Abstract
Background
Systemic ketamine can trigger apoptosis in the brain of rodents and primates during susceptible developmental periods. Clinically, spinally administered ketamine may improve the duration or quality of analgesia in children. Ketamine-induced spinal cord toxicity has been reported in adult animals, but has not been systematically studied in early development.
Methods
In anesthetized rat pups, intrathecal ketamine was administered by lumbar percutaneous injection. Changes in mechanical withdrawal threshold evaluated dose-dependent antinociceptive and carrageenan-induced anti-hyperalgesic effects in postnatal day (P)3 and 21 rat pups. Following intrathecal ketamine at P3, 7 or 21, spinal cords were examined for apoptosis (Fluoro-Jade C and activated caspase-3), histopathological change, and glial responses (ionized calcium binding adapter molecule 1 and glial fibrillary acid protein). Following maximal doses of ketamine or saline at P3 or P21, sensory thresholds and gait analysis were evaluated at P35.
Results
Intrathecal ketamine 3 mg/kg at P3 and 15 mg/kg at P21 reverses carrageenan-induced hyperalgesia. Baseline neuronal apoptosis in the spinal cord was greater at P3 than P7, predominantly in the dorsal horn. Intrathecal ketamine 3–10 mg/kg in P3 pups (but not 15 mg/kg at P21) acutely increased apoptosis and microglial activation in the spinal cord, and altered spinal function (reduced mechanical withdrawal threshold and altered static gait parameters) at P35.
Conclusions
As acute pathology and long-term behavioral change occurred in the same dose range as antihyperalgesic effects, the therapeutic ratio of intrathecal ketamine is less than one in the neonatal rat. This measure facilitates comparison of the relative safety of spinally-administered analgesic agents.
Introduction
There is increasing evidence that systemic general anesthetics with n-methyl-D-aspartate (NMDA) antagonist or γ-amino butyric acid agonist action can trigger apoptosis within the brain of rodents and primates1–3 during susceptible developmental periods. Apoptosis or programmed cell death is part of a normal developmental process for removal of redundant neurons, but increased apoptosis following systemic neonatal exposure to anesthetics has been associated with alterations in behavior as well as deficits in learning and memory4,5. Regional anesthesia has been suggested as an alternative to avoid or reduce general anesthetic exposure6, but the potential for apoptosis and local toxicity following spinal administration of anesthetic and analgesic drugs has not been evaluated in early developmental models. One agent of particular interest is the NMDA antagonist ketamine. Systemic administration of ketamine produces dose-dependent apoptosis in rodents7–9 and primates10–12. Clinically, ketamine may be administered not only systemically, but also spinally in combination with local anesthetic to prolong the duration or improve the quality of analgesia13. Surveys of pediatric anaesthetists in the United Kingdom reported that 32% added ketamine to caudal local anesthetic14, and that 15% added ketamine to epidural boluses or infusions15. However, ketamine-induced spinal cord toxicity has been reported in adult animals and the safety of spinal ketamine in children has been questioned16. While the majority of controlled trials investigating analgesic efficacy of spinal ketamine have been conducted in children over 6 months of age13, some studies have included infants17,18 and neonates19,20. In early life, the potential for spinally administered ketamine to produce apoptosis in the spinal cord is an additional concern, over and above issues of local toxicity related to different preparations of the drug. Prolonged general anesthesia in postnatal day 7 rats increased apoptosis in the spinal cord21, but effects at different ages were not evaluated. As age-related and regional differences in susceptibility to apoptosis following NMDA antagonists have been noted in the brain22, effects of spinally administered ketamine on apoptosis in the spinal cord at a range of postnatal ages requires investigation.
Using our recently developed model for preclinical safety evaluation of spinal drugs in neonatal rats,23 we examined the effects of intrathecal ketamine in rats aged postnatal day (P) 3, 7, or 21. Spinal cords were examined for: apoptosis using Fluoro-Jade C and activated caspase-3 immunohistochemistry; histopathological change with hematoxylin and eosin staining; and glial responses with ionized calcium binding adapter molecule 1 (Iba1) and glial fibrillary acidic protein (GFAP) immunohistochemistry. In addition, longer-term functional outcomes were assessed at P35 by changes in sensory thresholds and gait analysis. Finally, the antihyperalgesic dose of ketamine was determined, and a therapeutic index was calculated (toxic dose / analgesic dose), to allow comparison of the relative safety with other spinally administered analgesic agents.
Materials and Methods
All experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California San Diego, La Jolla, California (under the Guide for Care and Use of Laboratory Animals, National Institutes of Health publication 85-23, Bethesda, MD). Timed pregnant Holtzman rats were obtained (Sprague-Dawley, Harlan, Indianapolis, IN) and housed in a 12-h light-dark cycle with free access to food and water. On postnatal (P) day 3 or 7, pups were randomly assigned to treatment groups containing equal numbers of males and females, and if necessary litters were culled to a maximum of 12 pups. Pups were kept under radiant heat during treatment to maintain body temperature. The duration of maternal separation was minimized and was the same for control and treatment animals. Pups were weaned into same sex cages at P21. Separate groups of male and female P17–19 Holtzman rats were assigned to treatment groups at P21. Animals were regularly monitored and maintained until further testing at 5 weeks of age.
Intrathecal injection technique
Pups were anesthetized with isoflurane (3–5%) in oxygen and air. Percutaneous intrathecal injections were made at the low lumbar level (intervertebral space L4–5 or L5-L6) with a 30-gauge needle perpendicular to the skin. Injectate volumes of 0.5 mcl per gram bodyweight (previously determined to produce spread across lumbar and low thoracic segments in rat pups23), were delivered using a hand-driven microinjector (P3 and P7) or a 50 mcl Hamilton syringe (P21).
Dose-response of intrathecal ketamine in rat pups
Intrathecal ketamine doses are limited by motor weakness and/or excitation, but these effects have been reported at doses between 0.2 and 3 mg/kg in adult rodents24–26. Therefore, in pilot experiments increasing doses of preservative-free ketamine hydrochloride (Sigma-Aldrich, St Louis, MO) dissolved in sterile saline were administered to P3 and P21 pups to determine the maximum tolerated dose at each age. In P3 pups, 0.1 to 0.3 mg/kg produced no change in behavior or mechanical withdrawal threshold, 3 mg/kg and 10 mg/kg produced increasing initial sedation, and higher doses were lethal. In P21 pups, 15mg/kg intrathecal ketamine produced initial mild excitation, and higher doses were lethal. To assess acute toxicity, spinal cords were harvested 24 h after the following intrathecal ketamine injections:
P3 pups received 3 mg/kg or 10 mg/kg (mean body weight of 10 g giving a nominal injection volume of 5 mcl of 6 or 20 mg/ml ketamine and average total dose per animal of 30 or 100 mcg);
P7 pups received 3 mg/kg or 10 mg/kg (mean body weight of 16 g giving a nominal injection volume of 8 mcl of 6 or 20 mg/ml ketamine and average total dose per animal of 48 or 160 mcg);
P21 pups received 15 mg/kg (mean body weight 60 g giving nominal injection volume of 30 mcl of 30 mg/ml ketamine and average total dose per animal of 900 mcg).
Mechanical withdrawal thresholds were measured at baseline, 30 min after injection, and prior to sacrifice at 7 days. In addition, to assess apoptosis at an earlier time point P3 or P7 pups received 10 mg/kg intrathecal ketamine and spinal cords were harvested at 6 h.
In adult rodents, antihyperalgesic effects of intrathecal ketamine have been demonstrated in tissue injury models. Therefore, hindpaw inflammation was induced in P3 or P21 pups, and changes in mechanical withdrawal threshold were used to evaluate acute antihyperalgesic effects of intrathecal ketamine. Pups were lightly restrained on a flat bench surface and calibrated von Frey hairs (Stoelting, Wood Dale, IL) that deliver increasing mechanical stimuli (0.4 to 60 g) were applied to the dorsal surface of the hindpaw five times at 1-s intervals. The number of evoked flexion withdrawals was recorded, and the maximum force applied was that which evoked five withdrawal responses27. Animals were anesthetized with isoflurane (3–5%) in oxygen and air, and 1 mcl/g of 2% lambda carrageenan (Sigma-Aldrich) was injected into the mid-plantar surface of the left hindpaw. Mechanical withdrawal thresholds were again determined 3 h after carrageenan. Then, under brief anesthesia, 0.5 mcl/g intrathecal saline or ketamine (P3: 0.03, 0.3, or 3 mg/kg; P21: 1.5, 5, or 15 mg/kg; n = 4–6 all groups) was administered via percutaneous injection. Mechanical withdrawal thresholds were measured 30, 60, and 120 min following injection, with the investigator unaware of the treatment allocation.
Spinal cord preparation and staining
Details of the perfusion fixation and tissue preparation were as described in the companion manuscript23. In brief, terminally anesthetized animals were transcardially perfused with saline followed by 4% paraformaldehyde and spinal cords were dissected under a microscope. Distances from the injection site caudally to the end of the dissected cord and proximally to the lumbar enlargement were noted. Tissue was postfixed in 4% paraformaldehyde and then transferred to sucrose. Transverse blocks of the spinal cords caudal to the lumbar enlargement and just rostral to the level of injections were sectioned using a cryostat at 7 and 14 micrometer, mounted on Fisher Superfrost Plus (Fisher Scientific, Houston, TX) slides and then stored at −70°C.
Histology
Full details of the histopathology procedures are presented in the accompanying paper23. In brief, seven micron thick spinal cord sections from all experimental groups sacrificed at 1 and 7 days postinjection were evaluated by a neuropathologist (MG), who was unaware of the treatment group. Hematoxylin and eosin stained sections were examined for histopathological changes (evidence of cell injury or death, tissue necrosis, gliosis, inflammation or other changes). At least four sections were examined for each animal.
Fluoro-Jade
Fluoro-Jade C (Chemicon, Temecula, CA) staining was performed as previously described28 on 14 micron thick spinal cord sections prepared from tissue collected 6 or 24 h after intrathecal injection. Slides were coded, examined with the appropriate wavelength fluorescent microscopy, and an investigator unaware of treatment group counted the number of immunofluorescent cells and noted their distribution in the dorsal horn, ventral horn or adjacent to the central canal. Counts from at least four nonconsecutive sections of lumbosacral cord from each animal were averaged for statistical analysis.
Activated caspase-3
To further assess apoptosis, tissue obtained from P3 and P7 animals 6 or 24 h following intrathecal injection was stained for activated caspase-3 (1:100 Cell Signaling, Beverly, MA). Slides were coded and the number and location (dorsal horn, ventral horn or adjacent to central canal) of caspase-3 immunoreactive cells were counted under light microscopy by an investigator unaware of the treatment group.
Glial fibrillary acidic protein and ionized calcium binding Iba1
Tissue obtained 7 days following intrathecal injection was stained with primary antibodies against astrocyte (1:500 mouse anti-glial fibrillary acidic protein; Chemicon) and microglial (1:1,000 rabbit anti-Iba-1; WAKO, Richmond, VA) markers. Spinal cord sections were imaged using the same settings on a microscope (Olympus BX51 microscope with appropriate wavelength fluorescence illuminator; Olympus America, Inc., Center Valley, PA) equipped with a digital camera and image-capture software (Image Pro Plus software, Media Cybernatics Inc, Silver Spring, MD). As described in the accompanying paper23 spinal cord sections taken 3 days following intraspinal injection of 0.4 mcl 20 nM NMDA provided a positive control for glial activation.
Using Image-J,# coded Iba1 immunohistochemistry sections were analyzed after colorsplit using the green channel only, then were manually given an individual threshold for background subtraction and analyzed for area fraction (area of positively stained cells as a percentage of total area = 1,280 ×1,024 pixels). The mean of values from four nonconsecutive sections of lumbosacral cord for each animal were calculated, and treatment groups containing n = 4 animals were analysed statistically.
Evaluation of long-term functional outcomes following intrathecal ketamine
Maximum doses of intrathecal ketamine were administered to P3 (10 mg/kg) or P21 (15 mg/kg) pups. Preservative-free ketamine hydrochloride (Sigma-Aldrich) was prepared in sterile saline immediately prior to injection. Control animals received intrathecal saline. Each treatment group comprised 9 or 10 animals, with 5 males and 4 or 5 females. Pups were regularly monitored following injection. Body weight, hind limb mechanical withdrawal threshold and thermal latency and gait parameters were measured at 5 weeks of age, with investigators unaware of treatment allocation.
At P35, mechanical withdrawal thresholds were determined using a modified version of the up–down method with calibrated von Frey hairs applied to the plantar surface of the hindpaw as previously described29. Rats were allowed to acclimatize for at least 30 min in a clear plastic cage with a wire mesh bottom. The 50% paw withdrawal threshold was determined with a series of von Frey filaments (Stoelting) beginning with a buckling weight of 2.0 gm up to a maximum of 15 gm. If paw lifting occurred the next weaker filament was applied, but if application of the filament for 5 s did not elicit a withdrawal response the next stronger filament was used.
Thermal withdrawal latency was determined using a modified Hargreaves Box30 (University Anesthesia Research and Development Group, University of California San Diego, La Jolla, California), consisting of a glass surface (maintained at 30 °C) on which the rats were placed in individual Plexiglas cubicles. The thermal nociceptive stimulus originates from a focused projection bulb positioned below the glass surface. A timer is activated by the light source, and latency was defined as the time required for the paw to show a brisk withdrawal as detected by photodiode motion sensors that stopped the timer and terminated the stimulus. In the absence of a response within 20 s, the stimulus was terminated (cut-off time). Three measures were obtained from each hindpaw and latency expressed as mean ± SEM.
The CatWalk system (Noldus Information Technology, Wageningen, The Netherlands) was used to evaluate and quantify changes in static and dynamic gait parameters, as previously described31. Animals were placed on a glass runway containing light that is internally reflected until paws touch the glass and light up the area of contact. A video camera below the runway collected images and data was acquired using the CatWalk 7.1.6 software. At P22–25, rats were placed on one end of the runway and allowed to explore the environment for about 5 min for three consecutive days. Animals then commenced a training paradigm, as previously described31,32. Briefly, animals were deprived of food for at least 3 h prior to testing, and then were allowed to spontaneously cross the runway towards food rewards positioned at the farther end. Training continued for two weeks and when the animals reached P35, crossings were recorded which met the following two criteria: (i) a maximal time of 2 s for crossing the 60-cm-long part of the CatWalk used for gait recording, (2) runway crossings were required to be without intermediate stops in gait. Three crossings per animal were analyzed using the CatWalk® 7.1.6 software.
Data analysis
For evaluation of mechanical withdrawal threshold at P3 and P21, the number of withdrawal responses was plotted against the mechanical stimulus (force expressed as grams on log10 scale). A sigmoidal stimulus-response curve with nonvariable slope was constructed using nonlinear regression curve fit. The mid point of the curve (50% effective force; EF50) was determined and designated the threshold as previously described27. The effect of carrageenan on withdrawal threshold was assessed by Student's paired two-tailed t-test (sample normally distributed). Acute changes in behavioural thresholds at baseline and 30 min after ketamine were analysed by two-way repeated measures ANOVA with time and treatment as variables and Bonferroni post hoc comparisons. Percentage reversal of hyperalgesia was calculated from the thresholds as: (post-dose - inflamed) / (baseline - inflamed) × 100. At P35, mechanical withdrawal thresholds were evaluated using the up-down method and values were calculated as described33 and represented as mean ± SEM. Thermal withdrawal latency was designated as the mean of three values for each hindpaw. Data was normally distributed (D'Agostino and Pearson normality test) and treatment groups were compared with one way analysis of variance (ANOVA) followed by post hoc tests for multiple comparisons. Data was analyzed using Prism version 5.0 (GraphPad, San Diego, CA). P <0.05 was considered statistically significant.
Results
Analgesic action of intrathecal ketamine
Intrathecal ketamine did not produce antinociceptive effects in rat pups. Mechanical withdrawal threshold did not differ from baseline 30 min after injection of saline (1.3 ± 0.1g vs. 1.1 ± 0.1 g; n = 7), ketamine 3 mg/kg (1.3 ± 0.1 g vs. 1.5 ± 0.2 g; n = 6), or ketamine 10 mg/kg (1.3 ± 0.1 vs. 1.3 ± 0.2 g; n = 8) in P3 pups. Similarly in P21 pups, mechanical thresholds did not differ 30 min after injection of saline (22.3 ± 1.8 g vs. 21.4 ± 0.9 g; n = 4) or ketamine 15 mg/kg (21.6 ± 1.1 g vs. 24.1 ± 1.2 g; n = 8) (not significant, two-way repeated measures ANOVA with time and treatment as variables and Bonferroni post hoc comparisons).
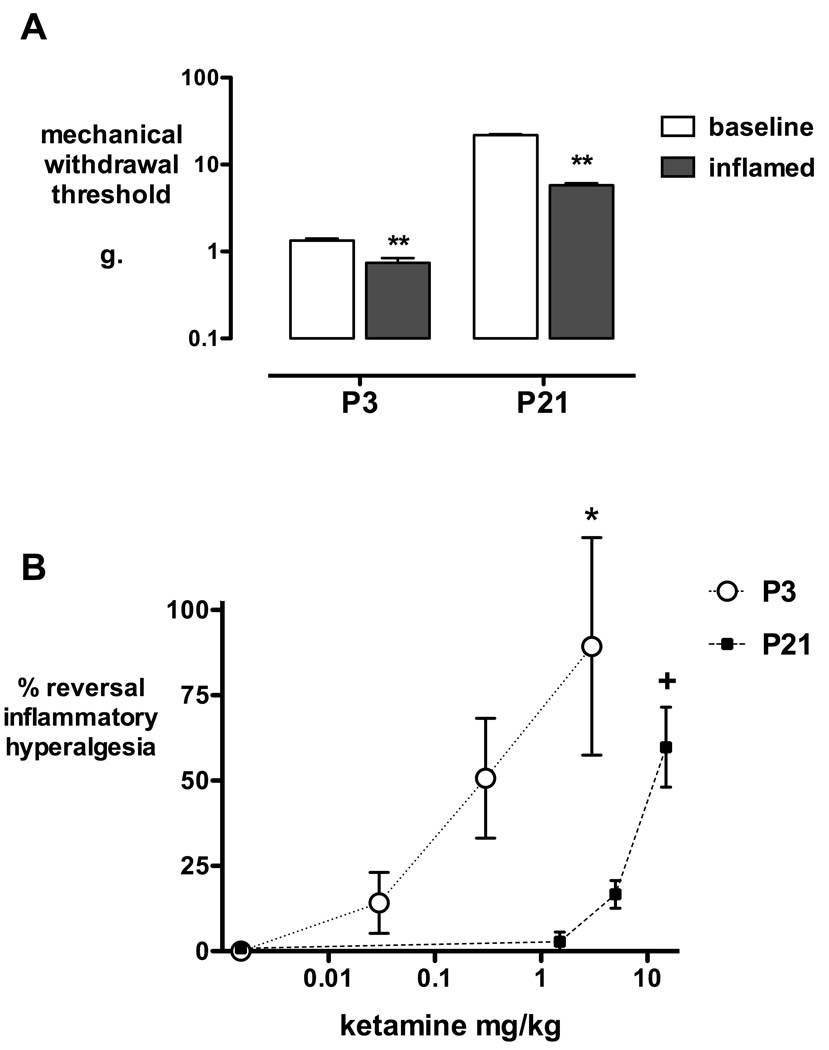
Intrathecal ketamine produced dose-dependent antihyperalgesic effects in P3 and P21 pups. There were no significant differences across treatment groups in thresholds measured at baseline or 3 h after carrageenan (not significant one-way ANOVA with Bonferroni post hoc comparisons). At both ages, hindpaw carrageenan produced inflammatory hyperalgesia as seen by a significant reduction in mechanical withdrawal threshold 3 h following injection (P < 0.01; fig.1A). As baseline mechanical thresholds vary with age (1.3 ± 0.07 g at P3, and 21.8 ± 0.6 g at P21), analgesic effects are expressed as percentage reversal of hyperalgesia to facilitate comparison of the degree of analgesia at different ages. Thirty minutes following intrathecal injection, hyperalgesia was significantly reversed by 3 mg/kg ketamine in P3 pups and 15 mg/kg ketamine in P21 pups (P < 0.05, one-way ANOVA with Bonferroni post hoc comparisons; fig. 1B).
Figure 1. Intrathecal ketamine and inflammatory hyperalgesia.
A: In P3 (n = 16) and P21 pups (n = 16), mechanical withdrawal thresholds are significantly decreased 3 h after hindpaw carrageenan. Data points = mean ± SEM; ** P < 0.01, Students two-tailed paired t-test. B: The dose-response for percentage reversal of hyperalgesia 30 min after intrathecal ketamine is shown for P3 and P21 rats. Data points = mean ± SEM; n = 4 per treatment group; * P < 0.05 saline versus ketamine 3 mg/kg; + P < 0.05 saline versus ketamine 15 mg/kg; one-way ANOVA with Bonferroni post-hoc comparisons.
Intrathecal ketamine and neuronal apoptosis
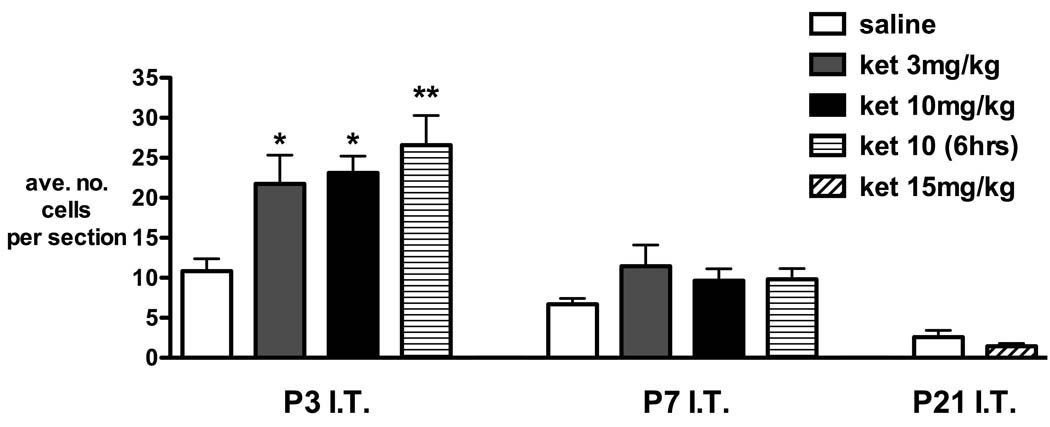
Figure 2 shows the effect of postnatal age (P3, P7, and P21) and treatment on the average number of Fluoro-Jade C positive cells in spinal cord sections. The degree of baseline apoptosis varies with postnatal age. In the saline group, the number of positive cells decreased with age (10.8 ± 1.5 at P3, 6.7 ± 0.7 at P7, 2.6 ± 0.8 at P21; mean ± SEM; P < 0.01 one-way ANOVA linear trend). We have previously shown that brief anesthesia for percutaneous injection does not influence apoptosis in the spinal cord, as cell counts did not differ between saline injected and naïve controls23.
Figure 2.
Fluoro-jade C staining following intrathecal injection of saline or ketamine in rats aged postnatal day (P) 3, 7, or 21. In P3 animals, positive cell counts were significantly increased 24 h after intrathecal (IT) injection of 3 and 10 mg/kg ketamine [ket 3 mg/kg, ket 10 mg/kg] and 6 h after 10 mg/kg ketamine [ket 10 (6 h)]. The number of positive cells for each animal was determined from the mean counts of at least four lumbosacral spinal cord sections. Bars = mean ± SEM, n = 4 animals each treatment group. * P < 0.05, ** P < 0.01 one-way ANOVA with Bonferroni post hoc comparisons versus saline.
In P3 pups, Fluoro-Jade C positive cell counts were significantly increased 24 h after injection of 3 or 10 mg/kg ketamine (P < 0.05, one-way ANOVA with Bonferroni post hoc comparisons). Numbers were slightly higher 6 h after injection of ketamine 10 mg/kg but did not differ significantly from values at 24 h. Following ketamine at P7 there was a slight increase in positive cell counts that was not statistically significant. Fluoro-Jade C positive cells were rarely seen in sections taken at P22 (24 h after P21 injection), and were not influenced by ketamine 15 mg/kg.
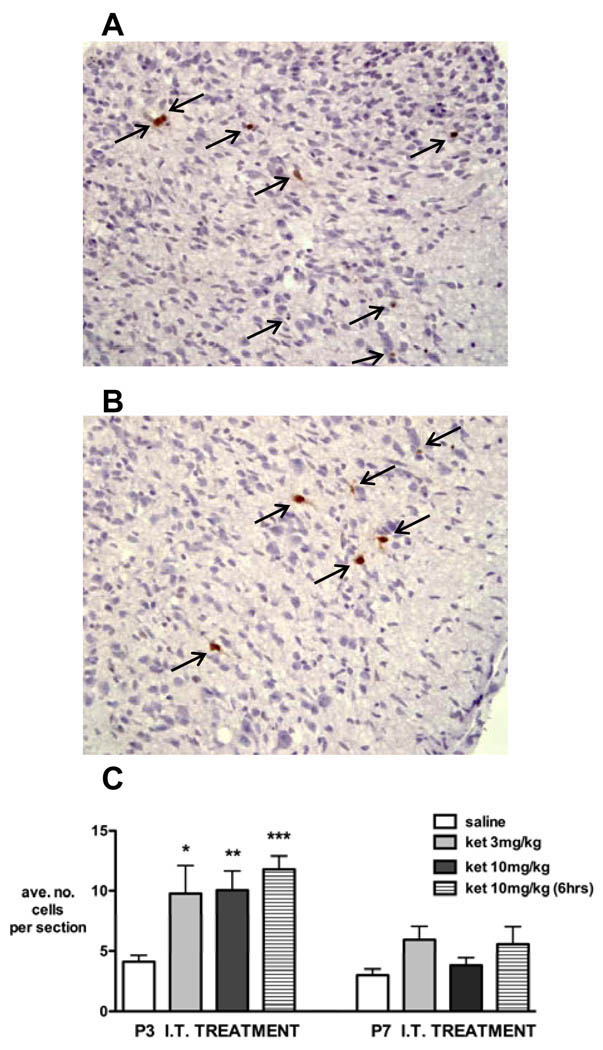
Consistent results were seen using activated caspase-3 to identify apoptotic neurons (fig. 3). The number of apoptotic cell profiles was significantly increased 24 h after ketamine 3 mg/kg, and 6 and 24 h after ketamine 10 mg/kg in P3 pups (P < 0.05, one-way ANOVA with Bonferroni post hoc comparisons). In P7 animals, increases following ketamine were not statistically significant.
Figure 3. Activated caspase-3 immunostaining after intrathecal injection of saline or ketamine in rats aged postnatal day (P) 3 or 7.
Representative sections from dorsal (A) and ventral (B) horn of spinal cord 24 h after injection of ketamine 3 mg/kg at P3.
C: In P3 animals, activated caspase-3 immunopositive cell counts 24 h after intrathecal (IT) injection of ketamine 3 mg/kg [ket 3 mg/kg, n = 4], ketamine 10 mg/kg [ket 10 mg/kg, n = 4] and 6 h after 10 mg/kg ketamine [ket 10 (6 h), n = 4] are increased compared with saline (n = 6). In P7 animals, numbers do not significantly differ following saline or ketamine (n = 4 all groups). The number of immunopositive cells for each animal was determined from the mean counts of at least four lumbosacral spinal cord sections. Bars = mean ± SEM, n = 4 animals each treatment group. * P < 0.05, ** P < 0.01, *** P < 0.001 one-way ANOVA with Bonferroni post hoc comparisons versus saline.
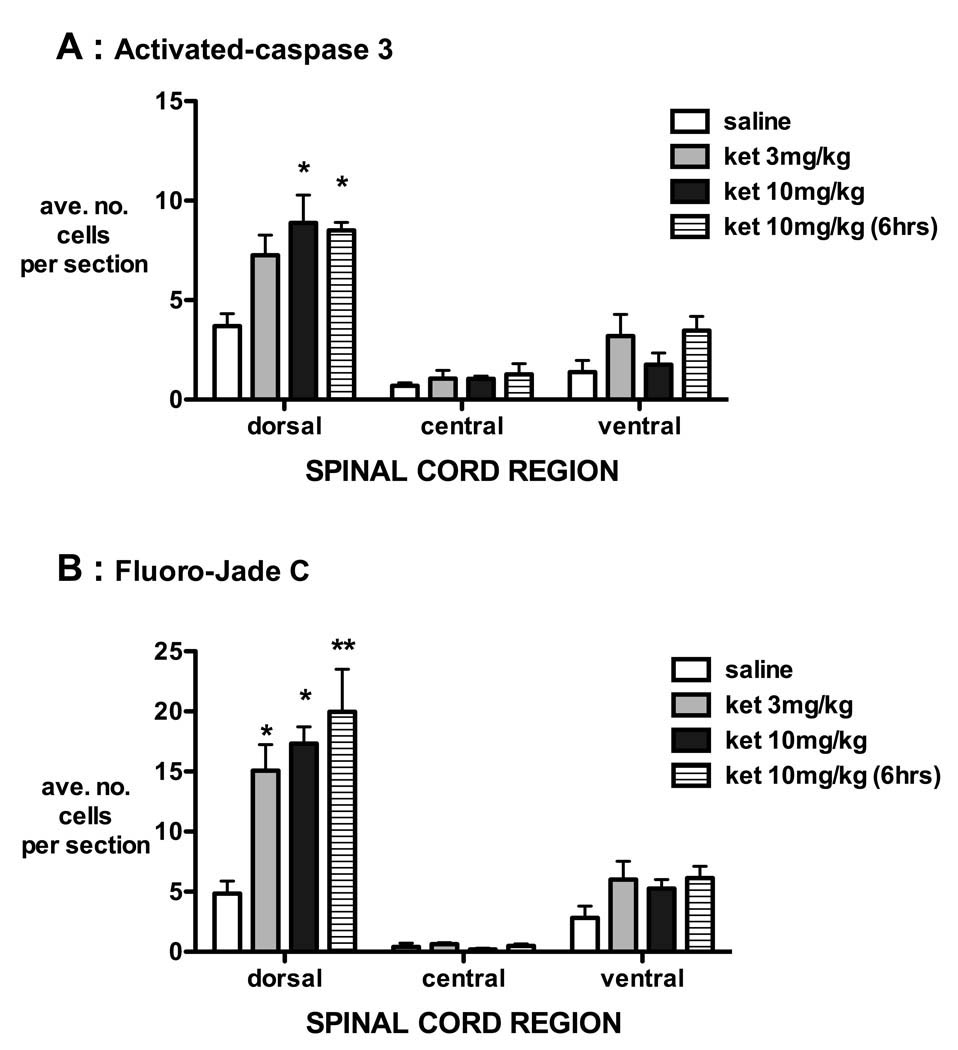
The majority of apoptotic cells were distributed throughout the dorsal horn of the spinal cord, and numbers in this region were significantly increased by intrathecal ketamine at P3 (fig. 4).
Figure 4.
Distribution of apoptotic cells in spinal cord sections of P3 rat pups after intrathecal injection of saline or ketamine. The location of positive cells was noted as being within the dorsal horn (dorsal), adjacent to the central canal (central) or within the ventral horn (ventral) following activated caspase-3 immunohistochemistry (A) or Fluoro-Jade C staining (B). The majority of apoptotic cells were distributed throughout the dorsal horn, and numbers within this region were significantly increased by 24 h after intrathecal (IT) injection of ketamine 3 mg/kg [ket 3 mg/kg], ketamine 10 mg/kg [ket 10 mg/kg] and 6 h after 10 mg/kg ketamine [ket 10 (6 h)]. The number of positive cells for each animal was determined from the mean counts of at least four lumbosacral spinal cord sections. Bars = mean ± SEM, n = 4 animals each treatment group. * P < 0.05 ** P < 0.01 one-way ANOVA with Bonferroni post-hoc comparison versus saline.
In P3 animals, apoptotic cells were also identified in haematoxylin and eosin sections of the spinal cord, and qualitative differences were not apparent between treatment groups. No necrosis, gliosis or inflammation was identified. One P21 animal (ketamine 15 mg/kg, 24-h survival) had focal subpial inflammation. No other histopathological changes were seen in the P21 animals.
Intrathecal ketamine and glial activation
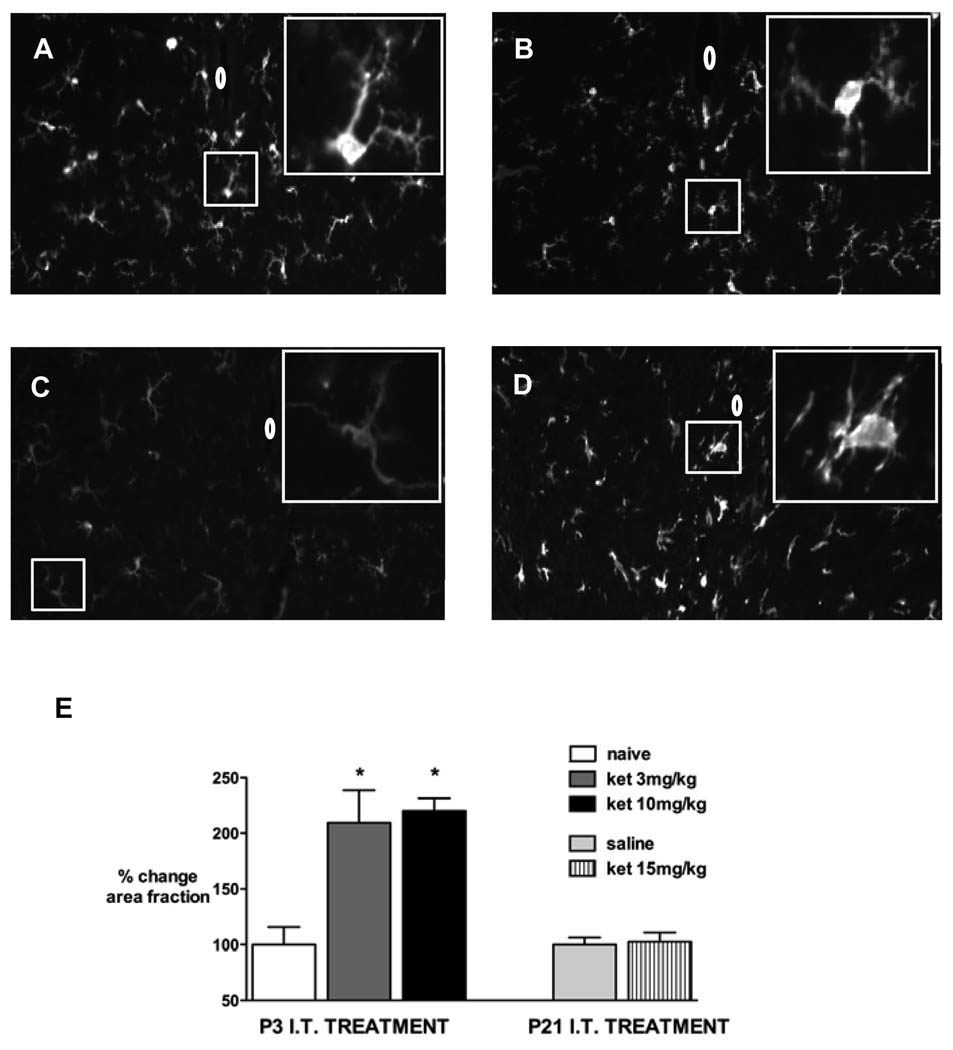
Iba1 immunoreactivity in the spinal cord was increased 7 days following injection of intrathecal ketamine 3 or 10 mg/kg in P3 pups (fig. 5). No change was apparent following ketamine injection in P21 pups.
Figure 5.
Representative spinal cord sections showing ionized calcium binding adapter molecule 1 (Iba1) immunoreactivity in the spinal cord 7 days following: P3 intrathecal (IT) injection of ketamine (ket) 3 mg/kg (A) or ketamine 10 mg/kg (B). Images are also shown from a naive age-matched P10 pup (C) and 3 days after intraspinal n-methyl-D-aspartate (NMDA) injection (D). Insets show high power magnification of microglia; 0 = position of central canal.
E: Immunofluorescence represented as % change in positive area fraction compared with control animals (naïve in P3, n = 3 and saline injection in P21, n = 3) 7 days after ketamine (ket) 3 mg/kg (n = 3) or ketamine 10 mg/kg (n = 4) at P3 or ketamine 15 mg/kg (n = 3) at P21. * P < 0.05 one-way ANOVA with Bonferroni post-hoc comparisons versus control.
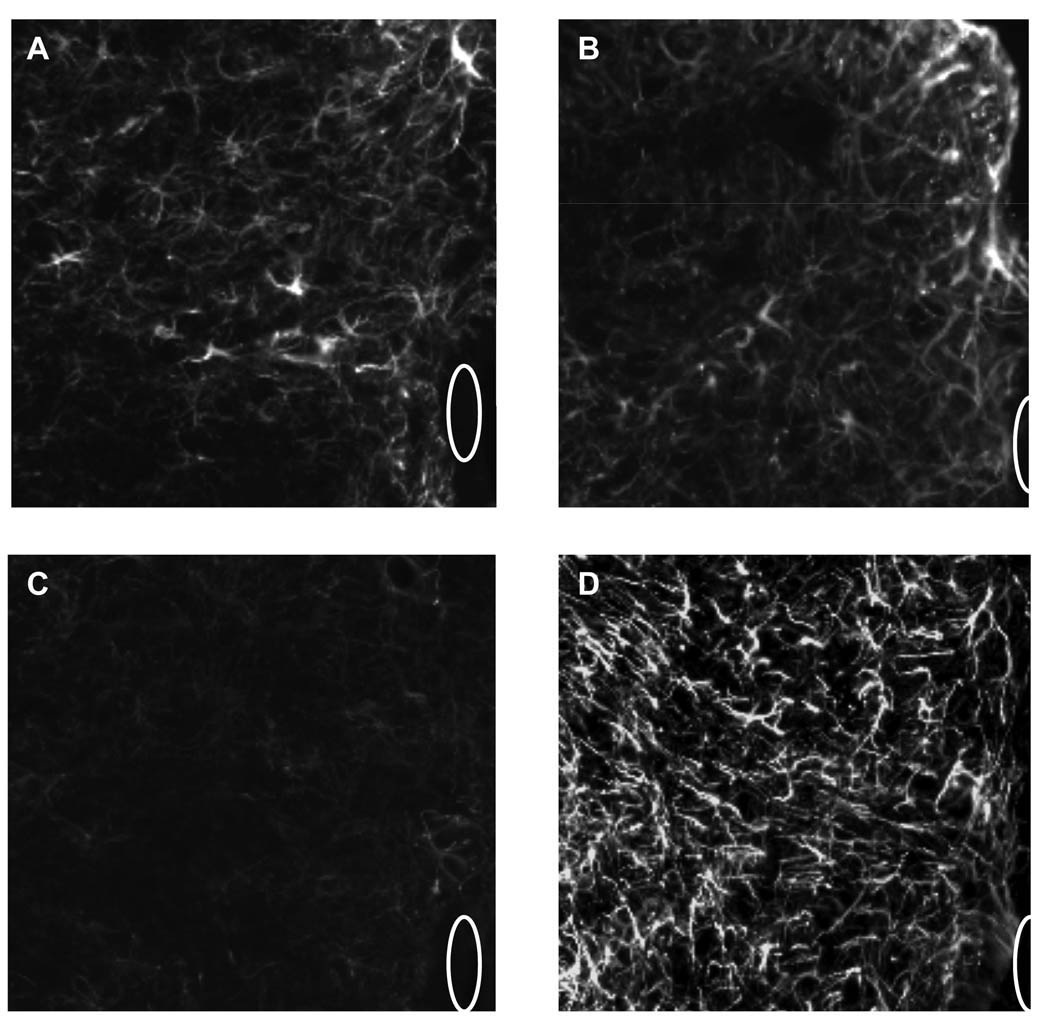
Seven days following injection of intrathecal ketamine in P3 or P21 rat pups there was no discernable increase in astrocyte staining as assessed by glial fibrillary acidic protein immunostaining (fig. 6), and no increased gliosis was visible with haematoxylin and eosin staining.
Figure 6.
Representative spinal cord sections showing glial fibrillary acidic protein (GFAP) immunoreactivity in the dorsal horn of the spinal cord 7 days after: P3 intrathecal injection of ketamine 3 mg/kg (A) or ketamine 10 mg/kg (B). Images are also shown from naive age-matched P10 pup (C) and 3 days after intraspinal n-methyl-D-aspartate (NMDA) injection (D). 0 = position of central canal.
Longer term functional effects following intrathecal ketamine
Mechanical withdrawal thresholds were measured in the above animals prior to sacrifice for histology. Mechanical withdrawal thresholds 7 days following P3 intrathecal injection of saline (3.0 ± 0.6 g; n = 3), ketamine 3 mg/kg (2.8 ± 0.8 g; n = 4), or ketamine 10 mg/kg (2.2 ± 0.5 g; n = 4) did not differ significantly. Although there was a trend to a lower threshold following high dose ketamine, the sample size was not sufficient to confirm this. Thresholds did not differ seven days following P21 injection of saline (25.1 ± 1.9 g; n = 4) or ketamine 15 mg/kg (27.9 ± 0.7 g; n = 4).
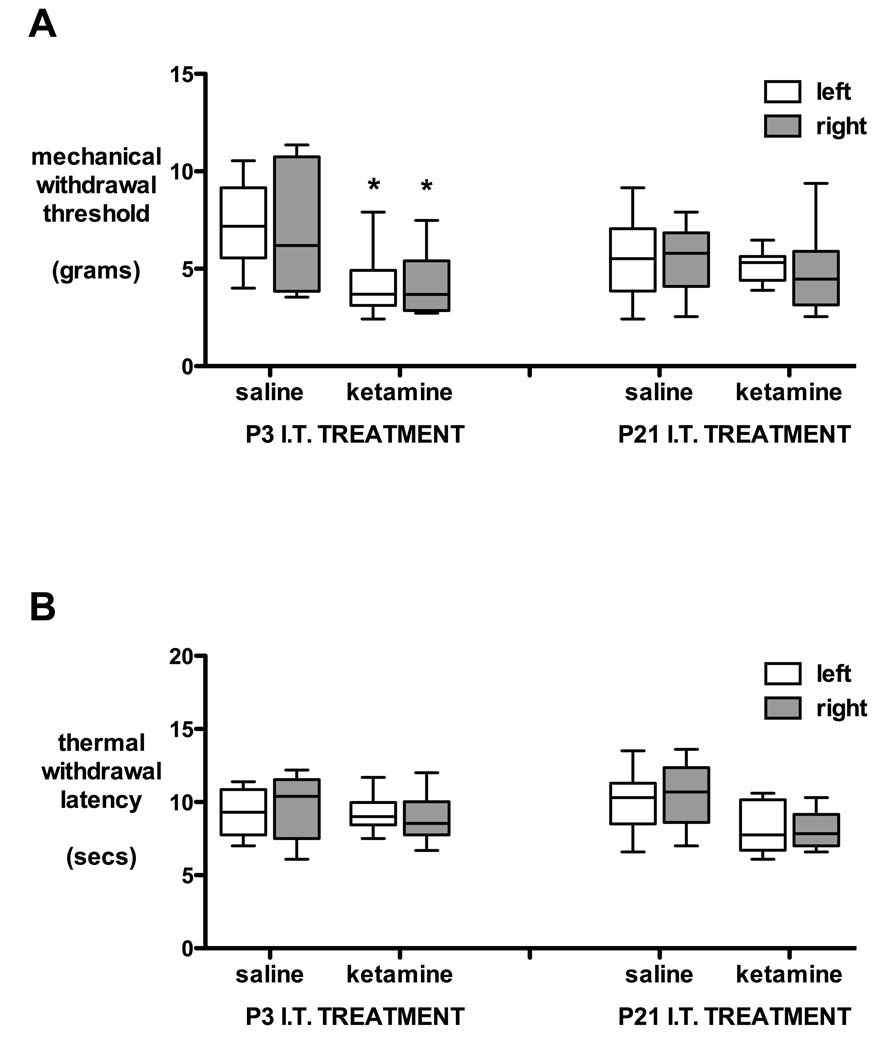
Additional groups of animals receiving saline or ketamine at P3 or P21 were evaluated at P35. Although females were lighter than males, overall body weight did not differ across groups (P3 saline = 129 ± 2 g, P3 10 mg/kg ketamine = 123 ± 3.1 g, P21 saline = 143 ± 5 g, P21 15 mg/kg ketamine = 136 ± 3.8 g; mean ± SEM; not significant one-way ANOVA). At P35, mechanical withdrawal thresholds were lower in the P3 ketamine group compared with the P3 saline group (P < 0.05 one way ANOVA; fig. 7A) but thermal withdrawal latencies (fig. 7B) did not differ in groups that had received intrathecal saline or ketamine at P3 or P21.
Figure 7.
Behavioral thresholds at 5 weeks of age after intrathecal injection of saline or ketamine on postnatal day (P) 3 or 21. Mechanical withdrawal thresholds (A) and thermal withdrawal latencies (B) of the left and right hindpaw are shown in animals that received saline or 10 mg/kg ketamine at P3, or saline or 15 mg/kg ketamine at P21. Box and whisker plot with 5–95% confidence intervals; n = 10 per group. * P < 0.05 one-way ANOVA with Bonferroni post hoc comparison P3 ketamine versus saline.
Gait analysis showed alterations in static parameters following injection of ketamine at P3. Both print area and print intensity were reduced at P35 following injection of ketamine at P3 (P<0.05 one way ANOVA with Bonferroni post-hoc comparisons; table 1). Duty cycle tended to be reduced following P3 ketamine, but differences from P3 saline were not statistically significant (P = 0.06). Intrathecal ketamine at P21 had no effect on gait. We have previously shown that gait parameters in saline injected animals did not differ from age-matched naïve animals23.
Table 1.
Gait Parameters at 5 Weeks Age after Intrathecal Ketamine or Saline at P3 or P21.
| TREATMENT | STATIC PARAMETERS | DYNAMIC PARAMETERS | ||||
|---|---|---|---|---|---|---|
| Print area | Print Intensity |
Coordination Regularity index |
Duty cycle | Stride length | Stability of gait |
|
| P3 saline (n = 11) |
40.3 ± 3.5 | 151.4 ± 4.6 | 99.8 ± 0.2 | 54.1 ± 1.4 | 107.5 ± 2.1 | 27.6 ± 0.7 |
| P3 ketamine 10 mg/kg (n = 10) |
25.5 ± 2.3* | 132 ± 3.0** | 99.8 ± 0.2 | 50.1 ± 1.5 | 106.9 ± 3.7 | 26.7 ± 0.6 |
| P21 saline (n = 13) |
38.2 ± 3.6 | 142.0 ± 4.5 | 99.3 ± 0.4 | 54.0 ± 1.5 | 108.4 ± 3.2 | 28.6 ± 0.7 |
| P21 ketamine 15 mg/kg (n = 11) |
32.9 ± 1.7 | 142.0 ± 2.7 | 100.0 ± 0 | 52.2 ± 0.9 | 109.0 ± 3.0 | 28.8 ± 0.7 |
Legend:
Duty cycle = ratio between stance duration and full stepcycle duration [stance phase duration / (stance + swing phase duration)]; P, postnatal age; Print area = surface area of floor contacted by hindpaw; Print intensity = intensity of pixels forming area of paw contact; Regularity index = index for degree of interlimb coordination during gait; Stability of gait = distance between two hindpaws measured perpendicular to walking direction; Stride length = distance between placement of hindpaw and subsequent placement of same paw
Data = mean ± SD;
P < 0.05
P < 0.01 one-way ANOVA with Bonferroni post-hoc comparisons P3 saline versus P3 ketamine.
Discussion
In the present studies we established that intrathecal ketamine produces an antihyperalgesic effect in the neonatal rat, and the minimum intrathecal dose producing significant reversal of inflammatory hyperalgesia was 3 mg/kg at P3. At this and higher doses, intrathecal ketamine in the P3 rat increased neuronal apoptosis in the dorsal horn of the cord, and was associated with persistent changes in spinal function as measured by mechanical withdrawal threshold and gait parameters at 35 days. These measures permit calculation of a therapeutic ratio (toxic dose/analgesic dose) that allows comparison of the relative safety of spinally administered drugs. As acute pathology and long-term behavioral changes occur in the same dose range as the antihyperalgesic effects of intrathecal ketamine, the therapeutic ratio is less than one in the neonatal (P3) rat. In rats receiving intrathecal ketamine at P21, apoptosis was not seen, and the therapeutic index is greater than one. Here however, dose-limiting side effects with single bolus injections precluded evaluation of histopathology at higher doses. These results contrast with our study of intrathecal morphine23 where bolus intrathecal delivery of up to 300 times the minimum analgesic dose in P3 rats, and 20 times in P21 rats, produced no evidence of drug related changes in acute spinal histopathology or chronic function. We believe that this therapeutic ratio has implications for the choice of spinal analgesic in pediatric clinical practice.
Spinal apoptosis
In the brain, vulnerability to the proapoptotic action of systemic NMDA antagonists varies with postnatal age and is restricted to regions displaying developmental apoptosis22. Following MK801, the relative increase in degenerating neurons is greatest in the dentate gyrus at P0, the hippocampus and thalamus at P3, and by P7 is also prevalent in the cortex22. Similarly, systemic ketamine (20 mg/kg × 6 at 2-h intervals) in the P7 rat increases apoptosis in the frontal cortex, striatum, hippocampus, thalamus and amygdala34. Therefore, many studies evaluating proapototic effects of ketamine have utilised rodents aged P7-P101. Although apoptosis has been reported in the spinal cord following general anesthesia at P721, the period of peak susceptibility may differ in the spinal cord. The pattern and degree of "naturally occurring cell death" or developmental apoptosis in the lumbar spinal cord changes with age, as the number of apoptotic cells is highest at P0 and P2, lower at P4 and P8, and negligible by P1035. Similarly, in the cervical spinal cord spontaneous apoptosis was detected at P2 to P5 and had decreased by P836. This is consistent with the current findings as spontaneous apoptosis was detected at P4 (i.e., 24 h after saline injection at P3), but numbers decreased by P8 and were negligible by P22. Therefore, the period of peak susceptibility to proapoptotic drugs may occur at an earlier developmental stage in the spinal cord than in the cortex.
Antibodies to activated caspase-3, an enzyme in the apoptotic cascade, identify neurons that have progressed beyond the point of commitment to cell death37 and Fluoro-Jade C is a sensitive marker of neuronal degeneration28. Both methodologies detected similar patterns of apoptosis in the brain following systemic ketamine8,34. Similarly, we used both activated caspase-3 immunohistochemistry and Fluoro-Jade C staining to confirm an increase in spinal cord apoptosis following intrathecal ketamine at P3. Apoptotic profiles were present in the saline group at P7, but numbers were lower than at P3, and increases following intrathecal ketamine were not statistically significant. Following systemic ketamine, apoptosis is not temporally related to plasma levels but can be detected from 6 h34, and by 24 h immunoreactivity to caspase-3 may be reduced as the cell decomposes37. Six hours following P3 intrathecal ketamine numbers of activated caspase-3 and Fluoro-Jade C positive neurons were slightly higher than at 24 h, but significant increases compared with saline were seen at both time points. Systemic ketamine produces dose-dependent apoptosis in the brain of rodents following single7,38 and repeated doses of 20 mg/kg8,22,34. Apoptosis in the spinal cord was produced by lower ketamine doses (3 and 10 mg/kg), which may relate to higher local concentrations following intrathecal administration.
Changes in spinal cord function
The degree of apoptosis in different brain regions varies following systemic ketamine. Repeated doses of 20 mg/kg ketamine (6 doses at 2-h intervals) increased activated caspase-3 positive cells by a factor of 10 in the frontal cortex and 2.5 fold in the hippocampus34, whereas single doses of ketamine increased cortical apoptosis by a factor of two following 20 mg/kg and by greater than four after 40 mg/kg7. In the spinal cord, the number of apoptotic profiles was similarly increased by a factor of 2–3 following intrathecal ketamine, and while statistically significant, the importance of the magnitude of such changes is evidently greater if associated with a persistent functional deficit39. For example, increased apoptosis in the hippocampus following NMDA antagonists and gamma-amino butyric acid agonists has been associated with long-term deficits in learning and memory5. Sensory thresholds and motor function can be used to evaluate long-term changes in spinal cord function. Following prolonged general anesthesia at P7, increased apoptosis in the cord was reported21. The sum of positive cells in four sections was reported, but if converted to mean values, numbers are similar to the current data in P7 pups (i.e., 1–2 positive cells per transverse section in the control group and 4–5 cells per section following nitrous oxide and isoflurane). This degree of change at P7 did not produce long-term changes in thermal tail-flick latency or motor function on the rota-rod21. Following intrathecal ketamine at P3, mechanical withdrawal threshold but not thermal latency was reduced at P35. Similarly, placement of a slow-release formulation of the NMDA antagonist MK-801 in Elvax (ethylene-vinyl acetate co-polymer) over the spinal cord of P0–1 pups40, produced persistent changes in mechanical but not thermal sensitivity, and the degree of change was greater with this prolonged blockade. The same intervention commencing at P6–7 did not produce long-term changes in mechanical reflex thresholds41. In addition to sensory thresholds, we evaluated weight bearing and sensorimotor coordination using a gait analysis system31. Body weight may influence print intensity measures, but weight did not differ across treatment groups. As the speed of gait may influence print area, stance and swing duration, data was only included from crossings with a maximal time of 2 s without intermediate stops. Hindpaw print area and print intensity were decreased following P3 ketamine, suggesting impairments in placement of the paw on the glass during the static phase of walking. More marked decreases in hindpaw print area and print intensity suggestive of allodynia have been shown in association with pain states such as monoarthritis42 and peripheral nerve lesions31. Following P3 ketamine, duty cycle was decreased as the time the hind paw was in contact with the surface during gait was decreased, but differences did not reach statistical significance, and there were no changes in stride length. In addition, the dynamic parameters of gait regularity and stability suggested that gait coordination was not impaired. During normal development, apoptosis in the ventral horn of the spinal cord occurs prenatally, but by P2 is maximal in the dorsal horn43. Similarly, in the current study, both numbers of apoptotic cells in the control group and increases following P3 ketamine were highest in the dorsal horn of the cord and were less marked in the ventral horn, which may reduce susceptibility to changes in motor function.
Dose-dependent effects
It has been stated that systemic ketamine produces apoptosis and long-term dysfunction only at "nonclinically relevant" doses as plasma levels in rodents are higher than measured in humans during clinical anaesthesia2. However, dose requirements are not comparable across species, and comparison of toxic and functional effects (e.g., dose to achieve specified level of anesthesia or analgesia) within the species being investigated may be more appropriate37. Prolonged infusion of ketamine at doses that produce a surgical plane of anesthesia increased apoptosis in P5–6 primates11 and apoptosis was increased by ketamine doses well below the ED50 for anesthesia in rodents7. Analgesic and anesthetic dose requirements also change with postnatal age44,45. Therefore, we evaluated toxic and analgesic doses of intrathecal ketamine in order to calculate a therapeutic ratio at different postnatal ages. Analgesia can be achieved with systemic ketamine doses lower than required for general anesthesia, and even lower doses may be required if specific spinally-mediated analgesia can be demonstrated following intrathecal administration. In adult rodents, intrathecal ketamine does not produce anti-nociceptive effects (ie. changes in baseline mechanical threshold or thermal latency), although one study reported mild prolongation of thermal latency after high doses46. Here, mechanical thresholds did not alter from baseline after intrathecal ketamine in P3 or P21 pups. However, anti-hyperalgesic effects can be demonstrated in injury models in adult rats24,25,47,48. Following hindpaw carrageenan, 0.5 mg intrathecal ketamine (approximately 2 mg/kg) reversed ipsilateral mechanical hyperalgesia and had no effect on the contralateral paw. A much higher systemic dose (50 mg/kg) was required to produce the same anti-hyperalgesic effect, suggesting that intrathecal ketamine produces spinally-mediated analgesic effects48. As hindpaw carrageenan produces quantifiable inflammatory hyperalgesia in rat pups27, we used this injury model to evaluate dose-dependent analgesic effects of intrathecal ketamine. In P3 pups, intrathecal ketamine 0.3 mg/kg partially reduced hyperalgesia at early time points, but 3 mg/kg was required to reverse inflammatory hyperalgesia. As this dose also produces significant apoptosis in the spinal cord the therapeutic ratio of intrathecal ketamine at P3 is less than one. This contrasts markedly with our data following intrathecal morphine23 which showed a therapeutic ratio of >300 at the same age.
Mechanisms of spinal ketamine effects
The primary action of ketamine is its non-competitive block of the NMDA ionophore. Within the spinal cord, there are significant postnatal changes in NMDA receptor distribution and density in the rat, and developmental changes in subunit expression are associated with changes in channel kinetics and an increased calcium influx44,49. This enhanced excitation may influence the potential for toxicity. Prolonged NMDA blockade, by spinal application of MK-801 in Elvax, disrupts normal maturation and produces long-term changes in the laminar40 and somatotopic41 distribution of primary afferent fibres, suggesting that normal maturation of spinal cord circuitry is activity-dependent. The mechanisms underlying anesthesia related apoptosis continue to be investigated39 and the hypothesis that cell death is solely precipitated by a reduction in synaptic activity has been questioned12. Ketamine has also been shown to impair dendritic growth of GABAergic neurons at lower concentrations and throughout a longer developmental period than proapoptotic effects50,51, but specific effects on inhibitory spinal circuitry have not been evaluated.
The potential for spinal cord toxicity following NMDA antagonists is not limited to effects on developmental apoptosis. Local toxicity has been demonstrated following intrathecal administration of ketamine in adult swine52, rabbits53,54 and dogs55. Although some studies have attributed changes to the preservative52,56, administration of preservative free S-ketamine for 7 days produced necrotizing lesions with cellular infiltrates in the cord54 and a 28-day infusion of preservative-free racemic ketamine produced pathologic changes ranging from mild inflammation and demyelination to marked necrosis55. Postulated mechanisms for spinal toxicity in adults include excitotoxicity, loss of the trophic role of NMDA receptors, and metabolic alterations in central nervous system levels of glutathione, glutamine and glutamate54,55. It has alternately been suggested that at high concentrations associated with intrathecal delivery, agents may form micelles which act as detergents and as such can have a direct damaging effect upon spinal cell membranes57. Similar histopathological effects may also become apparent with repeated dosing or infusion in younger animals, but in the current study, we used a single percutaneous administration of a higher dose of NMDA antagonist to avoid the confounding effects of surgery and catheter placement in small animals. Single doses of ketamine did not produce histopathological changes evident with light microscopy in P3 or P21 rats. However, neuronal apoptosis was increased by intrathecal ketamine, and this was associated with increased microglial activation 7 days later and changes in sensory threshold and gait several weeks later. This emphasises the importance of evaluating spinal toxicity in specific developmental models that include age-appropriate outcomes.
It has been postulated that apoptosis would be less marked when analgesia or anesthesia is used to balance the increased afferent input associated with intercurrent pain or surgery2. Following repeated hindpaw injections of formalin (each paw daily from P1 to P4) with or without ketamine (2 doses of 2.5 mg/kg daily P1 to P4), apoptosis in the cortex was increased in formalin only, but not in ketamine only or ketamine plus formalin groups58,59. However, ketamine was administered at an earlier postnatal age and at doses lower than associated with apoptosis in the brain in other studies. Further evaluation of dose-dependent proapoptotic effects in different injury models is warranted.
Clinical implications
Pediatric clinical studies report prolongation of analgesia with addition of ketamine to caudal local anesthetic13, and a reduction in the proportion of infants requiring acetaminophen (paracetamol) postoperatively17–20,60. While such studies provide evidence of an analgesic effect, the relative benefit of a reduction in postoperative acetaminophen may be outweighed by any potential risk of toxicity related to caudal ketamine. It has also been noted by authors that "conclusive safety studies will be required before this technique can be recommended for clinical practice"61, and "as yet, no permanent neurological injury has resulted from single-shot caudal ketamine use, but caution is warranted"18. Although many have focused on effects of preservatives, toxicity has been associated with prolonged use of preservative-free solutions in adults, and in addition we have now demonstrated increased apoptosis and long-term functional effects following preservative-free intrathecal ketamine in early development.
Translation of developmental ages from rodents to humans continues to be debated. Vulnerability to apoptosis in the brain coincides with rapid synaptogenesis or the brain growth spurt, which occurs predominantly in the first two postnatal weeks in the rodent, but may extend from midgestation to several years after birth in the human infant62. As peak apoptosis occurs at an earlier age in the spinal cord (P3 rather than P7) than the cortex, the period of susceptibility to pro-apoptotic drugs may be shorter, but confirmation of the critical period for long-term functional effects will require further studies at ages between P3 and P21. In terms of spinal processing many approximate a P3 rat with a preterm-term human neonate, P7 with an infant, P21 with an adolescent, and P35 with young adulthood; although ongoing structural and functional changes in synaptic function in the spinal cord occur throughout the first 3 postnatal weeks in the rat44,63. As the therapeutic ratio of ketamine is relatively narrow in the current neonatal rodent study and in several adult species, in the absence of definitive negative pathology and detailed follow-up in clinical studies, there is little to recommend this drug over alternative analgesics for routine perioperative analgesia in children16. While the majority of caudal ketamine studies have been conducted in children aged from 6 months to 12 yr13,64, some studies have included neonates and infants18–20, who are likely to be at increased risk of developmental neuroapoptosis. A reduction in the use of caudal ketamine has already occurred in many pediatric centers65.
In conclusion, the studies with ketamine in the present report and morphine in the accompanying paper provide support for the assertion that this neonatal model is capable of defining the relative degree of safety and toxicity of different spinal analgesics by comparing the minimum dose producing acute histopathology and persistent changes in function with the minimum dose which produces a “therapeutic” effect. The denominator was the spinal dose that alters acute nociception (e.g., opioid receptor agonism) or that which alters hyperalgesia (e.g., NMDA antagonism). These each represent the optimal expression of the principle mechanisms through which each drug (class) acts. This ratio must only be considered as an estimate of the safety of a given agent relative to another agent, given by that route, at the same postnatal age. Here we are able to say that morphine has no effect at doses that far exceed those required for antinociception, which is believed to reflect the clinical analgesic effect. In contrast, preservative-free ketamine even at doses required to produce an appropriate analgesic end point, produced acute apoptosis and evidence of persistent changes in function. Further studies will be required to define a ratio for other agents of the same class or different classes (e.g., α2-adrenergic agonists) of drugs for which spinal delivery has significant therapeutic advantages. As previously discussed23 evaluation of local anesthetic toxicity will require assessment of effects at additional sites of action, such as nerve roots, in addition to the spinal cord. Ongoing work is required to provide additional validation of the predictive validity of this preclinical model as a marker of safety and toxicity.
Acknowledgement
We would like to thank Noldus Information Technology, Wageningen, The Netherlands for the loan of a CatWalk® system
Financial Support: This research was supported by funding from National Institutes of Health NIH-DA15353 and NIH NS16541, Bethesda, Maryland (TLY); Association of Paediatric Anaesthetists of Great Britain and Ireland, London, United Kingdom (SW); County Council of Ostergotland, Ostergotland, Sweden and Swedish Society for Anaesthesiology and Intensive Care, Stockholm, Sweden (DW).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Image Processing and Analysis in Java; http://rsbweb.nih.gov/ij/. Last accessed August 31, 2009
Summary Statement: Bolus intrathecal ketamine in neonatal rats increases neuronal apoptosis in the spinal cord and alters long-term function at doses required for antihyperalgesic effects. The therapeutic ratio (toxic/analgesic dose) is less than one in early life.
REFERENCES
- 1.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–1707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 2.Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22:368–373. doi: 10.1097/aco.0b013e3283294c9e. [DOI] [PubMed] [Google Scholar]
- 3.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–520. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and γ-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 6.McGowan FX, Jr, Davis PJ. Anesthetic-related neurotoxicity in the developing infant: Of mice, rats, monkeys and, possibly, humans. Anesth Analg. 2008;106:1599–1602. doi: 10.1213/ane.0b013e31817330cf. [DOI] [PubMed] [Google Scholar]
- 7.Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scallet AC, Schmued LC, Slikker W, Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: Morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81:364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Sadovova N, Fu X, Schmued L, Scallet A, Hanig J, Slikker W. The role of the N-methyl-d-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132:967–977. doi: 10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 10.Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 11.Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27:727–731. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Slikker W., Jr Strategies and experimental models for evaluating anesthetics: Effects on the developing nervous system. Anesth Analg. 2008;106:1643–1658. doi: 10.1213/ane.ob013e3181732c01. [DOI] [PubMed] [Google Scholar]
- 13.Ansermino M, Basu R, Vandebeek C, Montgomery C. Nonopioid additives to local anaesthetics for caudal blockade in children: A systematic review. Paediatr Anaesth. 2003;13:561–573. doi: 10.1046/j.1460-9592.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- 14.Sanders JC. Paediatric regional anaesthesia: A survey of practice in the United Kingdom. Br J Anaesth. 2002;89:707–710. [PubMed] [Google Scholar]
- 15.Williams DG, Howard RF. Epidural analgesia in children. A survey of current opinions and practices amongst UK paediatric anaesthetists. Paediatr Anaesth. 2003;13:769–776. doi: 10.1046/j.1460-9592.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 16.Eisenach JC, Yaksh TL. Epidural ketamine in healthy children: What's the point? (letter) Anesth Analg. 2003;96:626. doi: 10.1097/00000539-200302000-00059. [DOI] [PubMed] [Google Scholar]
- 17.Marhofer P, Krenn CG, Plochl W, Wallner T, Glaser C, Koinig H, Fleischmann E, Hochtl A, Semsroth M. S(+)-ketamine for caudal block in paediatric anaesthesia. Br J Anaesth. 2000;84:341–345. doi: 10.1093/oxfordjournals.bja.a013436. [DOI] [PubMed] [Google Scholar]
- 18.Locatelli BG, Frawley G, Spotti A, Ingelmo P, Kaplanian S, Rossi B, Monia L, Sonzogni V. Analgesic effectiveness of caudal levobupivacaine and ketamine. Br J Anaesth. 2008;100:701–706. doi: 10.1093/bja/aen048. [DOI] [PubMed] [Google Scholar]
- 19.Hager H, Marhofer P, Sitzwohl C, Adler L, Kettner S, Semsroth M. Caudal clonidine prolongs analgesia from caudal S(+)-ketamine in children. Anesth Analg. 2002;94:1169–1172. doi: 10.1097/00000539-200205000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Weber F, Wulf H. Caudal bupivacaine and s(+)-ketamine for postoperative analgesia in children. Paediatr Anaesth. 2003;13:244–248. doi: 10.1046/j.1460-9592.2003.01018.x. [DOI] [PubMed] [Google Scholar]
- 21.Sanders RD, Xu J, Shu Y, Fidalgo A, Ma D, Maze M. General anesthetics induce apoptotic neurodegeneration in the neonatal rat spinal cord. Anesth Analg. 2008;106:1708–1711. doi: 10.1213/ane.0b013e3181733fdb. [DOI] [PubMed] [Google Scholar]
- 22.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 23.Westin BD, Walker SM, Deumens R, Grafe M, Yaksh TL. Validation of a preclinical spinal safety model: Effects of intrathecal morphine in the neonatal rat. Anesthesiology. 2010;112:XXX–XXX. doi: 10.1097/ALN.0b013e3181dcd6ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: Effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 25.Pelissier T, Infante C, Constandil L, Espinosa J, Lapeyra CD, Hernandez A. Antinociceptive effect and interaction of uncompetitive and competitive NMDA receptor antagonists upon capsaicin and paw pressure testing in normal and monoarthritic rats. Pain. 2008;134:113–127. doi: 10.1016/j.pain.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Kosson D, Klinowiecka A, Kosson P, Bonney I, Carr DB, Mayzner-Zawadzka E, Lipkowski AW. Intrathecal antinociceptive interaction between the NMDA antagonist ketamine and the opioids, morphine and biphalin. Eur J Pain. 2008;12:611–616. doi: 10.1016/j.ejpain.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Walker SM, Howard RF, Keay KA, Fitzgerald M. Developmental age influences the effect of epidural dexmedetomidine on inflammatory hyperalgesia in rat pups. Anesthesiology. 2005;102:1226–1234. doi: 10.1097/00000542-200506000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 29.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 30.Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- 31.Deumens R, Jaken RJ, Marcus MA, Joosten EA. The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J Neurosci Methods. 2007;164:120–130. doi: 10.1016/j.jneumeth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Deumens R, Koopmans GC, Honig WM, Hamers FP, Maquet V, Jerome R, Steinbusch HW, Joosten EA. Olfactory ensheathing cells, olfactory nerve fibroblasts and biomatrices to promote long-distance axon regrowth and functional recovery in the dorsally hemisected adult rat spinal cord. Exp Neurol. 2006;200:89–103. doi: 10.1016/j.expneurol.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 33.Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W, Wang C. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowrie MB, Lawson SJ. Cell death of spinal interneurones. Prog Neurobiol. 2000;61:543–555. doi: 10.1016/s0301-0082(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 36.de Louw AJ, de Vente J, Steinbusch HP, Gavilanes AW, Steinbusch HW, Blanco CE, Troost J, Vles JS. Apoptosis in the rat spinal cord during postnatal development: The effect of perinatal asphyxia on programmed cell death. Neuroscience. 2002;112:751–758. doi: 10.1016/s0306-4522(02)00134-3. [DOI] [PubMed] [Google Scholar]
- 37.Jevtovic-Todorovic V, Olney JW. PRO: Anesthesia-induced developmental neuroapoptosis: Status of the evidence. Anesth Analg. 2008;106:1659–1663. doi: 10.1213/ane.0b013e3181731ff2. [DOI] [PubMed] [Google Scholar]
- 38.Rudin M, Ben-Abraham R, Gazit V, Tendler Y, Tashlykov V, Katz Y. Single-dose ketamine administration induces apoptosis in neonatal mouse brain. J Basic Clin Physiol Pharmacol. 2005;16:231–243. doi: 10.1515/jbcpp.2005.16.4.231. [DOI] [PubMed] [Google Scholar]
- 39.Patel P, Sun L. Update on neonatal anesthetic neurotoxicity: Insight into molecular mechanisms and relevance to humans. Anesthesiology. 2009;110:703–708. doi: 10.1097/ALN.0b013e31819c42a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–1258. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- 41.Granmo M, Petersson P, Schouenborg J. Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci. 2008;28:5494–5503. doi: 10.1523/JNEUROSCI.0651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angeby-Moller K, Berge OG, Hamers FP. Using the CatWalk method to assess weight-bearing and pain behaviour in walking rats with ankle joint monoarthritis induced by carrageenan: Effects of morphine and rofecoxib. J Neurosci Methods. 2008;174:1–9. doi: 10.1016/j.jneumeth.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Lawson SJ, Davies HJ, Bennett JP, Lowrie MB. Evidence that spinal interneurons undergo programmed cell death postnatally in the rat. Eur J Neurosci. 1997;9:794–799. doi: 10.1111/j.1460-9568.1997.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald M, Walker SM. Infant pain management: A developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- 45.Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95:734–739. doi: 10.1097/00000542-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 46.Ahuja BR. Analgesic effect of intrathecal ketamine in rats. Br J Anaesth. 1983;55:991–995. doi: 10.1093/bja/55.10.991. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto T, Yaksh TL. Spinal pharmacology of thermal hyperesthesia induced by constriction injury of sciatic nerve: Excitatory amino acid antagonists. Pain. 1992;49:121–128. doi: 10.1016/0304-3959(92)90198-K. [DOI] [PubMed] [Google Scholar]
- 48.Kawamata T, Omote K, Sonoda H, Kawamata M, Namiki A. Analgesic mechanisms of ketamine in the presence and absence of peripheral inflammation. Anesthesiology. 2000;93:520–528. doi: 10.1097/00000542-200008000-00032. [DOI] [PubMed] [Google Scholar]
- 49.Pattinson D, Fitzgerald M. The neurobiology of infant pain: Development of excitatory and inhibitory neurotransmission in the spinal dorsal horn. Reg Anesth Pain Med. 2004;29:36–44. doi: 10.1016/j.rapm.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Vutskits L, Gascon E, Tassonyi E, Kiss JZ. Effect of ketamine on dendritic arbor development and survival of immature GABAergic neurons in vitro. Toxicol Sci. 2006;91:540–549. doi: 10.1093/toxsci/kfj180. [DOI] [PubMed] [Google Scholar]
- 51.Vutskits L, Gascon E, Potter G, Tassonyi E, Kiss JZ. Low concentrations of ketamine initiate dendritic atrophy of differentiated GABAergic neurons in culture. Toxicology. 2007;234:216–226. doi: 10.1016/j.tox.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Errando CL, Sifre C, Moliner S, Valia JC, Gimeno O, Minguez A, Boils P. Subarachnoid ketamine in swine: Pathological findings after repeated doses: Acute toxicity study. Reg Anesth Pain Med. 1999;24:146–152. doi: 10.1016/s1098-7339(99)90076-7. [DOI] [PubMed] [Google Scholar]
- 53.Malinovsky JM, Cozian A, Lepage JY, Mussini JM, Pinaud M, Souron R. Ketamine and midazolam neurotoxicity in the rabbit. Anesthesiology. 1991;75:91–97. doi: 10.1097/00000542-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 54.Vranken JH, Troost D, de Haan P, Pennings FA, van der Vegt MH, Dijkgraaf MG, Hollmann MW. Severe toxic damage to the rabbit spinal cord after intrathecal administration of preservative-free S(+)-ketamine. Anesthesiology. 2006;105:813–818. doi: 10.1097/00000542-200610000-00028. [DOI] [PubMed] [Google Scholar]
- 55.Yaksh TL, Tozier N, Horais KA, Malkmus S, Rathbun M, Lafranco L, Eisenach J. Toxicology profile of N-methyl-D-aspartate antagonists delivered by intrathecal infusion in the canine model. Anesthesiology. 2008;108:938–949. doi: 10.1097/ALN.0b013e31816c902a. [DOI] [PubMed] [Google Scholar]
- 56.Malinovsky JM, Lepage JY, Cozian A, Mussini JM, Pinaudt M, Souron R. Is ketamine or its preservative responsible for neurotoxicity in the rabbit? Anesthesiology. 1993;78:109–115. doi: 10.1097/00000542-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 57.Kitagawa N, Oda M, Nobutaka I, Satoh H, Totoki T, Morimoto M. A proposed mechanism for amitriptyline neurotoxicity based on its detergent nature. Toxicol Appl Pharmacol. 2006;217:100–106. doi: 10.1016/j.taap.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62:283–290. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- 59.Rovnaghi CR, Garg S, Hall RW, Bhutta AT, Anand KJ. Ketamine analgesia for inflammatory pain in neonatal rats: A factorial randomized trial examining long-term effects. Behav Brain Funct. 2008;4:35. doi: 10.1186/1744-9081-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhardwaj N, Yaddanapudi S, Ghai B, Wig J. Neostigmine does not prolong the duration of analgesia produced by caudal bupivacaine in children undergoing urethroplasty. J Postgrad Med. 2007;53:161–165. doi: 10.4103/0022-3859.33856. [DOI] [PubMed] [Google Scholar]
- 61.Lin C, Durieux ME. Ketamine and kids: An update. Paediatr Anaesth. 2005;15:91–97. doi: 10.1111/j.1460-9592.2005.01475.x. [DOI] [PubMed] [Google Scholar]
- 62.Creeley CE, Olney JW. The young: Neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2010;110:442–448. doi: 10.1213/ANE.0b013e3181c6b9ca. [DOI] [PubMed] [Google Scholar]
- 63.McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Beer DA, Thomas ML. Caudal additives in children: Solutions or problems? Br J Anaesth. 2003;90:487–498. doi: 10.1093/bja/aeg064. [DOI] [PubMed] [Google Scholar]
- 65.Eich C, Strauss J. Prompt and powerful effect of a practice guideline on caudal additives (letter) Paediatr Anaesth. 2009;19:271–272. doi: 10.1111/j.1460-9592.2009.02926.x. [DOI] [PubMed] [Google Scholar]