Abstract
Sex-specific traits that lead to the production of dimorphic gametes, sperm in males and eggs in females, are fundamental for sexual reproduction and accordingly widespread among animals. Yet the sex-biased genes that underlie these sex-specific traits are under strong selective pressure, and as a result of adaptive evolution they often become divergent. Indeed out of hundreds of male or female fertility genes identified in diverse organisms, only a very small number of them are implicated specifically in reproduction in more than one lineage. Few genes have exhibited a sex-biased, reproductive-specific requirement beyond a given phylum, raising the question of whether any sex-specific gametogenesis factors could be conserved and whether gametogenesis might have evolved multiple times. Here we describe a metazoan origin of a conserved human reproductive protein, BOULE, and its prevalence from primitive basal metazoans to chordates. We found that BOULE homologs are present in the genomes of representative species of each of the major lineages of metazoans and exhibit reproductive-specific expression in all species examined, with a preponderance of male-biased expression. Examination of Boule evolution within insect and mammalian lineages revealed little evidence for accelerated evolution, unlike most reproductive genes. Instead, purifying selection was the major force behind Boule evolution. Furthermore, loss of function of mammalian Boule resulted in male-specific infertility and a global arrest of sperm development remarkably similar to the phenotype in an insect boule mutation. This work demonstrates the conservation of a reproductive protein throughout eumetazoa, its predominant testis-biased expression in diverse bilaterian species, and conservation of a male gametogenic requirement in mice. This shows an ancient gametogenesis requirement for Boule among Bilateria and supports a model of a common origin of spermatogenesis.
Author Summary
While sexual reproduction is widespread among animals, it remains enigmatic to what extent sexual reproduction is conserved and when sex-specific gametogenesis (spermatogenesis and oogenesis) originated in animals. Here we demonstrate the presence of the reproductive-specific protein Boule throughout bilaterally-symmetric animals (Bilateria) and the conservation of its male reproductive function in mice. Examination of Boule evolution in insect and mammalian lineages, representing the Protostome and Deuterostome clades of bilateral animals, failed to detect any evidence for accelerated evolution. Instead, purifying selection is the major force behind Boule evolution. Further investigation of Boule homologs among Deuterostome species revealed reproduction-specific expression, with a strong prevalence of testis-biased expression. We further determined the function of a deuterostomian Boule homolog by inactivating Boule in mice (a representative mammal, a class of Deuterostomes). Like its counterpart in Drosophila (a representative of the opposing Protostome clade), mouse Boule is also required only for male reproduction. Loss of mouse Boule prevents sperm production, resulting in a global arrest of spermatogenesis in remarkable similarity to that of Drosophila boule mutants. Our findings are consistent with a common origin for male gametogenesis among metazoans and reveal the high conservation of a reproduction-specific protein among bilaterian animals.
Introduction
Evolution of sexual reproduction, consisting of the origin and maintenance of sex, has been a central focus of evolutionary biology since the time of Darwin. The origin of sex has generally been simplified to the question of the origin of meiosis, which is known to have a single origin among all eukaryotes [1], [2]. However, sexual reproduction in higher eukaryotes is more complex than meiosis alone, and has evolved independently in plants and animals. The fundamental component of animal sexual reproduction is gametogenesis, the differentiation of sexually dimorphic male sperm and female eggs. Unlike meiosis, which is required in both males and females, most other components of gametogenesis are sex-specific or sex-biased, such as sperm tail formation. These traits are subject to strong selective pressures from natural selection, sexual selection, and/or sexual antagonism [3], [4]. Because of these selective forces, sex-biased reproductive-specific traits are known to diverge rapidly. Such patterns of rapid divergence are not only prevalent among morphological traits like male genitalia, but also extend to the molecular level, including DNA sequences, the expression profiles of sex-biased reproductive genes and regulatory pathways underlying sex determination. [5]–[17].
What remains unknown is to what extent features of sexual reproduction can be conserved. Most animals produce sex-specific gametes distinct in size, morphology, motility and development. Animal sperm are predominantly small and motile, with compact nuclei and often a beating flagellum, which are produced through a series of male-specific developmental steps called spermatogenesis. Eggs are usually large in size and immotile, and are produced through a distinct developmental process called oogenesis. The evolutionary origin of such dimorphic features of animal sexual reproduction is intriguing yet difficult to address experimentally since they left no trace in the fossil record. However, the identification of conserved male or female-specific gametogenic proteins across large evolutionary distances could uncover molecular traces of any ancient gametogenic machinery, providing evidence for a common origin of sexually dimorphic traits among animals. While reproductive proteins with conserved homologs in different phyla are not uncommon, most of them are involved in general cellular functions, and are hence also required for other non-reproductive processes [18], [19]. Their sequence conservation likely results from their pleiotropic functional constraints (i.e. additional functions in non-reproductive tissues) rather than their reproductive functions. This is consistent with a model in which the pre-existing somatic cell machinery underwent reproductive specialization during the evolution of gametogenesis in multi-cellular ancestors. A few reproductive-specific proteins have more restricted roles in sexual reproduction in distant species. However they are either required in different sexes or different stages of sexual reproduction in distant lineages of metazoans, or functional information is only available for one metazoan lineage [20]–[25]. Furthermore, most of these proteins appear to be associated with common features of germ cells in both sexes, suggesting that the unique functions that differentiate germ cells from somatic cells are likely to be conserved in animals [26]–[29]. Like meiosis, germ cells are common to both sexes and are therefore not subjected to as strong selective pressures as sex-specific or sex-biased processes are.
Besides meiosis and the specification of germ cells, most of the other components of gametogenesis appear to be sex-biased or sex-specific. Despite a large number of sex-specific gametogenesis proteins uncovered in the model organisms Drosophila, C. elegans and mice, no conserved male- or female-specific gametogenic factors common to all lineages of animals have been clearly demonstrated [30]–[33]. However, the major steps of dimorphic gametogenesis among animals are very similar. For example, the major steps in spermatogenesis consist of the development of germline stem cells, mitotic proliferation of spermatogonial cells, preparation for and entry into meiosis, meiotic divisions, and finally differentiation of haploid spermatids into highly specialized motile sperm. Most, if not all, of the developmental steps and the developmental sequence of those steps are similar among animals from different phyla [32], [34]. These similarities raise the question of whether they evolved independently in different lineages by convergent evolution, or evolved from a single ancestral prototype. While the absence of a universal male- or female-specific reproductive factor is predictable due to the fast divergence of reproductive proteins and hence is compatible with the hypothesis of multiple origins of spermatogenesis and oogenesis, it does not exclude the alternative single-origin hypothesis. It remains possible that spermatogenesis and oogenesis each evolved from a single prototype, followed by the rapid divergence of most components of the reproductive machinery. However, a few core components of the ancient prototype critical for sperm or egg production could remain conserved.
Identification of such conserved core components is the key to distinguishing between these two possibilities. Such ancient core reproductive components should fulfill the following criteria: present in most, if not all, of the major lineages of animals undergoing sexual reproduction; originated around the time when gametogenesis likely evolved; demonstrated conservation at the sequence, expression and functional levels in species from diverse animal phyla. The most stringent criteria for a conserved male or female gametogenesis factor requires a clear demonstration that these conserved components are only required for gametogenesis in one sex among animals, and not for any other processes, thus excluding any possibility that such factors are conserved due to essential functions outside of gametogenesis. A strong candidate for a conserved male gametogenic factor in animals appears to be BOULE, the ancestral member of the human DAZ gene family. The human DAZ gene family consists of a Y-linked DAZ gene and the autosomal DAZ-Like (DAZL) and BOULE genes, all of which share a conserved RNA recognition motif (RRM) and a more divergent DAZ repeat consisting of 24 amino acids rich in N, Y, and Q residues [35]. All DAZ family proteins studied so far appear to be restricted to reproduction [35], [36], and the DAZ gene is commonly deleted in men with few or no sperm [37], [38]. Although no mutations in DAZL or BOULE have been shown to be responsible for infertility in men or women, homologs of DAZL and BOULE are required for fertility in other species, and over-expression of DAZ family proteins promote the differentiation of human embryonic stem cells towards the germ cell lineage [35], [39]–[43]. Furthermore, a human BOULE transgene rescued partial testicular defects of fly boule mutants, suggesting functional similarity between these two distant homologs [44].
However, the only two metazoan Boule homologs whose function has been characterized in depth revealed opposite gametogenic requirements [39], [40]. Loss-of-function phenotypes of Boule homologs in Drosophila and Caenorhabditis elegans reveal their divergent roles in reproduction, with boule required for male reproduction in flies and for oogenesis in the worm [39], [40]. The prevalence of Boule homologs among other metazoan phyla remains unexplored, raising the possibility that Boule may have undergone adaptive evolution like many other reproductive genes and subsequently diverged at the functional level among different metazoan branches. While partial rescue of the fly boule mutant defect by a human BOULE transgene suggests functional conservation, this may only reflect similar biochemical properties of all DAZ family members [45]. Indeed, frog Dazl is able to partially rescue the Drosophila boule mutant, despite the fact that frog Dazl performs a reproductive function distinct from fly boule [46], [47]. We sought to gain further insight into the metazoan evolution of Boule and to determine if it has a general conserved reproductive function, or also a conserved sex-specific function. We systematically examined the prevalence of Boule homologs in major animal phyla and also the molecular evolution of Boule in two distant bilaterian classes. To understand the functional evolution of bilaterian Boule, we surveyed the expression of Boule homologs in representative bilaterian species and determined the functional conservation of deuterostomian Boule through expression and genetic analyses of the mouse Boule homolog.
Results
Prevalence of Boule homologs in metazoans
We first asked what other animal lineages might have Boule homologs besides insects, mammals, and nematodes. In order to distinguish Boule from homologs of other DAZ family members as well as other general RNA binding proteins, we established the signature features of Boule that would allow us to identify Boule homologs in distant lineages with confidence. We separately aligned the protein sequences of known Boule homologs among two distant metazoan groups, mammals and insects, and established a consensus sequence for the RNA recognition motif (RRM) in each group (Figure S1). To determine general features of the Boule RRM we aligned the mammalian and insect consensus sequences to each other and found a 92-amino acid consensus sequence. The most conserved residues were in the two RNP motifs (PNRI(V)FVGG for RNP2 and DRAGV(I)SKGYGFV(I) for RNP1) that are known to be important for RNA binding in RRM proteins [48]. We also established a consensus sequence for the closely related Dazl RRM, and found it to be distinct from the Boule consensus sequence (Figure S2). Dazl homologs contain slightly different consensus sequences for both RNP2 (VFVGGI) and RNP1 (KGYGFVSF), have distinct sequences surrounding the RNPs, and have a conserved deletion of two amino acids (Figure S2) [35]. Interestingly, the mammalian Boule proteins appeared to share higher sequence similarity than insect homologs despite the fact that mammals have an additional Boule-like protein, Dazl, suggesting that the presence of Dazl did not relieve the selective pressure on Boule in any significant way. Not only is the sequence of the RRM highly conserved, but the proteins are similar in size, usually around 30 kDa, and contain a single RRM domain near the N-terminus [48]. While it is impossible to align all the exon-intron boundaries due to the extensive genomic divergence between distant species, we found that exon-intron structures spanning the region of the highly conserved RRM (exons 2, 3, 4, and 5) are conserved, except that Drosophila exons 3 and 4 are fused into a single exon (Figure S1C). Thus, comparison of the mammalian and insect Boule genes reveals conservation not only in specific protein sequences, but also in aspects of the genomic structure underlying these sequences.
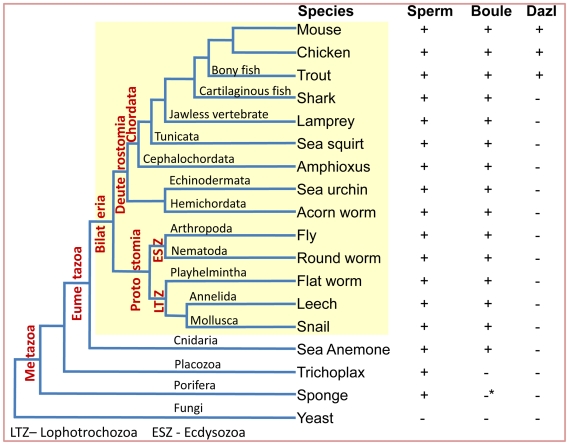
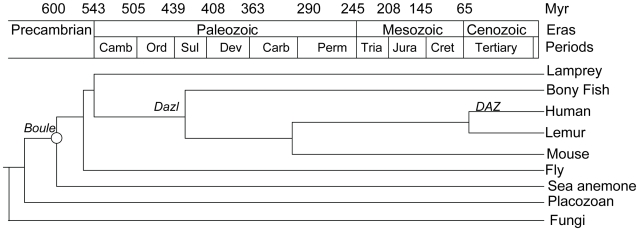
Since sex-biased genes often undergo lineage-specific loss during evolution [13], we assessed the prevalence of Boule homologs in each branch of metazoan evolution (Figure 1). Starting with the Boule RRM consensus sequence, we used Tblastn to search the genomes of species from major phyla representing the two clades of Bilaterians, deuterostomes and protostomes, for Boule homologs. Among deuterostomes, Boule homologs were identified in at least one species of every phylum (Figure 1): in Chordata (human, Homo sapiens; mouse, Mus musculus; chicken, Gallus gallus; rainbow trout, Oncorhynchus mykiss; elephant shark, Callorhinchus milii; lamprey, Petromyzon marinus;), Tunicata (sea squirt, Ciona intestinalis), Cephalochordata (lancelet or amphioxus, Branchiostoma floridae), Echinodermata (sea urchin, Strongylocentrotus purpuratus), and Hemichordata (Acorn worm, Saccoglossus kowalevskii). Boule homologs were present in many protostomian species of the Ecdysozoa and Lophotrochozoa superphyla (Figure 1, ESZ and LTZ, Figure 2A). Boule was found in fruit flies (D. melanogaster), mosquitoes (Anopheles gambiae), lobster (Homarus americanus), green shore crab (Carcinus Maenas), wasp (Nasonia vitripennis) and nematodes (C. elegans), representing the Arthropoda and Nematoda phyla (Figure 1, ESZ), and also in each phylum of the Lophotrochozoans such as Platyhelmintha (flatworm, Schistosoma japonicum), Annelida (leech, Helobdella robusta), and Mollusca (snail, Biomphalaria glabrata) (Figure 1, LTZ). Therefore, homologs of a known gametogenic protein—Boule—are present throughout both deuterostomes and protostomes.
Figure 1. Phylogenetic distribution of motile sperm, Boule and Dazl homologs among species from major lineages of the animal kingdom and fungi.
Sexual reproduction in which male animals produce motile sperm often with flagellum is found in all major phyla of metazoan animals [84]. Representative species of each major metazoan taxon (mostly phylum) were analyzed for the presence or absence of Boule and Dazl homologs. The common names for those species are listed with the names of the phyla or taxonomical groups they represent printed above their branches. Major superphyla groups and Chordata phylum are labeled vertically near the roots of their phylogenetic branches and bilaterian lineages (Bilateria) are highlighted in yellow. LTZ – Lophotrochozoa, ESZ – Ecdysozoa, Trout—rainbow trout (Oncorhynchus mykiss), Shark—elephant shark (Callorhinchus milii). All other species names are listed in supplementary information. *The absence of a Boule homolog in sponge is tentative since it is based on the draft genome of Amphimedon queenslandica (http://www.jgi.doe.gov/sequencing/statusreporter/psr.php?projectid=16318).
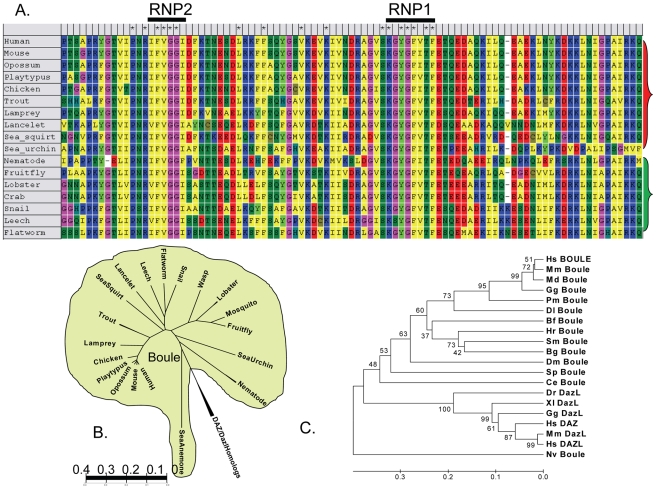
Figure 2. Conservation and prevalence of Boule proteins among animals.
(A) Alignment of Boule RRM domains from representative species of both deuterostomes and protostomes reveals conservation of the RNA binding domain, in particular in the regions surrounding RNP2 and RNP1 as well as the C-terminal region. Asterisks (*) mark amino acids conserved in Boule homologs from all species. The red bracket on the right indicates deuterostomian species and the green bracket protostomian species. Colors for individual amino acids are assigned based on similar properties of residues (Table S5). (B) Phylogenetic treatment of homologs from major metazoan branches of human DAZ gene family members (BOULE, DAZL and DAZ) using conserved RRM domains. Consistent with being the most ancient member of the DAZ family, the Boule clade is much more widespread and divergent, including members of the major phyla of protostomes and deuterostomes, while all DAZ/DAZL homologs are clustered together in one branch. The Dazl homolog clad and DAZ clad are much smaller and are restricted to vertebrates or primates, respectively. (C) Rooted tree showing the evolutionary distance among different homologs of the DAZ family. The numbers indicate bootstrap values. The evolutionary tree is drawn to scale, with branch lengths in units representing the number of amino acids substituted per site. Hs-Homo sapiens, Mm-Mus musculus, Md-Monodelphis domestica, Gg-Gallus gallus, Pm-Petromyzon marinus, Dl-Dicentrarchus labrax, Bf-Branchiostoma floridae, Hr-Helobdella robusta, Sm-Schistosoma mansoni, Bg-Biomphalaria glabrata, Dm-D. melanogaster, Sp-Strongylocentrotus purpuratus, Ce-C. elegans, Dr-Danio rerio, Xl-Xenopus laevis, Nv-Nematostella vectensis.
Origin of Boule in early animal evolution
Next, we asked when Boule arose during evolution by determining whether Boule homologs are present in basal, non-bilaterian metazoans or beyond the animal kingdom in plants or fungi.
Based on the consensus Boule features, we determined that Boule homologs are absent in fungi and plants, suggesting that Boule is restricted to the animal lineage (Figure S3A, Figure 1). We then explored the genomes of basal metazoan animal species and found that there is no Boule homolog in the most primitive animal, Trichoplax (Figure S3B, Figure 1). However, we identified a Boule homolog in the sea anemone, a species from the primitive Cnidaria phylum. Comparison of the consensus Boule sequence against the sea anemone genome (Nematostella vectensis) reveals two proteins with high similarity [49]. Surprisingly, the RRMs of both proteins contain characteristics of the Boule consensus sequence, while one of the sea anemone proteins has identical signature RNP1 and RNP2 motifs (PNRIFVGG and GVSKGYGSVT) to those of the Boule consensus domain (Figure 3C). Furthermore, unlike the fused exons 3 and 4 in the Drosophila boule genomic structure, the sea anemone boule gene has separate exons 3 and 4 as in humans, suggesting that the ancestral Boule gene contained separate exons 2, 3, 4 and 5 that encoded the RRM. This gene (XM_001637198) is predicted to encode a protein around 22 kDa, close to the typical size of Boule proteins. Hence, a Boule homolog is present in the sea anemone, a representative of Cnidaria (Figure S3C, Figure 1). A second sea anemone protein also has some similarity to the characteristic Boule RNP1 and RNP2, but there are multiple differences in critical positions and a greater divergence from the Boule consensus sequence (Figure S3C). Furthermore, the gene itself does not possess two conserved exon/intron junctions in the second half of the RRM domain that are present in all other species examined, including Drosophila. Therefore, the second protein is likely a more divergent duplicate of the ancient Boule gene, specific to the Cnidarian lineage.
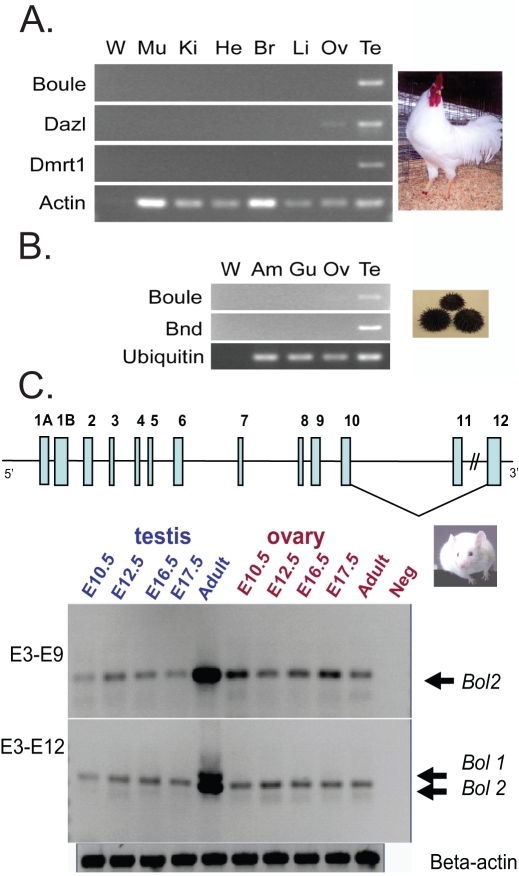
Figure 3. Conservation of reproduction-specific and testis-enriched expression of three deuterostome Boule homologs.
(A) RT-PCR survey using chicken Boule and Dazl specific primers on different tissues from adult rooster and hen (Gallus gallus). Dmrt1 (Doublesex and mab-3 related transcription factor 1) is a testis-specific gene and actin was used as a loading control. (B) RT-PCR survey using sea urchin-specific primers on different tissues from purple sea urchin (Strongylocentrotus purpuratus). Bnd (Bindin) is a testis-specific gene and ubiquitin was used as a loading control. W, water; Mu, muscle; Ki, kidney; He, heart; Br, brain; Li, liver; Ov, ovary; Te, testis; Am, Ampullae; Gu, guts. (C) Mouse Boule consists of 13 exons that encode multiple isoforms resulting from 5′ and 3′ alternative splicing. Two predominant mouse Boule isoforms – Bol1 and Bol2 – are male-biased and are restricted to or enriched in adult testes. Bol1 contains all 12 exons whereas Bol2 contains all exons except exon 11. RT-PCR analysis of RNA from mouse embryonic and adult gonads using primers corresponding to either exons 3 and 9 (upper panel, 501-bp band) or exons 3 and 12 (lower panel, 847-bp and 712-bp bands) reveals that full-length Bol1 is only expressed in postnatal testes, while Bol2 lacking exon 11 is also detectable at low levels throughout gonadal development in addition to its high adult testis expression. A similar pattern was confirmed using primers for exons 1 and exon 12.
The sea anemone is one of the most primitive metazoan species that undergoes sexual reproduction. It has separate sexes, inducible spawning and external fertilization [49]. Our finding places the origin of the Boule gene prior to the divergence of Bilateria from Cnidaria, but likely after Trichoplax branched from the common ancestor of eumetazoans, making Boule one of the few ancient animal gametogenic proteins known so far. Further analysis of Boule homologs in other basal metazoan lineages could better pinpoint the origin of metazoan Boule.
Dazl arose through a duplication of Boule, likely after protostomian and deuterostomian splitting, but the exact point of Dazl origin within deuterostome evolution has not been defined [35]. Homologs of Dazl have been identified in mammals, birds, reptiles, amphibians and fish [35], [50], [51], but whether Dazl is present in other non-vertebrate deuterostomes is unknown. Using the Dazl RRM domain, we searched for Dazl homologs in the genomes of acorn worm from Hemichordata (Saccoglossus kowalevskii), sea urchin from Echinodermata (Strongylocentrotus purpuratus), lancelet from Cephalochordata (Branchiostoma floridae), and sea squirt from Tunicata (Ciona intestinalis). We could not detect any canonical Dazl homologs (Figure 1). The highest BLAST hit from those genomes were Boule homologs, suggesting that Dazl is not present in either non-chordate deuterostomes or primitive chordates, and is likely restricted to the vertebrate lineage. To further determine the origin of Dazl in vertebrate evolution, we searched the genomes of the jawless fish, lamprey (Petromyzon marinus) and could not identify a Dazl homolog (http://genome.wustl.edu/genomes/view/petromyzon_marinus/) (Figure 1). Given that Dazl is present in bony fish such as zebrafish and medaka [52], [53], we then asked if Dazl is present in the cartilaginous fish, phylogenetically the oldest group of living jawed vertebrates. We searched the genome of the elephant shark (Callorhinchus milii) and found no evidence of a Dazl homolog, though a shark Boule homolog is present [54]. This analysis suggests that Dazl originated around the time of vertebrate radiation, likely in the ancestral lineage of bony fish (Figure 1).
To further determine the evolutionary relationship of metazoan Boule homologs, we performed phylogenetic analysis of Boule homologs from the major animal branches, together with homologs of the other members of the DAZ family, Dazl and DAZ (Figure 2B and 2C). Boule clearly represents the most ancient and widespread clade among the DAZ family members, present from sea anemone to human, whereas all Dazl and DAZ homologs can be clustered together in one branch. This is consistent with the distinct reproductive functions of DAZ and Dazl homologs, and the late arrival of Dazl in vertebrate evolution and DAZ in primate evolution (Figure 2B and 2C) [35], [38], [41], [47], [55]. Conservation of Boule homologs in major lineages of animals and their evolutionary relationship throughout animal evolution suggests that Boule is a fundamental component of eumetazoan reproductive machinery essential for the survival of most animal species.
Purifying selection, not positive selection, is predominant in the molecular evolution of Boule
The ancient origin and widespread presence of such a reproductive gene is in stark contrast with the pervasive rapid evolution usually associated with reproductive genes, especially male reproductive genes [5], [12]. The presence of Boule in various animals provided the rare opportunity to examine how selective forces shaped the molecular evolution of a reproduction-specific gene in distant lineages. We therefore examined Boule homologs that recently diverged from each other for any signs of adaptive evolution. We analyzed two separate groups of homologs to determine if Boule is under different selective pressure when Dazl homologs are present. We compared the entire boule coding sequences among seven Drosophila species (D. melanogaster, D. sechellia, D. yakuba, D. virilis, D. erecta, D. willistoni and D. ananassae) as well as eight representative mammalian species [56].
To determine if positive selection has played a role in Boule evolution, we compared the ratio of the rate of nucleotide changes that result in a non-synonymous amino acid substitution (Ka) to the rate of nucleotide changes that cause a synonymous amino acid substitution (Ks). Positive selection is a process that favors the retention of mutations that are beneficial to the reproductive success of an individual. Neutral theory predicts that the rate of non-synonymous substitutions (that by definition affect protein sequence) is equal to the rate of synonymous substitutions. If a protein has evolved under positive selection, there are more non-synonymous substitutions (Ka) than synonymous substitutions (Ks), and an accordingly high Ka/Ks ratio. If the protein evolved under purifying selection or negative selection, a process that removes deleterious alleles, there is a decrease or absence of non-synonymous substitutions, and therefore Ka/Ks is much smaller than that expected under neutral theory. A Ka/Ks greater than 1 is a strong indication of positive selection whereas only a Ka/Ks smaller than 0.1 usually suggests a role of purifying selection.
Among all pairwise comparisons among Drosophila species, we found that non-synonymous substitutions (Ka) were not in excess of synonymous substitution (Ks). Instead, Ka/Ks ratios for all pairwise comparisons were below 0.1 (Table S1), significantly lower than the ratio reported for rapidly diverging proteins [10], [12]. Similarly, all pairwise comparisons among mammalian species revealed Ka/Ks ratios below 0.1 (Table S2), indicating that the presence of Dazl homologs in mammals had little impact on the selective pressure on Boule homologs. Furthermore, this suggests that positive selection was not the major force driving the evolution of Boule either in Drosophila or mammals. Instead, the low Ka/Ks ratio suggests that purifying selection was responsible for the strong functional constraint on the entire protein, making Boule an exception to the rapid evolution commonly seen in reproductive genes [5], [9].
Male-biased gonadal expression among bilaterian Boule homologs
The prevalence and strong functional constraint of Boule throughout protostomes and deuterostomes suggests that Boule is likely a common reproductive factor with a critical function essential for the survival of bilaterian species. However the only Boule homologs functionally characterized exhibit divergent roles in reproduction, with Drosophila boule necessary for male reproduction and the C. elegans boule homolog, daz-1, required for egg production [39], [40]. Recently, the Boule homolog in the fish Medaka was reported to be expressed in both testes and ovaries [53]. Such divergent roles and expression during gametogenesis raised the question of what the ancestral function of Boule was, and whether the expression and function of Boule homologs might have diverged despite the high conservation of the functional motif. Since Boule function has only been examined in protostomes (C. elegans and Drosophila), we reasoned that by determining Boule expression patterns in deuterostomes we could ascertain whether or not the expression or function of Boule is conserved among bilaterians.
We chose two deuterostome species (chicken and sea urchin) from separate phyla and asked if Boule homologs are preferentially expressed in the testis or ovary. Like Drosophila and C. elegans, the sea urchin is also an invertebrate and has only the Boule gene, whereas chicken is a vertebrate with both Boule and Dazl. We identified homologs of Boule in chicken (G. gallus) and purple sea urchin (S. purpuratus) (Figure 1, Figure 2A) [51], [57], and found that chicken Boule is expressed specifically in the testis, and is not present in ovaries or any other organs we examined (Figure 3A, Figure S4). However, chicken Dazl is expressed in both testes and ovaries, similar to mammalian Dazl [41], [58]. The expression of the Boule homolog in sea urchin, a primitive deuterostomian species from the Echinodermata phylum, is also testis-biased and not expressed in any non-gonadal tissue (Figure 3B). A transcript that lacks a complete RRM domain was detectable at low levels in ovary (not shown). However, this ovarian transcript may not be functional and is likely the isoform previously reported in sea urchin ovary and eggs by in situ hybridization [57]. Together these results show that deuterostome homologs of Boule are also reproduction specific, like their protostome counterparts, but with a tendency toward testis-biased expression.
Since the nematode Boule homolog is only required for ovarian function but not male gametogenesis, and Boule transcripts have been detected in the ovaries as well as in the testes of some other species [53], [57], [59], we wondered if such ovarian expression in sporadic species is a lineage-specific phenomenon or if it is a common feature. Thus, we turned to the laboratory animal model, the mouse, for an in-depth gene expression and functional analysis. Although mammalian Boule is highly expressed in the adult mouse testis but not ovary, it is not known if Boule is expressed in the ovary during development [35]. In view of the different timing of meiotic initiation in female and male mammals, we determined the developmental expression profile of mouse Boule during both male and female embryonic gonadal development (embryonic day 10.5, E12.5, E16.5 and E17.5) in comparison with adult gonads.
We first characterized the entire mouse Boule genomic region and identified alternatively spliced isoforms (Text S1 and Figure 3C). Using primers spanning all 12 exons, we found two major Boule transcripts, both of which were most highly expressed in the adult testis. The primary Boule transcript contained all 12 exons (Bol1) and was expressed only in the adult testis, whereas a second transcript lacking exon 11 (Bol2) was highly expressed in adult testes but also detectable at low levels in early embryonic gonads of both sexes and the adult ovary (Figure 3C). We thus confirmed that the predominant expression of Boule during reproductive development is in the adult testis, including a testis-specific isoform, and also identified previously unreported low levels of Boule RNA in mouse ovaries. Together with previous findings, a total of seven out of eight bilaterian species examined (human, mouse, cattle, chicken, fish medaka, sea urchin and fruit fly) representing three different phyla express Boule in the adult testis [35], [53], [60]. Expression is in the same cell types (spermatocytes and spermatids) in the testes of the human, mouse and fish medaka, suggesting conservation of developmentally-regulated testicular expression of Boule in vertebrate animals [35], [53].
The observation that Boule homologs show predominantly testis-biased expression in diverse species is consistent with a conserved male gametogenic function in bilateral animals. However, the oogenic requirement seen in C. elegans taken together with detectable levels of ovarian expression in several species suggests the possibility that an additional oogenic function is also conserved. Alternative Boule transcripts detected in mouse ovaries or embryonic gonads, albeit at much lower levels, could still play an important physiological function and therefore contribute to its sequence conservation. To ascertain if Boule is functionally conserved in deuterostomes and if ovarian expression of Boule is physiologically significant, it is necessary to examine the physiological function of Boule in deuterostomes.
Generation of a deuterostomian Boule mutation
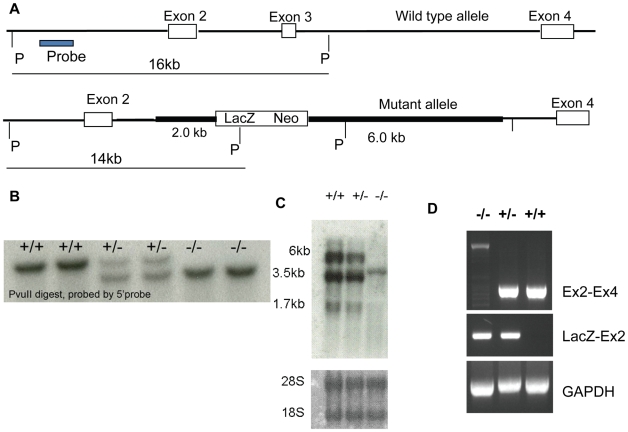
To determine if the male-specific requirement of Drosophila boule is functionally conserved among Bilateria, we set out to generate a mutation of a deuterostomian Boule homolog to investigate its physiological requirement. We used mice as a representative species of deuterostomes, and used gene targeting to delete the RNA binding domain, and thus disrupt the critical function of mouse Boule (Figure 4). We replaced exon 3, which encodes a part of the RNA binding domain and is present in all Boule isoforms (Figure 3C), with a lacZ-neo vector through homologous recombination in embryonic stem cells. The removal of exon 3 resulted in a deletion of the RNA binding domain and a frame-shift in the remaining transcript. This transcript is expected to produce a truncated BOULE protein missing both its RNA binding domain and the remaining C-terminal portion of the protein. Four chimeric mice were derived and correct homologous recombination as well as germline transmission of the Boule mutation was confirmed. Homozygote mice recovered from matings among heterozygotes were identified by genotyping and confirmed by Southern hybridization (Figure 4B).
Figure 4. Generation of a loss-of-function Boule mutation in mice.
(A) Gene targeting strategy with wild type genomic structure from exon 2 to exon 4 (top) and the successfully targeted locus (bottom). Thick bars represent the two genomic regions used for homologous recombination in the gene targeting construct. PvulI (P) restriction sites and the position of the 5′ probe are shown. (B) Southern hybridization with the 5′ probe shows that the wild type contains a 16-kb PvuII fragment, whereas homozygotes contain a 14-kb PvuII fragment and heterozygotes contain both 16-kb and 14-kb fragments, thus confirming homologous recombination. (C) Northern hybridization using Boule cDNA shows that all three wild type Boule transcripts are absent in the homozygotes; instead a novel transcript corresponding to exon 1, exon 2, and lacZ was detected. (D) RT-PCR using primers spanning exons 2 to 4 further confirmed the complete absence of wild type transcripts. Expected wild type products between exon 2 and 4 were present in heterozygotes and the wild type but absent in homozygotes. The weak amplification of a very large fragment in homozygotes represents the PCR product from the chimerical transcript spanning exon 2, LacZ, and exon 4 of the mutant allele.
We next determined if the mouse Boule mutation was a complete loss-of-function mutation. We performed Northern blot hybridization on RNA from the testes of wild-type, heterozygote, and homozygote mice with Boule cDNA as a probe and found that there are three Boule transcripts present in wildtype testes, all of which are absent in homozygous Boule mutants. Instead, a single novel transcript corresponding to the size of the predicted chimeric transcript consisting of the truncated Boule and beta-geo (a transcript containing exon 1, 2 and lacZ, Figure 4C) is present. We further confirmed the absence of wild type Boule transcripts by the more sensitive RT-PCR and did not detect exon 3 in the mutant transcript. Instead we only detected a much larger PCR product spanning exon 2 and 4 in homozygotes (Figure 4D). This large PCR product is absent in wildtype and contains the lacZ gene from the knockout vector. Hence we conclude that Boule expression is completely disrupted in the mutant, and we have established a loss-of-function allele in the mouse Boule homolog.
Mouse Boule is essential for male fertility but not for viability, growth, or female fertility
Homozygote Boule mutants exhibited normal viability, growth and mating behavior (Figure 5A). We recovered the expected number of homozygotes from heterozygote matings (wild type∶ heterozygotes∶ homozygotes = 44∶79∶43), indicating that there was no effect on survival. We next tested the fertility of mice homozygous for the Boule mutation to determine whether the Boule mutation affected male and/or female reproduction. Six homozygote males were each mated with two wild type females and individually produced no pups after four months. In contrast, wild type males sired at least two litters each during that time, suggesting that the homozygous Boule males were sterile. Female Boule homozygotes showed no obvious defects and were fertile, producing an average of 8.3 pups per litter (8.3±1.4, n = 12) with heterozygote males, similar to wild type or heterozygote females (7.7±2.1; n = 7 for wildtype; 7.6±2.4; n = 18 for heterozygotes). Homozygote females continued to be fertile up to the oldest age tested (12 months). Thus, mutation of mouse Boule disrupts male reproduction but does not affect normal development, growth or female fertility, suggesting that mammalian Boule is required only for male reproduction, similar to fly boule but different from worm daz-1.
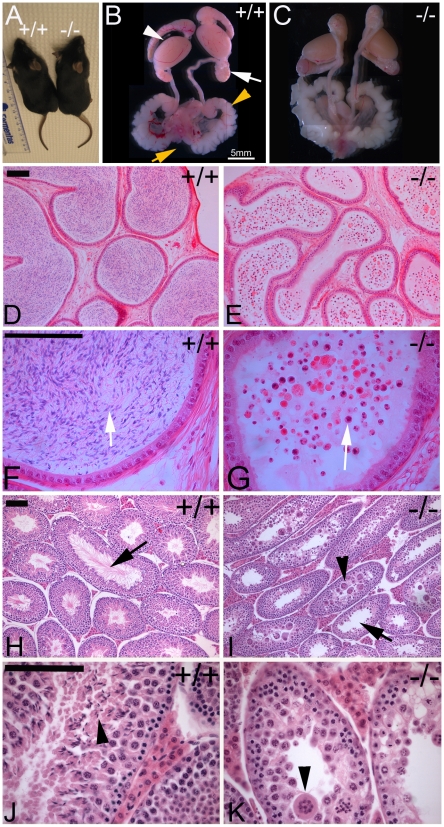
Figure 5. Mouse Boule mutant males are infertile, resulting from a global arrest of spermatogenesis.
(A) Knockout mice are indistinguishable from those of wild type in morphology and size. (B) The wild type male reproductive tract consists of a pair of oval-shape testes (white arrow head) attached to the epididymis (white arrow), seminal vesicle (yellow arrowhead), and urethra (yellow arrow). (C) The reproductive tract in mouse Boule mutants appears normal, similar to that of wild type mice. Wild type epididymis tubules contain lots of motile sperm (D), and condensed sperm nuclei and sperm tails (arrow) are clearly visible (F at higher magnification). In the Boule mutant, epididymal tubules lack mature sperm and some are half-empty (E). Instead, small degenerating round cells are found inside the tubules (arrow in G). (H) Testicular sections from wild type mice showing the presence of sperm in the lumen (arrow in H) and elongating spermatids (arrowhead in J). (I, K) Sections from Boule mutant testes showing the absence of both sperm from the lumen (arrow in I) and elongating spermatids. Mouse Boule mutant testes contain degenerating cysts of germ cells (arrowheads in I and K). The scale bars are equal to 100 µm unless noted.
Similar physiological requirements of Drosophila and mouse Boule homologs suggest possible conservation of an ancient male gametogenic requirement. However, it is also possible that such similarity is a mere coincidence since out of hundreds of bilaterian species, only homologs from three species are functionally characterized, with one out of the three being functionally divergent. We reasoned that if mouse and Drosophila Boule function is conserved, then the specific reproductive defects of the loss-of-function mutations in both species should be more likely to be similar than if they had evolved independently by chance. We therefore determined whether mouse Boule and fly boule function within similar processes of male reproduction. In both flies and mice, the major reproductive organs are clustered together in the male reproductive tract. The tract consists of a pair of testes for sperm production; a pair of sperm storage/maturation organs (the epididymis in mice and seminal vesicle in flies); accessory glands for providing proteins, other nutrients and seminal fluid that accompany sperm migration and fertilization (prostate, seminal vesicles and coagulating glands in mice and accessory glands in flies); and a sperm transport duct used for sperm transportation and maturation (vas deferens and urethra in mice and ejaculatory duct in flies) (Figure 5B) [30]. While the major components of the male reproductive tract in mammals and insects appear to serve similar reproductive functions, it is not known if any components are evolutionarily related between vertebrates and invertebrates. In mouse Boule mutants, all the components of the male reproductive tract are present and intact (compare Figure 5B and 5C), similar to that of the fly boule mutant [39]. Compared with wild type mice, the male reproductive tracts of Boule homozygous mutant mice were morphologically indistinguishable except for the testes, which are smaller by weight [61]. Hence the sterility defect is the result of a defect in the testis, similar to the sterility defect associated with the Drosophila boule mutation [39], [61].
Mouse Boule mutation causes a global arrest of sperm development
Further characterization of the reproductive defects revealed that mouse Boule mutant epididymides lacked mature sperm (Figure 5E), and instead contained degenerating cells that were not seen in the wild type (compare Figure 5D and 5F to Figure 5E and 5G). Therefore, the observed male sterility of the Boule homozygous mutant mice appears to be due to a complete absence of sperm in the epididymis.
Next, we examined the developmental impact of the mouse Boule mutation on sperm production. While the overall testicular structure was normal and all the somatic cell types were present in mouse Boule mutants, the effect of mouse Boule mutation on sperm development was dramatic, with a complete halt of spermatogenesis inside all seminiferous tubules of the testis. Both mature sperm and developing elongating spermatids were entirely absent from the lumen of individual seminiferous tubules (compare Figure 5H and 5J to Figure 5I and 5K). This indicates that the failure to produce sperm resulted from a major block in sperm production due to a global arrest of spermatogenesis prior to spermatid differentiation, similar to the spermatogenic defect seen in the testes of boule mutant flies [39], [61]. Such similar global, spermatogenic-specific impacts of mutations in orthologs in divergent phyla is surprising and unprecedented, given the vastly different organization of testicular structure, type of spermatogenesis and differences in the contribution of hormonal control in mammals and insects.
In Drosophila, spermatogenesis is cystic, where a single spermatogonial cell and its clonal descendants are encapsulated in a somatic cyst throughout sperm development. Though mouse spermatogenesis is acystic, we observed prominent ball-like structures containing degenerating cells in the mouse Boule mutant, resembling the degenerating cysts seen in the fly boule testis (Figure 5I and 5K, arrowheads). Although the cyst structure is not present in mammalian spermatogenesis, descendant cells from a single spermatogonial stem cell remain connected with each other through cytoplasmic bridges during mouse sperm development, similar to that in Drosophila spermatogenesis [62]–[65]. This phenomenon could lead to the merging of multiple interconnected arrested spermatogenic cells in Boule mutant testes, resulting in such giant “cysts” with multiple nuclei. Despite the distinct modes of spermatogenesis in mice and Drosophila, the mouse Boule and fly boule mutations caused a remarkably similar and specific global arrest of spermatogenesis. Though further characterization of the developmental and cellular defects in mouse Boule mutant testes is needed to determine the full extent of similarity in developmental and cellular defects between mouse and Drosophila Boule mutants [61], our data demonstrate a key physiological requirement of Boule in sperm development and conservation of its male reproduction function between two distant lineages of a protostome (Drosophila) and a deuterostome (mouse).
Discussion
Evolution of reproductive traits and genes is of the utmost interest to our understanding of the central questions in evolutionary biology such as speciation. However, the relatively rapid divergence of sex-biased reproductive genes in comparison with somatic cell proteins or non sex-biased reproductive proteins during evolution has made it difficult to study the evolution of sex-specific reproductive systems across extended evolutionary distances. Even though some reproductive genes are conserved beyond a given phyla, they are often also involved in other developmental processes. Such broad functionality compounds studies of their reproductive evolution because the selective pressures driving their evolution may be due to critical somatic functions, and not a reproduction-related function. The human DAZ family of reproductive genes, with homologs in diverse species, many of which are specifically expressed in reproductive tissues, are ideal candidates for the study of reproduction-specific gene evolution [35], [38], [40], [41], [46], [53], [60]. In particular, Boule, the ancestral gene member, is reproductive specific in flies and worms.
We identified homologs of Boule in the major phyla of metazoans, reconstructed the evolutionary history of Boule, and began to determine its functional divergence. We found that Boule, unlike other reproductive proteins, has been maintained in all major phyla of bilaterian animals as well as in Cnidarians, but are absent in the most primitive animals (the placozoan Trichoplax), fungi and plants (Figure 1). We found that Dazl homologs are only present in vertebrates, supporting the hypothesis that Boule is the ancestral member of the DAZ family [35]. Dazl homologs were absent in representative species of non-vertebrate deuterostomes and cartilaginous fish (elephant shark), but were present in bony fish and tetrapod animals (Figure 1). This places the origin of Dazl after the divergence of bony fish from cartilaginous fish but before the arrival of tetrapod animals (Figure 6). On the other hand, the widespread presence of Boule in eumetazoan animals indicates that the ancient Boule gene was present as early as 600 million years ago in the Precambrian era, in the common ancestors of Bilaterians (often called Urbilateria) as well as eumetazoans (Figure 6 and Figure 7) [66], [67].
Figure 6. Schematic representation of evolution history of the three DAZ family members—Boule, Dazl, and DAZ—during metazoan evolution.
The birth of Boule is estimated to be after the divergence of placozoan from the common ancestor (open circle) of Cnidaria (such as sea anemone) and Bilateria. Dazl arose through duplication from ancestral Boule during the evolution of bony fish, possibly after its split from lamprey and cartilaginous fish but prior to the divergence of ray-finned fish and lobe-finned fish (i.e. tetrapod animal lineage). After their divergence from New World monkeys such as lemur, Old World monkey Dazl duplicated and gave rise to DAZ [55]. Myr –millions of years. The geological scale is based on [67], [85].
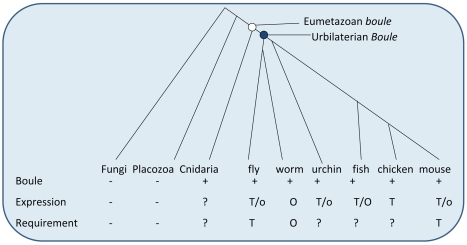
Figure 7. Expression and functional requirement of Boule homologs in metazoan species studied.
The presence of a Boule homolog (+), testis expression or functional requirement (T) or ovary expression or functional requirement (O) is marked. Low level expression in the ovary is marked as a lower-case “o” to distinguish it from abundant ovarian expression, marked as a capital “O”. The information on Boule homologs are based on this work and previous reports [35], [39], [40], [53]. The ancient Boule gene in the common ancestor of Bilateria (the Urbilaterian) is indicated as a filled circle, and ancestral Boule likely originated during the evolution of eumetazoans, shown as an open circle. The ancient bilaterian Boule was reproduction-specific, and we hypothesize that it functioned in spermatogenesis, based on a predominance of testis-biased expression in diverse lineages of bilaterian animals and the conservation of a male reproductive function in Drosophila and mouse.
Interestingly, human BOULE has previously been shown to be able to function in Drosophila testes, and can even rescue meiotic defects of boule mutant flies, suggesting a conservation of a spermatogenesis-specific function [35], [44]. However, the C. elegans boule homolog daz-1 is required only in oogenesis [40], making it unclear whether such a transgenic replacement in the fly actually represents a legitimate functional conservation. Furthermore, both C. elegans and Drosophila are protostomes, so whether Boule is even required for reproduction, let alone restricted to spermatogenesis, in any deuterostome species was not known. Using mice as a representative deuterostome, we generated a Boule null allele to address this question (Figure 4). Boule is required only for male reproduction in mice (Figure 5), similar to insect boule, revealing not only a conserved function, but suggesting an ancient requirement of Boule in gametogenesis. Furthermore, the requirement of mouse Boule for male reproduction and its dispensability for female fertility suggests that low level expression of Boule in embryonic germ cells and adult ovaries is not essential for either the development of germ cells or the production of female gametes. Similarly, Drosophila boule, initially thought to be testis-specific, has also been found to be alternatively spliced and expressed in the ovary and even some somatic tissues at a low level, though loss-of-function similarly only causes male sterility [59], [68]. Interestingly, this result shows that Boule has a spermatogenesis-specific requirement conserved in at least two distant lineages of bilateral animals, making it a strong candidate for a conserved male gametogenesis factor between Drosophila and mammals.
Given that mouse Boule is required for sperm production like fly boule but different from C. elegans daz-1, we propose that Urbilaterian Boule had an ancestral function in male gametogenesis which was lost during the evolution of the nematode lineage (Figure 7). This is consistent with the higher sequence divergence of the C. elegans daz-1 RNA binding motif than most other bilaterian Boule homologs (Figure 2). While we can not rule out the possibility that the similar male gametogenic requirement in mice and Drosophila is a coincidence and both evolved independently, the striking similarity in the reproductive defects of loss-of-function mutants of Drosophila and mouse Boule homologs (male specific infertility, global arrest of spermatogenesis, absence of elongating spermatids and mature sperm) argue against such a possibility. Furthermore, the predominance of testis-biased expression of Boule homologs among distinct bilaterian species (Figure 3), supports a model of an ancient male gametogenic function (Figure 7).
It is important to note, however, that this model does not exclude the possibility of an additional ancestral ovarian function of Urbilaterian Boule. Since ovary expression of Boule is also prevalent among diverse animals, and C. elegans daz-1 is required in females, the ancestral Boule gene may have also played a role in oogenesis, which may have been subsequently lost in specific lineages. Our data does not rule out this possibility, and such an ancestral oogenesis function of Boule could be in addition to our proposed ancient spermatogenesis function. Further functional analysis in other lineages, including medaka where strong ovarian Boule expression has been observed, could help determine the more likely scenario [53]. Additionally, characterization of Boule homolog(s) in the sea anemone, an outgroup to the bilaterian lineage, could provide further insights into the ancestral roles of Boule.
Whether or not an ovarian function of Boule is also conserved, our discovery that mammalian Boule is required only for sperm development like its fly counterpart is the first such demonstration of a conserved spermatogenesis-specific function in both lineages. While spermatogenesis occurs in the testes of different animal lineages, it is not known if either spermatogenesis or the testis itself is evolutionarily related between vertebrates and invertebrates. The fly testis, which is a single tube with a linear progression of spermatogenesis, appears different from the mouse testis, which is composed of many seminiferous tubules with a concentric progression of spermatogenesis from the periphery of the seminiferous tubules towards the lumen. However, if we focus on a single cycle of spermatogenesis within a segment of a mouse seminiferous tubule and compare it with a fly testis tubule, we see similar spermatogenic cell types present inside the fly testis tubule and the mouse seminiferous tubule segment [69]. Spermatogenesis in both species starts with spermatogonial stem cells located in a specific position of the tubule, attached to the apical end in fly and to basement membrane in mice, which move and progress into later stages of cell types in one direction, towards the basal end of the testis tubule in fly and towards the lumen in mice. All the major stages of sperm development appear to be present in both species and arranged in a similar spatial and temporal pattern. If both developmental processes evolved from an ancient primitive spermatogenesis prototype, one would predict the presence of at least some common male gametogenesis-specific regulators in both lineages. Yet no such common male gametogenesis factor has been demonstrated to be required exclusively for sperm production in both lineages. The lack of a universal male reproductive factor among all animal lineages, while consistent with rapid evolution of male reproductive genes, is in contrast to the prevalence of sexual reproduction and in particular to the similarity in male gametogenesis among metazoan animals [34], [69]. This paradox led to the question of whether such similarity in the reproductive traits arose from convergent evolution or from conservation of an ancient prototype in the common ancestor.
Furthermore, male reproductive traits and genes undergo rapid adaptive evolution in diverse lineages such as Drosophila, fish, rodents and primates [5], [9]–[12], [16], [17]. Male-biased genes exhibit a higher divergence of expression among closely related species than female-biased genes or genes expressed in both sexes [7], [13]. Additionally, testis-biased genes have the highest rate of extinction and species-specific de novo gene formation during evolution [13], [70], [71]. For example, the most widespread testis-specific proteins among both vertebrates and invertebrates appear to be sperm nuclear basic proteins (SNBP). Many organisms replace histones with a set of small basic structural proteins (SNBP) or protamines to establish a highly compact sperm chromatin structure [72], [73]. Although all metazoan SNBP homologs share their common ancestry with somatic histone H1 protein, the testis-specific SNBPs in different lineages have undergone extensive lineage-specific loss and dynamic evolution, including adaptive evolution [5], [73]. Furthermore it remains unclear if vertebrate and invertebrate protamine homologs are functionally conserved. Loss of one copy of either mouse Protamine-1 or Protamine-2 leads to male sterility, but in contrast, fly sperm carrying a deletion of both protamine-like homologs appears to be functional [74], [75]. Sexual selection has been proposed to be the major force driving this fast divergence of male reproductive traits, gene sequences, and their expression patterns [9], [10], [12]. Given that sexual reproduction is widespread among animals and sperm production appears to be present in all major phyla of metazoan animals, it raised a question whether any male-biased reproductive gene could be exempt from such selective pressure and remain conserved through extended evolutionary distances.
However, Boule homologs have been maintained throughout all major lineages of animals from a common eumetazoan ancestral gene and are required only for sperm development in both Drosophila and mice. We have shown that Boule proteins have resisted sexual selective pressure, and instead evolved under purifying selection. Though ancestral Boule may have also functioned in oogenesis, our findings that bilaterian Boule homologs tend toward male-biased expression, taken together with the similar spermatogenesis arrest phenotypes in both Drosophila and mouse mutants, supports the model of a common origin of bilaterian spermatogenesis.
While it remains to be seen if Boule homologs are restricted only to spermatogenesis or also function in the ovary, we have shown a clear case of conservation of a reproduction-specific gene across Bilateria. We found that among a broad representation of bilaterian animals, Boule expression was restricted to the gonads (Figure 3), indicating that it has remained reproduction-specific throughout evolution. In addition, DNA sequence analysis of multiple Drosophila and mammalian Boule homologs revealed that, unlike other reproductive proteins [11], [16], [17], Boule evolution has been driven not by positive selection, but by purifying selection. This establishes an unambiguous case of a reproduction-specific gene being driven predominantly by purifying selection, in two distinct animal lineages, suggesting a strong functional constraint. Interestingly, our in-depth analysis of the developmental defects in Boule null mice revealed a novel requirement in spermatid differentiation [61]. Such a postmeiotic function for boule is also likely present in Drosophila, though its requirement for spermatid differentiation would not have been revealed in the boule mutant flies due to an earlier block at meiosis [39], [61]. The previously established function of Boule in meiotic progression in both Drosophila and nematodes [39], [40] may also be conserved in mice, despite the lack of a similar meiotic defect in Boule null mice [61]. We proposed that Dazl and Boule may redundantly regulate meiosis, and that Dazl may compensate for Boule loss during meiosis in mice [61]. Yet despite this possibility of a partial redundancy of function with Dazl, mouse Boule has been maintained under purifying selection, further indicating that the presence of other DAZ family genes has had little impact on the functional constraint of Boule. While meiosis is fundamental to sexual reproduction and key components of meiotic machinery for chromosomal synapses and recombination are conserved from yeast to mammals [2], [76], the absence of Boule homologs in fungi together with the requirement of Boule homologs in only one sex of animals suggest that conservation of Boule is unlikely due to the same functional constraint that keeps components of meiotic machinery conserved. Another main functional constraint on metazoan reproduction appears to be associated with germ cell specification and maintenance. Mutations disrupting those conserved germ cell components, such as Vasa or Piwi, often result in a failure to form germ cells or a loss of germ cells before meiotic stages. Furthermore, the resulting infertility sometimes affects both males and females of the same species [21]–[23], [77]–[79]. These phenotypes differ from the sex-biased infertility of Boule mutations in all species examined, and the gametogenesis defects in Boule mutants are much less variable than those from either Vasa or Piwi mutants across species [21]–[23], [61], [78], [79]. Further characterization of the subcellular expression and molecular function of Boule will help to discern the relationship between Boule and these other highly conserved germ cell proteins.
We've shown the widespread presence of Boule homologs throughout bilaterian animals and the functional conservation of a reproductive-exclusive requirement among Drosophila, worm and mouse. This has revealed an ancient reproductive requirement in the Urbilaterian, the common ancestor of all bilaterian animals and highlights a fundamental reproductive function associated with Boule protein conserved over six hundred million years of evolution. With the identification of Boule and possibly more reproductive genes conserved across such large evolutionary distances, we can begin to compare the impact of sexual selection on the molecular evolution of the same components of reproductive traits in different animal lineages at both the microevolution and macroevolution levels.
Materials and Methods
Sequence and phylogeny analysis
For known Boule homologs, DNA sequences from various species were retrieved from the literature and the Genbank database. For species where the presence of Boule or Dazl homologs was unknown, we first searched the EST and cDNA database in Genbank using consensus RRM sequences of either Boule or Dazl using Tblastn and positively identified the homologs using our established criteria. The homolog sequences were further confirmed by the presence of Boule/Dazl homologs with high sequence similarity in other species within the same taxon. In the absence of EST or cDNA information, we then focused on a representative species from the same phylum whose genome had been completely sequenced. The specific genome databases were searched using the consensus Boule RRM sequences and Tblastn, and the top hits were analyzed to determine if they were Boule homologs based on criteria described above. The identified homologs were verified by BLASTing against the human protein database, which should identify human BOULE as the top hit sequence with highest similarity. New Boule and Dazl homologs we have identified as well as known homologs from previous publications are summarized in Table S3. Sequence alignment of RRMs and entire proteins was performed using ClustalW2 and ClustalX programs [80]. The parameters for alignment were protein Gap open penalty = 10, protein extension penalty = 0.2, and other parameters at default settings. Phylogenetic analysis was done using Mega 4.0 [81]. Ka and Ks were calculated as described [44].
Molecular biology
RNA was extracted by Trizol from tissues and reverse transcribed for amplification of Boule cDNA. For tissues collected in RNA later (Applied Biosystems/Ambion, Austin TX), samples were stored at 4°C overnight, solution was removed, and the tissues were stored at −80°C for later RNA extraction. A minimum of two pairs of primers spanning the RRM region and other regions were used to confirm the expression of the Boule gene (Table S4). All the Boule amplicons were confirmed by sequencing. Dmrt1 (Doublesex and mab-3 related transcription factor 1) was a testis-specific positive control in chicken [82] and Bnd (Bindin) was a testis-specific positive control in the sea urchin [83]. We used multiple sets of primers covering exons 2, 3, 4, 5 and 6, and determined that the main transcript is testis-specific (Table S4).
Animals
Mice (Mus musculus) were housed and bred in a barrier facility according to the guidelines approved by the ACUC committee at Northwestern University. Boule mutant mice were created in the mixed background of C57B6 and 129svj. Ripe purple sea urchins (Strongylocentrotus purpuratus) in spawning season (May, 2009) were collected from Pacific Ocean off Carslad, California (M-REP, Carslad, California) and shipped overnight on ice to Chicago. The sex of sea urchins was determined by the presence of eggs (often a milky spill on the outside of female urchins upon arrival) and by the presence of distinct gametes in the gonad tissue biopsies. Gonads, guts and ampullae from at least three male and three female purple sea urchins were collected and either stored in Trizol for immediate RNA extraction or snap-frozen in liquid nitrogen and stored at −80°C. Chicken gonadal and other tissues were collected from euthanized White leghorn chickens at the completion of a research project approved by UIUC animal committee at the University of Illinois at Urbana-Champaign Veterinary School. Tissues from two four-year old roosters and two three-year old hens were snap-frozen in liquid nitrogen or stored directly in RNA later.
Histology
Tissue histology was performed as described previously [35]. Testes were fixed in Bouins' solution overnight and sectioned at 5-µm thickness for hematoxylin/eosin staining. Bright field images were captured using a Leica DM 5000B compound microscope with a DFC320 camera and the Leica image capture suite software.
Generation of a mouse Boule mutation
We replaced exon 3 with lacZ-neo using the NZTK2 vector (Richard Palmiter, University of Washington, Seattle, WA). We designed primers with built-in SalI sites to amplify a 2-kb left arm next to exon 3, and primers with built-in XhoI and NotI sites to amplify a 5.9-kb right arm from 129svj mouse genomic DNA. High fidelity platinum PCR kits (Invitrogen) were used to amplify the fragment with minimal PCR error. The amplified fragments were cloned into Topo vectors and later released with appropriate enzymes for subcloning into the NZTK2 vector. The clones with correct orientation of left and right arm insertions were chosen for sequencing. Sequencing of the genomic arms in the selected clones indicated that both arms had greater than 99% sequence identity with the genomic sequence. Gene targeting was performed on 200 ES cell clones (129svj E14 feeder cell-less ES cells) and four positive ES clones (1D5, 1H5, 2A4 and 2D7) were identified by the presence of both the 2-kb and 6-kb arms using primers outside each arm and on the vector. The 1D5 clone was used to inject blastocysts at the Northwestern University Transgenic Core Facility. Four chimerical mice produced lacZ-positive progeny and mice from two independent founders were used to generate mutant mice for the analysis. The phenotypes were identical among the mutant mice from two independent lines and we did not distinguish our analyses between the two lines.
Supporting Information
Alignments for RNA binding domains (RRM) from insects (A) and mammals (B) reveal strong conservation among homologs. Chicken Boule RRM was added as an outgroup to mammalian domain alignment for comparison. (C) Based on the sequences from mammals and insects, the consensus RRM sequences (RNP1 and RNP 2) for Boule are likely representative of bilaterian Boule. The site of exon/intron junction is marked by the arrows and is also conserved among Boule homologs. The seven Drosophila species used are D. melanogaster, D. sechellia, D. yakuba, D. virilis, D. erecta, D. willistoni and D. ananassae. Mosquito (Aedes aegypti), Honeybee (Apis mellifera), Beetle (Tribolium castaneum), Wasp (Nasonia vitripennis), SMonkey (Squirrel Monkey, Saimiri sciureus), Marmoset (Callithrix jacchus), Tamarin (Saquinus Oedipus), mouse (Mus musculus), Rat (Rattus norvegicus), Lemur (Microcebus murinus), Human (Homo sapiens) Frog (Xenopus laevis), Chimp (Pan troglodytes) PChimp (Pygmy Chimp, Pan paniscus), RMonkey (Rhesus Monkey, Macaca mulatta), Hedgehog (Erinaceus europaeus), Microbat (Myotis lucifugus), GuineaPig (Cavia porcellus), Dog (Canis familiaris), Cow (Bos Taurus), Horse (Equus caballus), Bushbaby (Otolemur garnettii), Playtypus (Ornithorhynchus anatinus). Opossum (Monodelphis domestica), Chicken (Gallus gallus). Color scheme: blue– A,I,L,M,F,W,V; red—R,K; green—N,Q,S,T; pink—C; Magenta—E,D; orange—G; cyan—H,Y; yellow—P [80].
(5.46 MB TIF)
Dazl homologs share similar signature RRM motifs but distinct from that of Boule. (A). RRM sequence alignment of Dazl homologs from diverse vertebrate species. The Dazl RRM is two amino acids shorter than the Boule RRM because of a two amino acid deletion. The consensus sequence for Dazl RRM was generated and is distinct from Boule RRM (lower panel). XL Frog (Xenopus laevis), XT Frog (Xenopus tropicalis), Cow (Bos taurus), Human (Homo sapiens), PP Chimpanzee (Pan paniscus), PT Chimpanzee (Pan troglodytes), SS Monkey (squirrel monkey, Saimiri sciureus), RhesusMonkey (Macaca mulatta), Rat (Rattus norvegicus), mouse (Mus musculus), chick (Gallus gallus), DR Zebrafish (Danio rerio), OL Killifish (Oryzias latipes), MM Lemur (Microcebus murinus), SO tamarin (Saguinus Oedipus), LS Seabass (Lates calcarifer), RP Frog (Rana pipiens), CP Newt (Cynops pyrrhogaster), CF Dog (Canis familiaris), EE Hedgehog (Erinaceus europaeus), Opossum (Monodelphis domestica), Platypus (Ornithorhynchus Anatinus), Bushbaby (Otolemur garnettii), SA Shrew (Sorex araneus), TB Shrew (Tupaia Belangeri), FuguFish (Takifugu rubripes). (B). Alignment of Boule and Dazl RRM consensus sequences.
(5.91 MB TIF)
Absence in yeast and Trichoplax but presence in sea anemone of Boule homologs. (A) The yeast protein with highest similarity to the Boule consensus sequence is Hrp1, but it is not a Boule homolog. Sequence alignment of Boule and Hrp1 is shown. The Hrp1 RRM does not contain the characteristic amino acids of the Boule RRM. When the yeast Hrp1 sequence is compared to that of the fly or human genome, the fly gene with the greatest similarity is not boule but hRNP. Although yeast Hrp1 has a sequence similarity to Boule in the RRM and probably represents the closest RRM protein to Boule, Hrp1 is unlikely to be a Boule ortholog. Boule homologs are also absent in other single-cell eukaryotes and in plants, including Schizosaccharomyces pombe, Dictyostelium discoideum and Arabidopsis thaliana. We thus concluded that Boule homologs are restricted to animals. (B) In Trichoplax, the protein with highest sequence similarity to the Boule consensus sequence does not contain the key signature amino acids of Boule and is not a Boule homolog. Furthermore, the RNA binding domains of these proteins do not contain any introns, whereas Boule genes contain a conserved genomic structure with at least two introns separating the RRM-encoding exons at conserved junctions. Hence, a Boule homolog appears to be absent in the Trichoplax [86]. (C) There are two sea anemone proteins with high sequence similarity to the Boule consensus sequence. The sea anemone (Sa) Boule1 shows greater similarity and also shares the three internal exon-intron junctions as the consensus Boule, whereas sea anemone Boule2 only shares two exon-intron junctions. For phylogenetic tree construction (Figure. 1), sea anemone Boule1 was used as the Boule homolog sequence. The arrows mark the sites of exon-intron junctions based on genomic sequences. RNP2 and RNP1 are underlined.
(1.84 MB TIF)
Real-time PCR analysis of chicken gene expression. Dmrt1 is testis-specific control in the chicken. In the chicken, Boule is highly enriched in the testis, and Dazl is 10-fold more abundant in the testis than the ovary. Dmrt1 RNA was not detectable in muscle, kidney or ovary, while Dazl RNA was not detectable in muscle or kidney.
(0.53 MB TIF)
Ka/Ks ratio analysis revealed no evidence for accelerated evolution among Drosophila boule homologs. We performed pair-wise comparison of Ka and Ks for the entire boule coding sequences among seven Drosophila species (Dmel-D. melanogaster, Dsec-D. sechellia, Dsec-D. yakuba, Dvir-D. virilis, Dere-D. erecta, Dwil-D. willistoni, and Dana-D. ananassae). All Ka/Ks ratios were significantly below 1.
(0.05 MB DOC)
Ka/Ks ratio analysis also failed to identify any evidence for accelerated evolution among mammalian Boule homologs. Molecular evolutionary analysis of the entire coding sequence in the eight representative mammalian species: Monotremes (platypus), Marsupials (opossum) and Eutherians (mouse, rat, dog, rhesus monkey, chimpanzee and human), revealed no excessive non-synonymous nucleotide changes in comparison with synonymous changes. A total of 477 nucleotides were used from each homolog. Ka/Ks ratios were all below 1, with the highest value less than 0.1. These data suggest that not only was the RRM conserved through mammalian evolution, but the entire Boule gene, including less conserved regions, did not undergo rapid evolution.
(0.08 MB DOC)
Summary table listing information for all genes used in the analysis. Homologs of Boule, Dazl and DAZ used in this analysis are listed with species names and their sequence ID from genbank other databases.
(0.09 MB DOC)
List of primers used for RT-PCR and qRT-PCR analyses of Chicken, Sea urchin and mouse Boule homologs.
(0.06 MB DOC)
Supplemental data.
(0.04 MB DOC)
Acknowledgments
EYX is indebted to Renee Reijo Pera for her generous support in the initial phase of this project and comments on the manuscript. We are grateful to Janice Bahr and Arthur Veis for their assistance in collection of chicken and sea urchin tissues. We thank Debu Chakravarti, Erwin Goldberg, Bob Holmgren, Manyuan Long, David Page, and Lily Xu for discussion and comments and Rhoda Chang, Vinh Nguyen, Takeshi Kurita, Y. M. Kuo, and Miranda Bernhardt for technical assistance.
Footnotes
The authors have declared that no competing interests exist.
This study is supported by NIH NICHD HD045871 and Northwestern Memorial Hospital EAM grants (EYX). MJWV is supported by Cellular and Molecular Basis of Disease (CMBD) training grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ramesh MA, Malik SB, Logsdon JM., Jr A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr Biol. 2005;15:185–191. doi: 10.1016/j.cub.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Villeneuve AM, Hillers KJ. Whence meiosis? Cell. 2001;106:647–650. doi: 10.1016/s0092-8674(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth B. The evolution of sex chromosome. Science. 1991;251:1030–1033. doi: 10.1126/science.1998119. [DOI] [PubMed] [Google Scholar]
- 4.Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. 2007;8:689–698. doi: 10.1038/nrg2167. [DOI] [PubMed] [Google Scholar]
- 5.Wyckoff G, Wang W, Wu C-I. Rapid evolution of reproductive genes in the descent of man. Nature. 2000;403:304–309. doi: 10.1038/35002070. [DOI] [PubMed] [Google Scholar]
- 6.Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–1233. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- 7.Meiklejohn CD, Parsch J, Ranz JM, Hartl DL. Rapid evolution of male-biased gene expression in Drosophila. Proc Natl Acad Sci U S A. 2003;100:9894–9899. doi: 10.1073/pnas.1630690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin I, Baker BS. The evolutionary dynamics of sex determination. Science. 1998;281:1990–1994. doi: 10.1126/science.281.5385.1990. [DOI] [PubMed] [Google Scholar]
- 9.Clark NL, Aagaard JE, Swanson WJ. Evolution of reproductive proteins from animals and plants. Reproduction. 2006;131:11–22. doi: 10.1530/rep.1.00357. [DOI] [PubMed] [Google Scholar]
- 10.Haerty W, Jagadeeshan S, Kulathinal RJ, Wong A, Ravi Ram K, et al. Evolution in the Fast Lane: Rapidly Evolving Sex-Related Genes in Drosophila. Genetics. 2007;177:1321–1335. doi: 10.1534/genetics.107.078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurminsky DI, Nurminskaya MV, De Aguiar D, Hartl DL. Selective sweep of a newly evolved sperm-specific gene in Drosophila [see comments]. Nature. 1998;396:572–575. doi: 10.1038/25126. [DOI] [PubMed] [Google Scholar]
- 12.Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Sturgill D, Parisi M, Kumar S, Oliver B. Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature. 2007;450:233–237. doi: 10.1038/nature06323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Good JM, Nachman MW. Rates of protein evolution are positively correlated with developmental timing of expression during mouse spermatogenesis. Mol Biol Evol. 2005;22:1044–1052. doi: 10.1093/molbev/msi087. [DOI] [PubMed] [Google Scholar]
- 15.Oliver PL, Goodstadt L, Bayes JJ, Birtle Z, Roach KC, et al. Accelerated evolution of the Prdm9 speciation gene across diverse metazoan taxa. PLoS Genet. 2009;5:e1000753. doi: 10.1371/journal.pgen.1000753. doi: 10.1371/journal.pgen.1000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalamegham R, Sturgill D, Siegfried E, Oliver B. Drosophila mojoless, a retroposed GSK-3, has functionally diverged to acquire an essential role in male fertility. Mol Biol Evol. 2007;24:732–742. doi: 10.1093/molbev/msl201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loppin B, Lepetit D, Dorus S, Couble P, Karr TL. Origin and neofunctionalization of a Drosophila paternal effect gene essential for zygote viability. Curr Biol. 2005;15:87–93. doi: 10.1016/j.cub.2004.12.071. [DOI] [PubMed] [Google Scholar]
- 18.Dorus S, Busby SA, Gerike U, Shabanowitz J, Hunt DF, et al. Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nat Genet. 2006;38:1440–1445. doi: 10.1038/ng1915. [DOI] [PubMed] [Google Scholar]
- 19.Chu DS, Liu H, Nix P, Wu TF, Ralston EJ, et al. Sperm chromatin proteomics identifies evolutionarily conserved fertility factors. Nature. 2006;443:101. doi: 10.1038/nature05050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarkower D. Establishing sexual dimorphism: conservation amidst diversity? Nat Rev Genet. 2001;2:175. doi: 10.1038/35056032. [DOI] [PubMed] [Google Scholar]
- 21.Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–747. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 22.Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, et al. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000;14:841–853. [PMC free article] [PubMed] [Google Scholar]
- 24.Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- 25.Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 2008;135:311–321. doi: 10.1242/dev.015156. [DOI] [PubMed] [Google Scholar]
- 26.Cinalli RM, Rangan P, Lehmann R. Germ cells are forever. Cell. 2008;132:559–562. doi: 10.1016/j.cell.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Extavour CGM. Evolution of the bilaterian germ line: lineage origin and modulation of specification mechanisms. Integrative & Comparative Biology. 2007:icm027. doi: 10.1093/icb/icm027. [DOI] [PubMed] [Google Scholar]
- 28.Pepling ME, de Cuevas M, Spradling AC. Germline cysts: a conserved phase of germ cell development? Trends Cell Biol. 1999;9:257–262. doi: 10.1016/s0962-8924(99)01594-9. [DOI] [PubMed] [Google Scholar]
- 29.Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–393. doi: 10.1126/science.1140846. [DOI] [PubMed] [Google Scholar]
- 30.Fuller MT. Spermatogenesis. In: Bate M, Martinez-Arias A, editors. Development of Drosophila melanogaster. Cold Spring Harbor: 1993. pp. 71–147. [Google Scholar]
- 31.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41–49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 32.White-Cooper H, Doggett K, Ellis R. Evolution of Spermatogenesis. In: Pitnick HaB., editor. Sperm Evolution: An evolutionary perspective. Academic Press; 2008. 151- [Google Scholar]
- 33.Kimble J, Page DC. The mysteries of sexual identity. The germ cell's perspective. Science. 2007;316:400–401. doi: 10.1126/science.1142109. [DOI] [PubMed] [Google Scholar]
- 34.Roosen-Runge EC. Comparative aspects of spermatogenesis. Biol Reprod. 1969;1:(Suppl 1):24–31. doi: 10.1095/biolreprod1.supplement_1.24. [DOI] [PubMed] [Google Scholar]
- 35.Xu EY, Moore FL, Pera RA. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7414–7419. doi: 10.1073/pnas.131090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen PH. Putative biological functions of the DAZ family. Int J Androl. 2004;27:125–129. doi: 10.1111/j.1365-2605.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 37.Reijo R, Alagappan RK, Patrizio P, Page DC. Severe oligozoospermia resulting from deletions of azoospermia factor gene. Lancet. 1996;347:1290–1293. doi: 10.1016/s0140-6736(96)90938-1. [DOI] [PubMed] [Google Scholar]
- 38.Reijo R, Lee TY, Salo P, Alagappan R, Brown LG. Diverse spermatogenic defects in humans caused by Y chromosome deletions. Nature Genetics. 1995;10:383–393. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- 39.Eberhart CG, Maines JZ, Wasserman SA. Meiotic cell cycle requirement for a fly homologue of human Deleted in Azoospermia. Nature. 1996;381:783–785. doi: 10.1038/381783a0. [DOI] [PubMed] [Google Scholar]
- 40.Karashima T, Sugimoto A, Yamamoto M. Caenorhabditis elegans homologue of the human azoospermia factor DAZ is required for oogenesis but not for spermatogenesis. Development. 2000;127:1069–1079. doi: 10.1242/dev.127.5.1069. [DOI] [PubMed] [Google Scholar]
- 41.Ruggiu M, Speed R, Taggart M, McKay SJ, Kilanowski F, et al. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 42.Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Z, Ji P, Cao J, Zhu S, Li Y, et al. Dazl promotes germ cell differentiation from embryonic stem cells. J Mol Cell Biol. 2009;1:93–103. doi: 10.1093/jmcb/mjp026. [DOI] [PubMed] [Google Scholar]
- 44.Xu EY, Lee DF, Klebes A, Turek PJ, Kornberg TB, et al. Human BOULE gene rescues meiotic defects in infertile flies. Human Molecular Genetics. 2003;12 doi: 10.1093/hmg/ddg017. [DOI] [PubMed] [Google Scholar]
- 45.Otori M, Karashima T, Yamamoto M. The Caenorhabditis elegans homologue of deleted in azoospermia is involved in the sperm/oocyte switch. Mol Biol Cell. 2006;17:3147–3155. doi: 10.1091/mbc.E05-11-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houston DW, Zhang J, Maines JZ, Wasserman SA, King ML. A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development. 1998;125:171–180. doi: 10.1242/dev.125.2.171. [DOI] [PubMed] [Google Scholar]
- 47.Houston DW, King ML. A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development. 2000;127:447–456. doi: 10.1242/dev.127.3.447. [DOI] [PubMed] [Google Scholar]
- 48.Bandziulis RJ, Swanson MS, Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989;3:431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- 49.Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, et al. Sea Anemone Genome Reveals Ancestral Eumetazoan Gene Repertoire and Genomic Organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 50.Bachvarova RF, Crother BI, Manova K, Chatfield J, Shoemaker CM, et al. Expression of Dazl and Vasa in turtle embryos and ovaries: evidence for inductive specification of germ cells. Evol Dev. 2009;11:525–534. doi: 10.1111/j.1525-142X.2009.00360.x. [DOI] [PubMed] [Google Scholar]
- 51.van de Lavoir MC, Diamond JH, Leighton PA, Mather-Love C, Heyer BS, et al. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–769. doi: 10.1038/nature04831. [DOI] [PubMed] [Google Scholar]
- 52.Maegawa S, Yasuda K, Inoue K. Maternal mRNA localization of zebrafish DAZ-like gene. Mech Dev. 1999;81:223–226. doi: 10.1016/s0925-4773(98)00242-1. [DOI] [PubMed] [Google Scholar]
- 53.Xu H, Li Z, Li M, Wang L, Hong Y. Boule is present in fish and bisexually expressed in adult and embryonic germ cells of medaka. PLoS One. 2009;4:e6097. doi: 10.1371/journal.pone.0006097. doi: 10.1371/journal.pone.0006097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venkatesh B, Kirkness EF, Loh YH, Halpern AL, Lee AP, et al. Survey sequencing and comparative analysis of the elephant shark (Callorhinchus milii) genome. PLoS Biol. 2007;5:e101. doi: 10.1371/journal.pbio.0050101. doi: 10.1371/journal.pbio.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saxena R, Brown LG, Hawkins T, Alagappan RK, Skaletsky H, et al. The DAZ gene cluster on the human Y chromosome arose from an autosomal gene that was transposed, repeatedly amplified and pruned. Nature Genetics. 1996;14:292–299. doi: 10.1038/ng1196-292. [DOI] [PubMed] [Google Scholar]
- 56.Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- 57.Juliano CE, Voronina E, Stack C, Aldrich M, Cameron AR, et al. Germ line determinants are not localized early in sea urchin development, but do accumulate in the small micromere lineage. Dev Biol. 2006;300:406–415. doi: 10.1016/j.ydbio.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 58.Dorfman DM, Genest DR, Reijo Pera RA. Human DAZL1 encodes a candidate fertility factor in women that localizes to the prenatal and postnatal germ cells. Human Reproduction. 1999;14:2531–2536. doi: 10.1093/humrep/14.10.2531. [DOI] [PubMed] [Google Scholar]
- 59.Hoopfer ED, Penton A, Watts RJ, Luo L. Genomic analysis of Drosophila neuronal remodeling: a role for the RNA-binding protein Boule as a negative regulator of axon pruning. J Neurosci. 2008;28:6092–6103. doi: 10.1523/JNEUROSCI.0677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q, Li J, Li Q, Li X, Liu Z, et al. Cloning and characterization of the gene encoding the bovine BOULE protein. Mol Genet Genomics. 2009;281:67–75. doi: 10.1007/s00438-008-0394-6. [DOI] [PubMed] [Google Scholar]
- 61.Vangompel MJ, Xu EY. A novel requirement in mammalian spermatid differentiation for the DAZ-family protein Boule. Hum Mol Genet. 2010;19:2360–2369. doi: 10.1093/hmg/ddq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brill JA, Hime GR, Scharer-Schuksz M, Fuller MT. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development. 2000;127:3855–3864. doi: 10.1242/dev.127.17.3855. [DOI] [PubMed] [Google Scholar]
- 63.Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophys Biochem Cytol. 1959;5:453–460. doi: 10.1083/jcb.5.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenbaum MP, Ma L, Matzuk MM. Conversion of midbodies into germ cell intercellular bridges. Dev Biol. 2007;305:389–396. doi: 10.1016/j.ydbio.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. Journal of Cell Science. 1996;109:2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- 66.Hedges SB. The origin and evolution of model organisms. Nat Rev Genet. 2002;3:838–849. doi: 10.1038/nrg929. [DOI] [PubMed] [Google Scholar]
- 67.Knoll AH, Carroll SB. Early Animal Evolution: Emerging Views from Comparative Biology and Geology. Science. 1999;284:2129–2137. doi: 10.1126/science.284.5423.2129. [DOI] [PubMed] [Google Scholar]
- 68.Joiner M-LA, Wu C-F. Nervous system function for the testis RNA-binding protein Boule in Drosophila. Journal of Neurogenetics. 2004;18:341–363. doi: 10.1080/01677060490477435. [DOI] [PubMed] [Google Scholar]
- 69.Fuller MT. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Seminars in Cell and Developmental Biology. 1998;9:433–444. doi: 10.1006/scdb.1998.0227. [DOI] [PubMed] [Google Scholar]
- 70.Levine MT, Jones CD, Kern AD, Lindfors HA, Begun DJ. Novel genes derived from noncoding DNA in Drosophila melanogaster are frequently X-linked and exhibit testis-biased expression. Proc Natl Acad Sci U S A. 2006;103:9935–9939. doi: 10.1073/pnas.0509809103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vibranovski MD, Lopes HF, Karr TL, Long M. Stage-specific expression profiling of Drosophila spermatogenesis suggests that meiotic sex chromosome inactivation drives genomic relocation of testis-expressed genes. PLoS Genet. 2009;5:e1000731. doi: 10.1371/journal.pgen.1000731. doi: 10.1371/journal.pgen.1000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braun RE. Packaging paternal chromosomes with protamine. Nat Genet. 2001;28:10–12. doi: 10.1038/ng0501-10. [DOI] [PubMed] [Google Scholar]
- 73.Eirin-Lopez JM, Ausio J. Origin and evolution of chromosomal sperm proteins. Bioessays. 2009;31:1062–1070. doi: 10.1002/bies.200900050. [DOI] [PubMed] [Google Scholar]
- 74.Cho C, Willis WD, Goulding EH, Jung-Ha H, Choi YC, et al. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat Genet. 2001;28:82–86. doi: 10.1038/ng0501-82. [DOI] [PubMed] [Google Scholar]
- 75.Rathke C, Baarends WM, Jayaramaiah-Raja S, Bartkuhn M, Renkawitz R, et al. Transition from a nucleosome-based to a protamine-based chromatin configuration during spermiogenesis in Drosophila. J Cell Sci. 2007;120:1689–1700. doi: 10.1242/jcs.004663. [DOI] [PubMed] [Google Scholar]
- 76.Keeney S. Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol. 2001;52:1–53. doi: 10.1016/s0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- 77.Hay B, Jan LY, Jan YN. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 1990;109:425–433. doi: 10.1242/dev.109.2.425. [DOI] [PubMed] [Google Scholar]
- 78.Lasko PaMA. Posterior localization of vsa protein correlates with, but is not sufficient for, pole cell development. Genes and Development. 1990;4:905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- 79.Lin H, Spradling AC. A novel goup of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development. 1997;124:2463–2476. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- 80.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 81.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 82.Shan Z, Nanda I, Wang Y, Schmid M, Vortkamp A, et al. Sex-specific expression of an evolutionarily conserved male regulatory gene, DMRT1, in birds. Cytogenet Cell Genet. 2000;89:252–257. doi: 10.1159/000015626. [DOI] [PubMed] [Google Scholar]
- 83.Cameron RA, Minor JE, Nishioka D, Britten RJ, Davidson EH. Locale and level of bindin mRNA in maturing testis of the sea urchin, Strongylocentrotus purpuratus. Dev Biol. 1990;142:44–49. doi: 10.1016/0012-1606(90)90149-d. [DOI] [PubMed] [Google Scholar]
- 84.Roosen-Rung EC. The process of spermatogenesis in animals. Cambridge and New York: Cambridge University Press; 1977. [Google Scholar]
- 85.Futuyma DJ. Evolutionary Biology. Sunderland, Massachusetts: Sinauer Associates, Inc; 1986. [Google Scholar]
- 86.Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, et al. The Trichoplax genome and the nature of placozoans. Nature. 2008;454:955–960. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignments for RNA binding domains (RRM) from insects (A) and mammals (B) reveal strong conservation among homologs. Chicken Boule RRM was added as an outgroup to mammalian domain alignment for comparison. (C) Based on the sequences from mammals and insects, the consensus RRM sequences (RNP1 and RNP 2) for Boule are likely representative of bilaterian Boule. The site of exon/intron junction is marked by the arrows and is also conserved among Boule homologs. The seven Drosophila species used are D. melanogaster, D. sechellia, D. yakuba, D. virilis, D. erecta, D. willistoni and D. ananassae. Mosquito (Aedes aegypti), Honeybee (Apis mellifera), Beetle (Tribolium castaneum), Wasp (Nasonia vitripennis), SMonkey (Squirrel Monkey, Saimiri sciureus), Marmoset (Callithrix jacchus), Tamarin (Saquinus Oedipus), mouse (Mus musculus), Rat (Rattus norvegicus), Lemur (Microcebus murinus), Human (Homo sapiens) Frog (Xenopus laevis), Chimp (Pan troglodytes) PChimp (Pygmy Chimp, Pan paniscus), RMonkey (Rhesus Monkey, Macaca mulatta), Hedgehog (Erinaceus europaeus), Microbat (Myotis lucifugus), GuineaPig (Cavia porcellus), Dog (Canis familiaris), Cow (Bos Taurus), Horse (Equus caballus), Bushbaby (Otolemur garnettii), Playtypus (Ornithorhynchus anatinus). Opossum (Monodelphis domestica), Chicken (Gallus gallus). Color scheme: blue– A,I,L,M,F,W,V; red—R,K; green—N,Q,S,T; pink—C; Magenta—E,D; orange—G; cyan—H,Y; yellow—P [80].
(5.46 MB TIF)
Dazl homologs share similar signature RRM motifs but distinct from that of Boule. (A). RRM sequence alignment of Dazl homologs from diverse vertebrate species. The Dazl RRM is two amino acids shorter than the Boule RRM because of a two amino acid deletion. The consensus sequence for Dazl RRM was generated and is distinct from Boule RRM (lower panel). XL Frog (Xenopus laevis), XT Frog (Xenopus tropicalis), Cow (Bos taurus), Human (Homo sapiens), PP Chimpanzee (Pan paniscus), PT Chimpanzee (Pan troglodytes), SS Monkey (squirrel monkey, Saimiri sciureus), RhesusMonkey (Macaca mulatta), Rat (Rattus norvegicus), mouse (Mus musculus), chick (Gallus gallus), DR Zebrafish (Danio rerio), OL Killifish (Oryzias latipes), MM Lemur (Microcebus murinus), SO tamarin (Saguinus Oedipus), LS Seabass (Lates calcarifer), RP Frog (Rana pipiens), CP Newt (Cynops pyrrhogaster), CF Dog (Canis familiaris), EE Hedgehog (Erinaceus europaeus), Opossum (Monodelphis domestica), Platypus (Ornithorhynchus Anatinus), Bushbaby (Otolemur garnettii), SA Shrew (Sorex araneus), TB Shrew (Tupaia Belangeri), FuguFish (Takifugu rubripes). (B). Alignment of Boule and Dazl RRM consensus sequences.
(5.91 MB TIF)
Absence in yeast and Trichoplax but presence in sea anemone of Boule homologs. (A) The yeast protein with highest similarity to the Boule consensus sequence is Hrp1, but it is not a Boule homolog. Sequence alignment of Boule and Hrp1 is shown. The Hrp1 RRM does not contain the characteristic amino acids of the Boule RRM. When the yeast Hrp1 sequence is compared to that of the fly or human genome, the fly gene with the greatest similarity is not boule but hRNP. Although yeast Hrp1 has a sequence similarity to Boule in the RRM and probably represents the closest RRM protein to Boule, Hrp1 is unlikely to be a Boule ortholog. Boule homologs are also absent in other single-cell eukaryotes and in plants, including Schizosaccharomyces pombe, Dictyostelium discoideum and Arabidopsis thaliana. We thus concluded that Boule homologs are restricted to animals. (B) In Trichoplax, the protein with highest sequence similarity to the Boule consensus sequence does not contain the key signature amino acids of Boule and is not a Boule homolog. Furthermore, the RNA binding domains of these proteins do not contain any introns, whereas Boule genes contain a conserved genomic structure with at least two introns separating the RRM-encoding exons at conserved junctions. Hence, a Boule homolog appears to be absent in the Trichoplax [86]. (C) There are two sea anemone proteins with high sequence similarity to the Boule consensus sequence. The sea anemone (Sa) Boule1 shows greater similarity and also shares the three internal exon-intron junctions as the consensus Boule, whereas sea anemone Boule2 only shares two exon-intron junctions. For phylogenetic tree construction (Figure. 1), sea anemone Boule1 was used as the Boule homolog sequence. The arrows mark the sites of exon-intron junctions based on genomic sequences. RNP2 and RNP1 are underlined.
(1.84 MB TIF)
Real-time PCR analysis of chicken gene expression. Dmrt1 is testis-specific control in the chicken. In the chicken, Boule is highly enriched in the testis, and Dazl is 10-fold more abundant in the testis than the ovary. Dmrt1 RNA was not detectable in muscle, kidney or ovary, while Dazl RNA was not detectable in muscle or kidney.
(0.53 MB TIF)
Ka/Ks ratio analysis revealed no evidence for accelerated evolution among Drosophila boule homologs. We performed pair-wise comparison of Ka and Ks for the entire boule coding sequences among seven Drosophila species (Dmel-D. melanogaster, Dsec-D. sechellia, Dsec-D. yakuba, Dvir-D. virilis, Dere-D. erecta, Dwil-D. willistoni, and Dana-D. ananassae). All Ka/Ks ratios were significantly below 1.
(0.05 MB DOC)
Ka/Ks ratio analysis also failed to identify any evidence for accelerated evolution among mammalian Boule homologs. Molecular evolutionary analysis of the entire coding sequence in the eight representative mammalian species: Monotremes (platypus), Marsupials (opossum) and Eutherians (mouse, rat, dog, rhesus monkey, chimpanzee and human), revealed no excessive non-synonymous nucleotide changes in comparison with synonymous changes. A total of 477 nucleotides were used from each homolog. Ka/Ks ratios were all below 1, with the highest value less than 0.1. These data suggest that not only was the RRM conserved through mammalian evolution, but the entire Boule gene, including less conserved regions, did not undergo rapid evolution.
(0.08 MB DOC)
Summary table listing information for all genes used in the analysis. Homologs of Boule, Dazl and DAZ used in this analysis are listed with species names and their sequence ID from genbank other databases.
(0.09 MB DOC)
List of primers used for RT-PCR and qRT-PCR analyses of Chicken, Sea urchin and mouse Boule homologs.
(0.06 MB DOC)
Supplemental data.
(0.04 MB DOC)