Short abstract
The respiratory effects of dexmedetomidine were retrospectively examined in 33 postsurgical patients involved in a randomised, placebo-controlled trial after extubation in the intensive care unit (ICU). Morphine requirements were reduced by over 50% in patients receiving dexmedetomidine. There were no differences in respiratory rates, oxygen saturations, arterial pH and arterial partial carbon dioxide tension (PaCO2) between the groups. Interestingly the arterial partial oxygen tension (PaO2) : fractional inspired oxygen (FIO2) ratios were statistically significantly higher in the dexmedetomidine group. Dexmedetomidine provides important postsurgical analgesia and appears to have no clinically important adverse effects on respiration in the surgical patient who requires intensive care.
Keywords: α2-Adrenoceptor agonist, analgesia, dexmedetomidine, intensive care, postoperative, respiratory, sedation
Abstract
Introduction:
The α2-agonist dexmedetomidine is a new class of sedative drug that is being investigated for use in ICU settings. It is an effective agent for the management of sedation and analgesia after cardiac, general, orthopaedic, head and neck, oncological and vascular surgery in the ICU [1]. Cardiovascular stability was demonstrated, with significant reductions in rate-pressure product during sedation and over the extubation period.
Dexmedetomidine possesses several properties that may additionally benefit those critically ill patients who require sedation. In spontaneously breathing volunteers, intravenous dexmedetomidine caused marked sedation with only mild reductions in resting ventilation at higher doses [2]. Dexmedetomidine reduces the haemodynamic response to intubation and extubation [3,4,5] and attenuates the stress response to surgery [6], as a result of the α2-mediated reduction in sympathetic tone. Therefore, it should be possible to continue sedation with dexmedetomidine over the stressful extubation period without concerns over respiratory depression, while ensuring that haemodynamic stability is preserved.
The present study is a retrospective analysis of the respiratory response to dexmedetomidine in 33 postsurgical patients (who were involved in a randomized, double-blind, placebo-controlled trial [1]) after extubation in the ICU.
Methods:
Patients who participated in the present study were admitted after surgery to our general or cardiothoracic ICUs, and were expected to receive at least 6 h of postsurgical sedation and artificial ventilation.
On arrival in the ICU after surgery, patients were randomized to receive either dexmedetomidine or placebo (normal saline) with rescue sedation and analgesia being provided, only if clinically needed, with midazolam and morphine boluses, respectively. Sedation was titrated to maintain a Ramsay Sedation Score [7] of 3 or greater while the patients were intubated, and infusions of study drug were continued for a maximum of 6 h after extubation to achieve a Ramsay Sedation Score of 2 or greater.
The patients were intubated and ventilated with oxygen-enriched air to attain acceptable arterial blood gases, and extubation occurred when clinically indicated. All patients received supplemental oxygen after extubation, which was delivered by a fixed performance device. Assessment of pain was by direct communication with the patient.
Results are expressed as mean ± standard deviation unless otherwise stated. Patient characteristics, operative details and morphine usage were analyzed using the Mann-Whitney U-test. Statistical differences for respiratory measurements between the two groups were determined using analysis of variance for repeated measures, with the Bonferroni test for post hoc comparisons.
Results:
Of the 40 patients who participated in the study, seven patients could not be included in the analysis of respiratory function because they did not receive a study drug infusion after extubation. Consequently, data from 33 patients are used in the analysis of respiratory function; 16 received dexmedetomidine and 17 placebo. Inadequate arterial blood gas analysis was available in five patients (two from the dexmedetomidine group, and three from the placebo group). There were no significant differences in patient characteristics and operative details between the groups.
Requirements for morphine were reduced by more than 50% in patients receiving dexmedetomidine when compared with placebo after extubation (0.003 ± 0.004 vs 0.008 ± 0.006 mg/kg per h; P= 0.040).
There were no statistically significant differences between placebo and dexmedetomidine for oxygen saturations measured by pulse oximetry (P= 0.26), respiratory rate (P= 0.16; Fig. 1), arterial pH (P= 0.77) and PaCO2 (P= 0.75; Fig. 2) for the 6 h after extubation.
The dexmedetomidine group showed significantly higher PaO2: FIO2 ratios throughout the 6-h intubation (P= 0.036) and extubation (P= 0.037) periods (Fig. 3). There were no adverse respiratory events seen in either the dexmedetomidine or placebo group.
Discussion:
Lack of respiratory depression in patients sedated with α2-adrenoceptor agonists was first reported by Maxwell [8] in a study investigating the respiratory effects of clonidine. However, more recent data suggests that clonidine may cause mild respiratory depression in humans [9], and α2-adrenoceptor agonists are well known to produce profound intraoperative hypoxaemia in sheep [10,11]. The effects of dexmedetomidine on other ventilation parameters also appear to be species specific [12].
Belleville et al [2] investigated the ventilatory effects of a 2-min intravenous infusion of dexmedetomidine on human volunteers. According to those investigators, minute ventilation and arterial PaCO2 were mildly decreased and increased, respectively. There was a rightward shift and depression of the hypercapnic response with infusions of 1.0 and 2.0 μg/kg.
Previous studies that investigated the respiratory effects of dexmedetomidine have only been performed in healthy human volunteers, who have received either single intramuscular injections or short (= 10 min) intravenous infusions of dexmedetomidine. It is therefore reassuring that no deleterious clinical effects on respiration and gas exchange were seen in the patients we studied, who were receiving long-term infusions. However, there are important limitations to the present results. No dose/response curve for dexmedetomidine can be formulated from the data, and further investigation is probably ethically difficult to achieve in the spontaneously ventilating intensive care patient. We also have no data on the ventilatory responses to hypercapnia and hypoxia, which would also be difficult to examine practically and ethically. The placebo group received more than twice as much morphine as patients receiving dexmedetomidine infusions after extubation, but there were no differences in respiratory rate or PaCO2 between the groups. We can not therefore determine from this study whether dexmedetomidine has any benefits over morphine from a respiratory perspective.
There were no differences in oxygen saturations between the groups because the administered oxygen concentration was adjusted to maintain satisfactory gas exchange. Interestingly, however, there were statistically significant higher PaO2 : FIO2 ratios in the dexmedetomidine group. This ratio allows for the variation in administered oxygen to patients during the study period, and gives some clinical indication of alveolar gas exchange. However, this variable was not a primary outcome variable for the present study, and may represent a type 1 error, although post hoc analysis reveals that the data have 80% power to detect a significant difference (α value 0.05). Further studies are obviously required.
Sedation continued over the extubation period, has been shown to reduce haemodynamic disturbances and myocardial ischaemia [13]. We have previously shown [1] that dexmedetomidine provides cardiovascular stability, with a reduction in rate-pressure product over the extubation period. A sedative agent that has analgesic properties, minimal effects on respiration and offers ischaemia protection would have enormous potential in the ICU. Dexmedetomidine may fulfill all of these roles, but at present we can only conclude that dexmedetomidine has no deleterious clinical effects on respiration when used in doses that are sufficient to provide adequate sedation and effective analgesia in the surgical population requiring intensive care.
Introduction
The α2-agonist dexmedetomidine is a new class of sedative drug that is being investigated in ICU settings. It is an effective agent for the management of sedation and analgesia after cardiac, general, orthopaedic, head and neck, oncological and vascular surgery in the ICU [1]. Cardiovascular stability was demonstrated with significant reductions in rate-pressure product during sedation and over the extubation period.
Dexmedetomidine possesses several properties that may additionally benefit those critically ill patients who require sedation. In spontaneously breathing volunteers intravenous dexmedetomidine caused marked sedation, with only mild reductions in resting ventilation at higher doses [2]. In animal studies dexmedetomidine did not appear to potentiate the respiratory depression of opioids when used in combination [14], and these results were reproduced with another α2-agonist, clonidine, in human volunteers [15]. Dexmedetomidine reduces the haemodynamic response to intubation and extubation [3,4,5] and attenuates the stress response to surgery [6], as a result of the α2-mediated reduction in sympathetic tone. It should therefore be possible to continue sedation with dexmedetomidine over the stressful extubation period without concerns over respiratory depression, while ensuring that haemodynamic stability is preserved. After extubation dexmedetomidine can continue to provide analgesia to the postoperative patient, thus reducing the need for opioids and their side effects.
We retrospectively analyzed the respiratory response to dexmedetomidine in postsurgical patients after extubation in the ICU. These patients were involved in a multicentre trial conducted to examine the safety and efficacy of dexmedetomidine in the ICU [1], and only data from our centre has been included.
Materials and methods
Forty patients from our hospital, who had been recruited into a multicentre, randomized, double blind, placebo-controlled trial, were retrospectively analyzed. Patients participating in this study were admitted after surgery to our general or cardiothoracic ICUs, and were expected to receive at least 6 h of postsurgical sedation and artificial ventilation. Exclusion criteria included the following: serious central nervous system trauma; requirement for an infusion of a neuromuscular blocking agent; any contraindications or allergy to any of the trial drugs; gross obesity (over 50% above ideal body weight); prior enrollment in a trial with any experimental drug during the past 30 days; and uncontrolled diabetes.
Anaesthetic technique was not controlled during surgery, although benzodiazepines were not allowed as the sole anaesthetic agent.
On arrival in the ICU after surgery, patients were randomized to receive either dexmedetomidine or placebo (normal saline), with rescue sedation and analgesia being provided, only if clinically needed, with midazolam and morphine boluses, respectively. A detailed description of the methods has previously been reported [1]. Essentially, all patients received a loading dose of study drug within 1 h of arriving in the ICU, followed by a variable infusion, to maintain a Ramsay Sedation Score [7] of 3 or greater while intubated. After extubation, infusions of study drug were continued for a maximum of 6 h to target a Ramsay Sedation Score of 2 or greater. In this latter period, morphine and paracetamol boluses could be used for analgesia. Patients were only allowed the study drug infusion for a maximum of 24 h, and were converted to the usual sedation regimen of the unit if there was a continued need for sedation.
The patients were intubated and ventilated with oxygen-enriched air to attain acceptable arterial blood gases, and could receive any other therapeutic interventions that were deemed necessary by the resident ICU staff. Extubation occurred when it was clinically indicated. All patients received supplemental oxygen after extubation, which was delivered by a fixed performance device. Assessment of pain was by direct communication with the patient.
Heart rate, blood pressure, central venous pressure, pulse oximetry and respiratory rate were monitored continuously and recorded hourly. Arterial blood gases were recorded at 2-h intervals over the extubation period.
Statistical analysis
Results are expressed as mean ± standard deviation unless otherwise stated. Patient characteristics, operative details and morphine usage were analyzed using the Mann-Whitney U-test. Statistical differences for respiratory measurements between the two groups were determined using analysis of variance for repeated measures. Post hoc comparisons were made using the Bonferroni test to determine differences among groups at individual time points, as well as differences over time within individual groups.
Power analysis for the numbers studied at our centre would be expected to show a difference of 25% in respiratory rate and PaCO2 between the two groups (α value 0.05, β value 0.8).
Results
Of the 40 patients who participated in the study, seven could not be included in the analysis of respiratory function because they did not receive a study drug infusion after extubation. One patient required ventilation for longer than 24 h, and five patients were withdrawn from the study before extubation: two patients returned to the operating theatre because of bleeding; one had residual neuromuscular blockade; one had bradycardia with hypotension requiring a pacemaker; and one was withdrawn at the surgeon's request because of operative complications. A further patient had the study drug discontinued in error before extubation.
Consequently, data from 33 patients were used in the analysis of respiratory function, and 16 of these patients received dexmedetomidine and 17 received placebo. Inadequate arterial blood gas analysis was available in five patients (two from the dexmedetomidine group, three from the placebo group) because of problems with sampling from the arterial cannula. There were no significant differences with respect to type of operation, age, sex, weight, height, intubation time, weaning time from the ventilator and study drug infusion time (Table 1).
Table 1.
Patient characteristics and operative details for the dexmedetomidine and placebo groups
| Dexmedetomidine | Placebo | |
| Type of surgery (cardiac/general) | 9/7 | 10/7 |
| APACHE II score for general surgical patients (median [interquartile range]) | 10 (5) | 10 (2) |
| Parsonnett score for cardiac surgery patients (median [interquartile range]) | 10 (6) | 13 (7) |
| Age (years) | 63 ± 15 | 63 ± 15 |
| Sex (male/female) | 12/4 | 11/6 |
| Wean-extubation time (h) | 2.6 ± 2.7 | 2.4 ± 2.5 |
| Intubation time (h) | 10.5 ± 4.5 | 9.0 ± 4.4 |
| Total infusion (intubation + extubation; h) | 17.5 ± 3.7 | 15.1 ± 4 |
Values are expressed as mean ± standard deviation or as numbers, unless otherwise stated. APACHE, Acute Physiology and Chronic Health Evaluation.
Mean infusion rates for dexmedetomidine before extubation and during the extubated period were 0.42 ± 0.18 and 0.17 ± 0.13 μg/kg per h, respectively, and there were no differences in the distribution of Ramsay Sedation Scores between the placebo and dexmedetomidine groups. Requirements for morphine were reduced by half in patients receiving dexmedetomidine while intubated when compared with placebo (0.011 ± 0.02 versus 0.022 ± 0.02 mg/kg per h; P = 0.024), and this morphine-sparing effect of dexmedetomidine was even more pronounced during the extubation period (0.003 ± 0.004 versus 0.008 ± 0.006 mg/kg per h; P= 0.040; Fig. 4). In the dexmedetomidine group 50% (eight out of 16) patients required no morphine at all after extubation, compared with 24% (four out of 17) in the placebo group.
Figure 4.
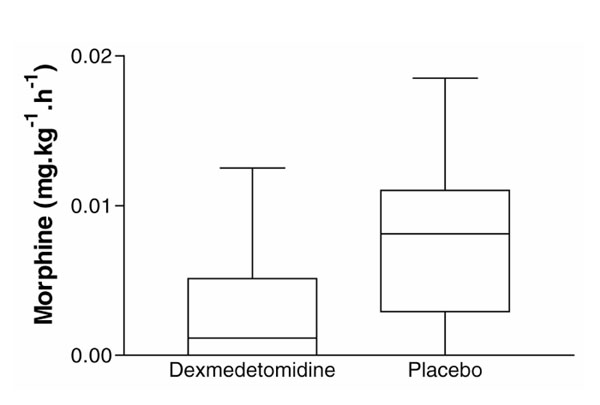
Requirements for rescue analgesia with morphine during the extubation period for patients receiving dexmedetomidine and placebo. Values are medians; boxes indicate 25-75th percentiles and bars indicate the range.
There were no statistically significant differences between the placebo and dexmedetomidine groups for oxygen saturations measured by pulse oximetry (P= 0.26) and respiratory rate (P= 0.16) for the 6 h following extubation (Figs 1 and 5). There were no differences in arterial pH (P= 0.77) and PaCO2 (P= 0.75) measurements between the groups, and within the dexmedetomidine group, over the same 6-h period (Figs 2 and 6).
Figure 1.
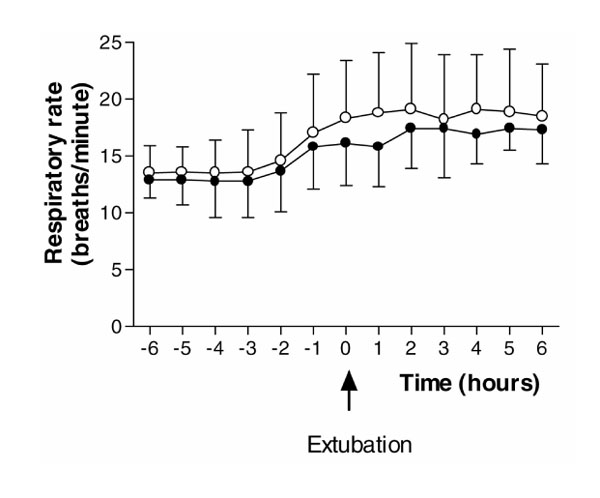
Respiratory rate for the 6-h periods before and after extubation. (Filled circle) Dexmedetomidine; (Empty circle) placebo. Values are expressed as mean ± standard deviation.
Figure 5.
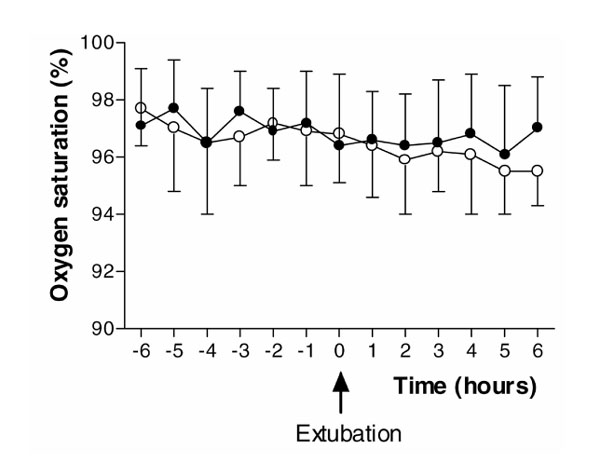
Oxygen saturation measured by pulse oximetry for the 6-h periods before and after extubation. (Filled circle) Dexmedetomidine; (Empty circle) placebo. Values are expressed as mean ± standard deviation.
Figure 2.
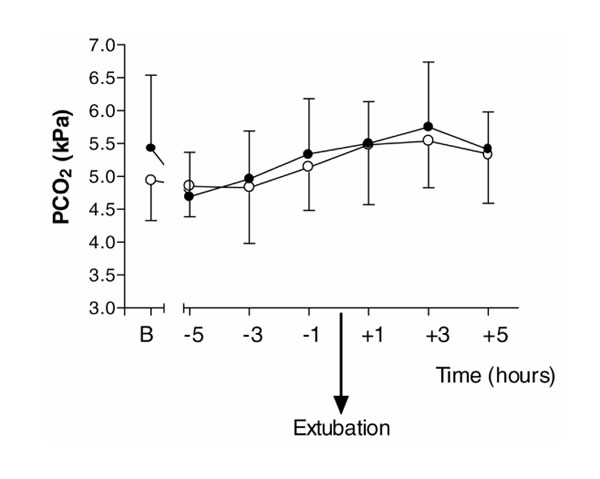
PaCO2 (PCO2) for the 6-h periods before and after extubation, and baseline values (B) on admission to ICU immediately after surgery. (Filled circle) Dexmedetomidine; (Empty circle) placebo. Values are expressed as mean ± standard deviation.
Figure 6.
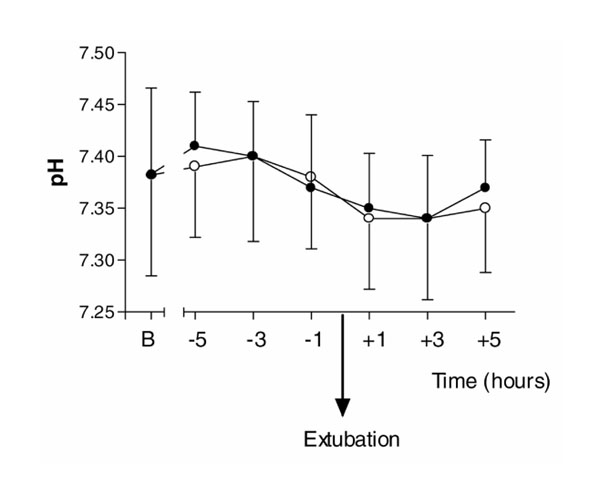
Arterial pH for the 6 hour periods before and after extubation, and baseline values (B) on admission to ICU immediately after surgery. (Filled circle) Dexmedetomidine; (Empty circle) placebo. Values are expressed as mean ± standard deviation.
Because all patients received oxygen therapy throughout the study period, the PaO2 : FIO2 ratio was used to compare oxygenation. There were significant differences in the PaO2 : FIO2 ratios for the 6-h extubation period (P= 0.037) and for the 6-h period while intubated before extubation between the two groups (P= 0.036). The dexmedetomidine group showed significantly higher PaO2 : FIO2 ratios throughout these periods (Fig. 3). There were no adverse respiratory events seen in either the dexmedetomidine or placebo group.
Figure 3.
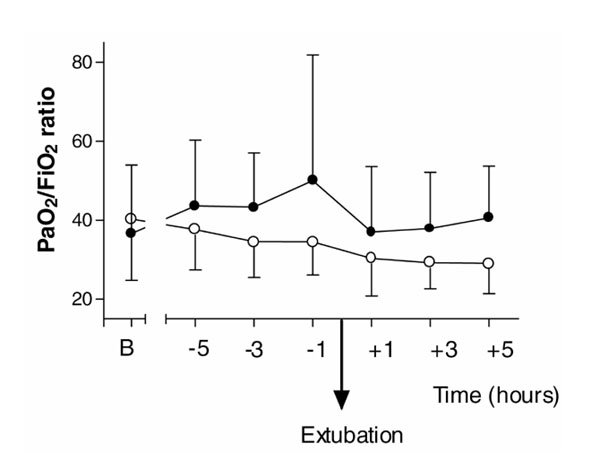
PaO2 : FIO2 ratio for the 6-h periods before and after extubation, and baseline values (B) on admission to ICU immediately after surgery. (Filled circle) Dexmedetomidine; (Empty circle) placebo. Values are expressed as mean ± standard deviation.
Discussion
The present results show that dexmedetomidine appears to have no clinically important adverse effects on respiratory rate and gas exchange when used in spontaneously breathing ICU patients after surgery.
Lack of respiratory depression in patients sedated with α2-adrenoceptor agonists was first reported by Maxwell [8] in a study conducted to investigate the respiratory effects of clonidine. However more recent data [9] suggest that clonidine may cause mild respiratory depression in humans. Furthermore, α2-adrenoceptor agonists have been reported to produce profound intraoperative hypoxaemia, which is species specific and is particularly seen in sheep [10,11].
The effects of dexmedetomidine on other ventilation parameters similarly appear to be species specific. Thus, in dogs dexmedetomidine causes an increase in minute ventilation, without any change in arterial blood gases, but causes a decreased response to inhaled carbon dioxide [12]. However, in New Zealand white rabbits dexmedetomidine caused marked and dose-dependent reductions in respiratory rate and PaO2 and significant rises in arterial PaCO2, which persisted 60 min after cessation of the dexmedetomidine infusion [15]. Idazoxan, a specific α2-adrenoceptor antagonist, reversed the effects on sedation and PaCO2.
Belleville et al [2] investigated the ventilatory effects of a 2-min intravenous infusion of dexmedetomidine at four dose levels (maximum 2.0 μg/kg) in human volunteers. Immediately after the maximum infusion of 2.0 μg/kg, irregular breathing patterns were noticed with short periods of apnoea, although there were no episodes of significant arterial oxygen desaturation to below 90%. According to those authors, minute ventilation and arterial PaCO2 were mildly decreased and increased, respectively, in relation to the depth of sedation as a result of dexmedetomidine infusions of 1.0 and 2.0 μg/kg. The reduction in minute ventilation was predominantly a result of a reduction in tidal volume, although there was a small but nonsignificant reduction in respiratory rate. There was a rightward shift and depression of the hypercapnic response at these infusion doses.
The mechanisms for these changes in ventilation are unknown, but it is possible that they are a result of central respiratory depression, given the distribution of α2-adrenoceptors in the brainstem [16]. However, the effects of dexmedetomidine on human respiration are much less marked than those of opioids and other intravenous and volatile anaesthetic agents, and appear to be similar in order of magnitude to those seen in the heavy sleep state.
Previous studies that investigated the respiratory effects of dexmedetomidine have only been performed in healthy human volunteers, who have received either single intramuscular injections or short (≤ 10 min) intravenous infusions of dexmedetomidine. It is therefore reassuring that no deleterious clinical effects on respiration and gas exchange were seen in our patients, who received long-term infusions of dexmedetomidine.
However, there are important limitations to the present results. In this study patients were sedated to a Ramsay Sedation Score of 2 or greater after extubation, and so we do not have respiratory function data on patients who were more heavily sedated. Consequently, no clear dose/response curve for dexmedetomidine can be formulated, and further investigation is probably ethically difficult to achieve in the spontaneously ventilating intensive care patient. We also have no data on the ventilatory responses to hypercapnia and hypoxia, which have been studied in healthy volunteers, but again this would be difficult to examine practically and ethically. The placebo group received over twice as much morphine as did patients who received dexmedetomidine infusions after extubation, but there were no differences in respiratory rate or PaCO2 between the groups. We can not therefore determine from the present study whether dexmedetomidine has any benefits over morphine from a respiratory perspective.
There were no differences in oxygen saturations between the groups, because the administered oxygen concentration was adjusted to maintain satisfactory gas exchange. Interestingly, however, there were statistically significant higher PaO2: FIO2 ratios in the dexmedetomidine group after extubation and for the intubated period before extubation. This ratio allows for the variation in administered oxygen to patients during the study period, and gives us some clinical indication of alveolar gas exchange. However, this variable was not a primary outcome variable for the study, and may represent a type 1 error, although post hoc analysis revealed that the present data have 80% power to detect a significant difference (α value 0.05). While intubated, ventilation was adjusted to maintain satisfactory gas exchange, and so mode of ventilation, tidal volumes, positive end-expiratory pressure, etc, may not have been equivalent between the groups, and this may explain some of the differences. However, this can not explain the improvements in gas exchange seen during the extubation period, when the only ventilation variable was the FIO2. Circulatory variables were not measured (oxygen uptake, cardiac output and mixed venous oxygen content), and again these may have been responsible for some of the differences in PaO2 : FIO2 ratio that were observed. Further investigations are required to exclude a type 1 error and to elucidate the exact mechanism for these effects if this phenomenon is reproduced in other studies. α2-Adrenergic-induced hypoxaemia, which is seen particularly in sheep, does not appear to occur, and a great deal is still to be learned about organ-specific and species-specific distribution and density of α2-adrenoceptors, and the individual specificity of α2-adrenoceptor agonists.
The clinical benefits of a sedative and analgesic agent in the ICU that has only mild respiratory depressant effects are immediately apparent. Sedation continued over the extubation period has been shown [13] to reduce haemodynamic disturbances and myocardial ischaemia, but is currently rarely performed because of the known negative effects on respiration from the sedative agents that are currently available. We have previously shown that dexmedetomidine provides cardiovascular stability, with a reduction in rate-pressure product over the extubation period, and so might protect against ischaemia, independently of its sedative properties, as a consequence of its α2-agonist activity [1]. A sedative agent that has analgesic properties, minimal effects on respiration and may offer ischaemia protection would also have enormous potential outside the ICU. Dexmedetomidine may fulfill all of these roles, but at present we can only conclude that dexmedetomidine has no deleterious clinical effects on respiration when used in doses that provide adequate sedation and effective analgesia in the surgical population requiring intensive care.
Acknowledgments
Acknowledgement
This study was supported by Abbott Laboratories. The results of this study were presented in part at the 20th International Symposium on Intensive Care and Emergency Medicine, Brussels, 2000.
References
- Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, Vedio A, Singer M, Feneck R, Treacher D, Willatts SM, Grounds RM. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. . Anesthesiology. 1992;77:1125–1133. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- Jaakola ML, Ali-Melkkila T, Kanto J, Kallio A, Scheinin H, Scheinin M. Dexmedetomidine reduces intraocular pressure, intubation responses and anaesthetic requirements in patients undergoing ophthalmic surgery. . Br J Anaesth. 1992;68:570–575. doi: 10.1093/bja/68.6.570. [DOI] [PubMed] [Google Scholar]
- Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. . Br J Anaesth. 1992;68:126–131. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- Aho M, Lehtinen AM, Erkola O, Kallio A, Korttila K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. . Anesthesiology. 1991;74:997–1002. doi: 10.1097/00000542-199106000-00005. [DOI] [PubMed] [Google Scholar]
- Aantaa R, Jaakola ML, Kallio A, Kanto J, Scheinin M, Vuorinen J. A comparison of dexmedetomidine, and alpha 2-adrenoceptor agonist, and midazolam as i.m. premedication for minor gynaecological surgery. . Br J Anaesth. 1991;67:402–409. doi: 10.1093/bja/67.4.402. [DOI] [PubMed] [Google Scholar]
- Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell GM. The effects of 2-(2,6-dichlorphenylamine)-2-imidazoline hydrochloride (Catapres) upon the systemic and coronary haemodynamics and metabolism of intact dogs. . Arch Int Pharmacodyn Ther. 1969;181:7–14. [PubMed] [Google Scholar]
- Ooi R, Pattison J, Feldman SA. The effects of intravenous clonidine on ventilation. Anaesthesia. 1991;46:632–633. doi: 10.1111/j.1365-2044.1991.tb09709.x. [DOI] [PubMed] [Google Scholar]
- Celly CS, McDonnell WN, Black WD. Cardiopulmonary effects of the alpha-2 adrenoceptor agonists medetomidine and ST-91 in anesthetized sheep. . J Pharmacol Exp Ther. 1999;289:712–720. [PubMed] [Google Scholar]
- Bacon PJ, Jones JG, Taylor P, Stewart S, Wilson-Nunn D, Kerr M. Impairment of gas exchange due to alveolar oedema during xylazine sedation in sheep; absence of a free radical mediated inflammatory mechanism. Res Vet Sci. 1998;65:71–75. doi: 10.1016/s0034-5288(98)90030-3. [DOI] [PubMed] [Google Scholar]
- Bloor BC, Abdul-Rasool I, Temp J, Jenkins S, Valcke C, Ward DS. The effects of medetomidine, an alpha 2-adrenergic agonist, on ventilatory drive in the dog. Acta Vet Scand Suppl. 1989;85:65–70. [PubMed] [Google Scholar]
- Conti J, Smith D. Haemodynamic responses to extubation after cardiac surgery with and without continued sedation. . Br J Anaesth. 1998;80:834–836. doi: 10.1093/bja/80.6.834. [DOI] [PubMed] [Google Scholar]
- Furst SR, Weinger MB. Dexmedetomidine, a selective alpha 2-agonist, does not potentiate the cardiorespiratory depression of alfentanil in the rat. Anesthesiology. 1990;72:882–888. doi: 10.1097/00000542-199005000-00019. [DOI] [PubMed] [Google Scholar]
- Jarvis DA, Duncan SR, Segal IS, Maze M. Ventilatory effects of clonidine alone and in the presence of alfentanil, in human volunteers. Anesthesiology. 1992;76:899–905. doi: 10.1097/00000542-199206000-00005. [DOI] [PubMed] [Google Scholar]
- Khan Z, Ferguson C, Jones R. Alpha-2 and imidazoline receptor agonists: their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]


