers WO, Doolan DL, Charoenvit Y, Jones TR, Majam VF, Belmonte A, Lu M, Belmonte M, Carucci DJ, Hoffman SL. Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8(+)-T-cell populations. Infect Immun. 2002;70:3493–3499. doi: 10.1128/IAI.70.7.3493-3499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt NW, Butler NS, Harty JT. CD8 T cell immunity to Plasmodium permits generation of protective antibodies after repeated sporozoite challenge. Vaccine. 2009;27:6103–6106. doi: 10.1016/j.vaccine.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klotz FW, Scheller LF, Seguin MC, Kumar N, Marletta MA, Green SJ, Azad AF. Co-localization of inducible-nitric oxide synthase and Plasmodium berghei in hepatocytes from rats immunized with irradiated sporozoites. J Immunol. 1995;154:3391–3395. [PubMed] [Google Scholar]
- 55.Berg RE, Crossley E, Murray S, Forman J. Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J Immunol. 2005;175:1751–1757. doi: 10.4049/jimmunol.175.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]