Abstract
Small vessel vasculitides such as microscopic polyangiitis and Wegener’s granulomatosis commonly involve the kidney and lung, with alveolar haemorrhage being the commonest manifestation of pulmonary involvement. Here we describe a patient who developed acute renal failure and pulmonary haemorrhage with positive autoantibodies against myeloperoxidase 1 year after a diagnosis of usual interstitial pneumonia had been made and we discuss the uncommon association of pulmonary fibrosis and anti-myeloperoxidase positive vasculitis.
Keywords: anti-neutrophil cytoplasmic antibodies, microscopic polyangiitis, myeloperoxidase, pulmonary fibrosis
Introduction
Small vessel vasculitides are multisystem disorders in which the commonest pulmonary manifestation is alveolar haemorrhage. Pulmonary fibrosis (PF) has been less frequently observed and may precede other disease features. We describe a patient who was diagnosed with PF 1 year prior to presentation with anti-myeloperoxidase positive vasculitis.
Case history
A 69-year-old woman was admitted to the hospital in February 2009 with 3 days’ history of left-sided pleuritic chest pain, productive cough (without haemoptysis) and dyspnoea at rest. She was a lifelong smoker but had no previously diagnosed chest disease; her only past medical history was of hypercholesterolaemia. She had previously kept canaries and budgerigars but had not done so for over 20 years, and she had no history of asbestos exposure. Her regular medications consisted of aspirin and simvastatin. On examination, she was apyrexial but hypotensive (80/55 mmHg) and hypoxic with an arterial pO2 of 8.99 kPa on 28% inspired oxygen. Chest auscultation revealed crepitations and bronchial breathing at the left lung base, and urine dipstick showed 4+ blood and 2+ protein. Her neutrophil count was elevated at 19.7 × 109/L and C-reactive protein 176 mg/L (reference range 0–10). She had a normal serum creatinine of 89 µmol/L. Urine microscopy showed leucocytes and erythrocytes but no casts, and culture was negative. Chest radiograph revealed left lower lobe consolidation together with reticular shadowing in the periphery of the right zone and nodules in the left upper zone; a previous study in October 2007 had been normal. Based on these findings, a diagnosis of bacterial pneumonia was made and she was commenced on antibiotics with good clinical response.
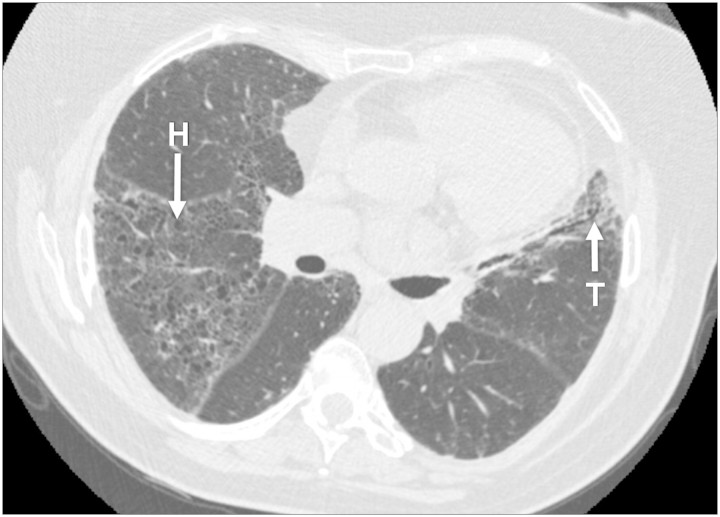
On review 1 month later, her cough and chest discomfort had resolved. However, she had ongoing exertional dyspnoea, for example, when carrying out housework. Chest auscultation revealed crackles in both axillae. A repeat chest radiograph showed resolution of her left basal consolidation but the reticulonodular changes were still present. Spirometry revealed a normal FEV1/FVC ratio of 79% but reduced total lung capacity at 80% of predicted. Anti-nuclear antibodies and avian precipitins were negative. Her urine albumin concentration was high at >300 mg/L but with a normal serum creatinine of 92 µmol/L. In view of the clinical suspicion of pulmonary fibrotic disease, a high-resolution chest computed tomography scan was performed (Figure 1). This showed diffuse fibrotic change with honeycombing in all lobes together with subpleural ground glass shadowing and traction bronchiectasis in the lingula. A diagnosis of usual interstitial pneumonia (UIP) was made. However, as her symptoms were stable and relatively mild, it was not felt that treatment was indicated.
Fig. 1.
High-resolution computed tomography scan demonstrating honeycomb fibrosis (H) and traction bronchiectasis (T).
She remained well until January 2010 when she presented with a 1-month history of anorexia, weight loss, lethargy and exertional dyspnoea together with haemoptysis for 2 weeks. She was apyrexial and clinically euvolaemic with a blood pressure of 138/92 mmHg and oxygen saturations of 98% on air. Urine dipstick showed 3+ blood and 2+ protein. She had acute renal failure with a serum creatinine of 630 µmol/L and haemoglobin 10.9 g/dL. Blood and urine cultures were negative. Her perinuclear anti-neutrophil cytoplasmic antibodies (ANCA) were strongly positive with an anti-myeloperoxidase (MPO) antibody level of 403 U/L (reference range <6); anti-proteinase 3 and anti-glomerular basement membrane antibodies were not detected. There was new right lower zone shadowing on her chest radiograph. Percutaneous renal biopsy showed a pauci-immune necrotizing glomerulonephritis with crescent formation. A diagnosis of acute renal failure and pulmonary haemorrhage secondary to microscopic polyangiitis (MPA) was made and she was treated with prednisolone, cyclophosphamide and plasma exchange. She had no further haemoptysis and a repeat chest radiograph a few days later showed resolution of the right basal shadowing. Her serum creatinine fell to around 200 µmol/L. We wondered whether her presentation with PF the previous year may have been an early manifestation of MPA, particularly given the urinary abnormalities documented at the time. We therefore obtained a stored serum sample from March 2009; however, this was negative on testing for anti-MPO antibodies.
Discussion
UIP is a term used to describe the condition previously known as cryptogenic fibrosing alveolitis or idiopathic PF. Patients commonly present with progressive dyspnoea and a non-productive cough, and pulmonary function tests show a restrictive pattern with reduced lung volumes but a normal FEV1/FVC ratio. Plain chest radiographs usually demonstrate interstitial opacities and reduced lung volumes, with high-resolution computed tomography features characteristically including reticulonodular opacities, honeycomb changes and traction bronchiectasis.
MPA is a form of systemic vasculitis defined according to the Chapel Hill classification [1] as a necrotizing vasculitis (with few or no immune deposits) affecting small vessels such as capillaries, venules or arterioles. This condition is usually associated with the presence of ANCA, most commonly with a perinuclear pattern on immunofluorescence and specificity against MPO. Renal involvement usually results in rapidly progressive renal impairment with a pauci-immune necrotizing crescentic glomerulonephritis on renal biopsy. Lung involvement is also frequent, pulmonary haemorrhage being the usual manifestation; PF is a rarely reported finding. In one Japanese study of patients presenting with glomerulonephritis in association with anti-MPO antibodies, 27 out of 40 had evidence of non-infective interstitial pneumonitis, with coexisting pulmonary haemorrhage in 7 [2]. However, the occurrence of PF is much lower in most other reported series. Table 1 summarizes the cases we identified in the literature describing patients with both PF and anti-MPO positive systemic vasculitis. Of the 58 cases where sufficient data were available, PF had been diagnosed before the development of vasculitis in 22, vasculitis preceded the identification of PF in 1 and both conditions were identified simultaneously in 35. However, it is possible that, in patients in whom both conditions were diagnosed at the same time, PF may have been present beforehand but not clinically apparent. Similarly, patients presenting initially with PF may have had evidence of vasculitis that was not identified until later (possibly including our case).
Table 1.
Summary of cases in the literature of PF in association with anti-MPO positive vasculitis
| Reference | No. of cases | Initial presentation (PF, pulmonary fibrosis; V, vasculitis; S, simultaneous) | Positive anti-MPO preceding vasculitis (where PF occurred first) |
|---|---|---|---|
| [7] | 2 | PF, 2 | Unknown |
| [2] | 27 | Unknown | |
| [8] | 3 | Unknown | |
| [9] | 1 | PF | Unknown |
| [10] | 1 | S | |
| [11] | 4 | PF, 4 | Unknown |
| [12] | 1 | PF | Unknown |
| [13] | 1 | PF | Unknown |
| [14] | 6 | PF, 2; S, 4 | Unknown |
| [15] | 2 | S | |
| [16] | 1 | PF | Yes |
| [17] | 8 | Unknown | |
| [18] | 19 | S, 19 | |
| [19] | 15 | Unknown | |
| [4] | 1 | PF | Unknown |
| [20] | 1 | PF | Unknown |
| [21] | 1 | S | |
| [3] | 5 | PF, 4; S, 1 | Yes, 2; unknown, 2 |
| [22] | 1 | PF | Unknown |
| [23] | 12 | PF, 3; V, 1; S, 8 | Unknown |
| This case | 1 | PF | No |
In the 22 patients where PF was identified first, anti-MPO antibodies were detected prior to the diagnosis of vasculitis in 3; in the remaining 19, they were identified when the patients presented with vasculitis but had not been measured before this. Our patient was anti-MPO negative when the diagnosis of PF was made but became antibody positive when she developed acute renal failure and pulmonary haemorrhage secondary to MPA, perhaps suggesting two unrelated pathologies. Interestingly however, in the series reported by Foulon et al. [3], one patient who presented simultaneously with vasculitis and PF was anti-MPO negative at the time, and autoantibodies were only detected 26 months after this initial presentation. It thus remains possible that the two conditions were indeed related in our case, and the presence of haematuria and proteinuria when she presented with PF would be consistent with this. To our knowledge, this is the first reported case documenting a negative autoantibody titre at the time of PF diagnosis.
Two main mechanisms have been proposed for the development of PF in patients with small vessel vasculitis. The first is that fibrosis occurs in response to pulmonary haemorrhage which may itself be subclinical [4]. The second is that ANCA antigens such as MPO undergo translocation to the surface of neutrophils (possibly in response to proinflammatory cytokines), and subsequent binding of circulating ANCA results in neutrophil degranulation and the release of reactive oxygen species, causing injury and consequent fibrosis [5,6]. In our patient, if the PF was a manifestation of vasculitis, this would suggest that either the latter mechanism is not an absolute requirement for the development of fibrosis or that autoantibody levels too low to be detected by conventional assays may still be sufficient to cause pathology.
In summary, PF is an uncommon but documented finding in some patients with small vessel vasculitis. Whether this is a genuine manifestation of vasculitis or a coincidental finding remains unclear; however, the fact that most such patients have autoantibodies against MPO suggests that there may be a true association in at least some cases. PF may be identified at the same time as the diagnosis of vasculitis is made or, alternatively, may precede it by months or years. At present, there are few reports examining the presence of anti-MPO antibodies during this interim period, and further study of this aspect may contribute to the understanding of the mechanisms underlying this possible uncommon manifestation of systemic vasculitis.
Conflict of interest statement. None declared.
References
- 1.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 2.Arimura Y, Minoshima S, Tanaka U, et al. Pulmonary involvement in patients with myeloperoxidase specific-antineutrophil cytoplasmic antibody. Ryumachi. 1995;35:46–55. [PubMed] [Google Scholar]
- 3.Foulon G, Delaval P, Valeyre D, et al. ANCA-associated lung fibrosis: analysis of 17 patients. Respir Med. 2008;102:1392–1398. doi: 10.1016/j.rmed.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum J, Danoff S, Askin FB, et al. Microscopic polyangiitis presenting as a “pulmonary-muscle” syndrome: is subclinical alveolar hemorrhage the mechanism of pulmonary fibrosis? Arthritis Rheum. 2007;56:2065–2071. doi: 10.1002/art.22633. [DOI] [PubMed] [Google Scholar]
- 5.Falk RJ, Terrell RS, Charles LA, et al. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins CE, Quismorio FP., Jr Pulmonary involvement in microscopic polyangiitis. Curr Opin Pulm Med. 2005;11:447–451. doi: 10.1097/01.mcp.0000170520.63874.fb. [DOI] [PubMed] [Google Scholar]
- 7.Nada AK, Torres VE, Ryu JH, et al. Pulmonary fibrosis as an unusual clinical manifestation of a pulmonary-renal vasculitis in elderly patients. Mayo Clin Proc. 1990;65:847–856. doi: 10.1016/s0025-6196(12)62575-0. [DOI] [PubMed] [Google Scholar]
- 8.Gaudin PB, Askin FB, Falk RJ, et al. The pathologic spectrum of pulmonary lesions in patients with anti-neutrophil cytoplasmic autoantibodies specific for anti-proteinase 3 and anti-myeloperoxidase. Am J Clin Pathol. 1995;104:7–16. doi: 10.1093/ajcp/104.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Becker-Merok A, Nossent JC, Ritland N. Fibrosing alveolitis predating microscopic polyangiitis. Scand J Rheumatol. 1999;28:254–256. doi: 10.1080/03009749950155643. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Suda S, Takayama M, et al. A case of MPO ANCA associated glomerulonephritis with interstitial pneumonitis complicated with lung tuberculosis and pericarditis. Nippon Jinzo Gakkai Shi. 2000;42:591–596. [PubMed] [Google Scholar]
- 11.Hiromura K, Nojima Y, Kitahara T, et al. Four cases of anti-myeloperoxidase antibody-related rapidly progressive glomerulonephritis during the course of idiopathic pulmonary fibrosis. Clin Nephrol. 2000;53:384–389. [PubMed] [Google Scholar]
- 12.Mansi IA, Opran A, Sondhi D, et al. Microscopic polyangiitis presenting as idiopathic pulmonary fibrosis: is anti-neutrophilic cytoplasmic antibody testing indicated? Am J Med Sci. 2001;321:201–202. doi: 10.1097/00000441-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Takeda Y, Aoki A, Tsuji T, et al. A case of non-specific interstitial pneumonia in patient with microscopic polyangiitis. Ryumachi. 2003;43:654–659. [PubMed] [Google Scholar]
- 14.Eschun GM, Mink SN, Sharma S. Pulmonary interstitial fibrosis as a presenting manifestation in perinuclear antineutrophilic cytoplasmic antibody microscopic polyangiitis. Chest. 2003;123:297–301. doi: 10.1378/chest.123.1.297. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Matsuo K, Ishikawa S, et al. Two autopsy cases of ANCA-associated glomerulonephritis manifested after contrast medium use. Nippon Jinzo Gakkai Shi. 2004;46:365–370. [PubMed] [Google Scholar]
- 16.Inoue T, Tanaka E, Kato T, et al. A case of MPO-ANCA-related vasculitis after asbestos exposure with progression of a renal lesion after improvement of interstitial pneumonia. Nihon Kokyuki Gakkai Zasshi. 2004;42:496–501. [PubMed] [Google Scholar]
- 17.Homma S, Matsushita H, Nakata K. Pulmonary fibrosis in myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitides. Respirology. 2004;9:190–196. doi: 10.1111/j.1440-1843.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 18.Ando Y, Okada F, Matsumoto S, et al. Thoracic manifestation of myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA)-related disease. CT findings in 51 patients. J Comput Assist Tomogr. 2004;28:710–716. doi: 10.1097/01.rct.0000135280.79012.c7. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Yu F, Zhang Y, et al. Characteristics of Chinese patients with Wegener’s granulomatosis with anti-myeloperoxidase autoantibodies. Kidney Int. 2005;68:2225–2229. doi: 10.1111/j.1523-1755.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Matsushita H, Uji M, et al. A case of interstitial pneumonia preceding microscopic polyangiitis. Nihon Kokyuki Gakkai Zasshi. 2007;45:615–620. [PubMed] [Google Scholar]
- 21.Sugimoto T, Kanasaki K, Koyama T, et al. A case of myeloperoxidase-antineutrophil cytoplasmic antibody positive-polyarteritis nodosa complicated by interstitial pneumonia and rapidly progressive renal failure. Clin Rheumatol. 2007;26:429–432. doi: 10.1007/s10067-005-0142-9. [DOI] [PubMed] [Google Scholar]
- 22.Gompelmann DR, Wenz H, Heussel CP, et al. Microscopic polyangiitis as etiology for lung fibrosis—a known but often late-diagnosed cause. Med Klin (Munich) 2009;104:476–479. doi: 10.1007/s00063-009-1097-4. [DOI] [PubMed] [Google Scholar]
- 23.Hervier B, Pagnoux C, Agard C, et al. Pulmonary fibrosis associated with ANCA-positive vasculitides. Retrospective study of 12 cases and review of the literature. Ann Rheum Dis. 2009;68:404–407. doi: 10.1136/ard.2008.096131. [DOI] [PubMed] [Google Scholar]