Abstract
In most individuals, language production and visuospatial skills are subserved predominantly by the left and right hemispheres, respectively. Functional Transcranial Doppler (fTCD) provides a noninvasive and relatively low-cost method for measuring functional lateralization. However, while the silent word generation task provides an accurate and reliable paradigm for investigating lateralization of language production, there is no comparable gold-standard method for measuring visuospatial skills. Thirty undergraduate students (19 females) completed a task of spatial memory while undergoing fTCD recording. Participants completed this task at two different time points, separated by between 26 to 155 days. The relative activation between hemispheres averaged across all participants was found to be consistent across testing sessions. This was observed at the individual level also, with a quantitative index of lateralization showing high reproducibility. These findings indicate that the use of the spatial memory task with fTCD is a robust methodology for examining laterality of visuospatial skills.
Keywords: Cerebral lateralization, Visuospatial, Functional transcranial Doppler ultrasonography, Reliability, Reproducibility, Spatial memory
INTRODUCTION
In most, but not all, cases, functional differences are observed between the two hemispheres: language production is subserved predominantly by the left hemisphere and visuospatial skills by the right hemisphere. Determining the lateralization of each function is of both theoretical and practical interest, providing insight into the development of functional specialization (Whitehouse & Bishop, 2009), as well as informing clinical decision-making, as in the case of preoperative evaluations of epileptic patients (Loring, Meador, Lee, & King, 1992).
Traditionally, cerebral lateralization has been determined with the Wada test, an invasive procedure in which function of one cerebral hemisphere is transiently disrupted by the administration of sodium amobarbital via a carotid or femoral artery (Wada & Rasmussen, 1960). However, while the Wada test is highly effective in determining functional lateralization, the invasive nature of the procedure is a major drawback. Although functional magnetic resonance imaging (fMRI) is starting to replace the Wada test in clinical settings, it remains too expensive for routine use in research projects. An alternative procedure that has become increasingly popular is functional Transcranial Doppler (fTCD). fTCD uses ultrasound to measure event-related changes in blood flow through the left and right middle cerebral arteries (MCA). Interest in fTCD has spiked in the recent years with recent software advancements that considerably enhance the accuracy of measurement (Deppe, Knecht, Henningsen, & Ringelstein, 1997).
To date, research with fTCD has focused mainly on determining lateralization of language production. A widely used paradigm for this is the word generation task, in which participants are asked to silently generate words that begin with a given letter. Using this paradigm, Deppe et al. (2000) found that around 90% of 188 right-handed individuals have these abilities lateralized predominantly to the left hemisphere, which is similar to the proportion of individuals identified to have a leftward asymmetry for language using fMRI (Deppe et al., 2000) or the Wada test (Knecht et al., 1998). Further examination has found the reproducibility for this paradigm to be excellent. Knecht et al. retested 10 individuals between one hour and 14 months after their initial assessment and found the correlation of quantitatively defined laterality indices between testing sessions to be extremely high, r = .95.
Considerably less research has evaluated visuospatial paradigms and currently no task has been found to have the rigorous properties exhibited by the word generation paradigm. Whitehouse and Bishop (2009) developed a spatial memory task, which requires participants to memorize the location of a number of red circles randomly interspersed among a greater number of white circles. Around 80% of the 75 participants were found to exhibit greater right- than left-hemisphere activation. This figure is congruent with findings from studies of brain-damaged patients, which estimate that visuospatial functions are lateralized predominantly to the right hemisphere in around 75% of cases (Bryden, Hécaen, & DeAgostini, 1983). The current study investigated the test-retest reliability of fTCD activation elicited by the spatial memory task reported in Whitehouse and Bishop (2009). We also sought to determine whether this paradigm was subject to habituation of cerebral blood flow over time, a phenomenon that would limit the applicability of this task.
METHOD
Participants
Participants were staff or students of Oxford University. All were part of a larger sample, recruited for a previous study (Whitehouse & Bishop, 2009). In the previous study, participants were administered the task of spatial memory along with a word generation task that determines laterality for language. Selected members of this sample were contacted again for the current study. The cohort included in the current study was over-sampled for those individuals with atypical (i.e., left-hemisphere) lateralization for the spatial memory task, in order to provide an evaluation of the full range of cerebral activation. The 30 participants (19 females) for the current study were aged between 18 and 29 years (M = 22.07, SD = 3.32). All were healthy and without any history of neurological disorder. Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971), with scores of 40 or above denoting right-handedness, 40 or below denoting left-handedness, and scores in between denoting ambidexterity. The sample included 16 right-handed (10 females), 13 left-handed (9 females), and 1 ambidextrous (male) individual. The interval between individual testing sessions ranged from 26 to 155 days (M = 63.33, SD = 40.2).
Apparatus and Stimuli
Blood flow velocity through the right and left MCAs was measured with a Doppler ultrasonography device (DWL Multidop T2; DWL Elektronische Systeme, Singen, Germany). Participants were fitted with a flexible head-set, which held in place a 2-MHz transducer probe over each temporal skull window. The spatial memory task was controlled by Presentation Software (version 0.43, 2001; Neurobehavioral System Inc., San Francisco, CA) on a Dell laptop computer, which sent markers to the fTCD to denote the start of each epoch. All participants had at least 15 accepted epochs in each session. A paired t test found no difference in the number of accepted epochs between the two time points (Time 1: M = 18.8, SD = 1.16; Time 2: M = 18.87, SD = 1.2), t = .32, p = .75.
Design and Procedure
Procedure for the spatial-memory experiment was identical to that outlined in Whitehouse and Bishop (2009). Participants were seated in a quiet laboratory, with a computer placed roughly 80 cm in front of them. After receiving information about the experiment, participants were cued by a tone to attend the computer screen. White (n = 17) and red (n = 9) circles appeared on the screen, overlaid on a black background. The circles were distributed approximately evenly across the screen, but were not aligned in rows or columns. Participants were instructed to memorize the location of the red circles, which were randomly located around the screen. The circles remained on the screen for 5 seconds, and were then replaced by a blank screen for 10 seconds. Following another cuing tone, the circle array appeared for another 5 seconds. In half of the 20 trials, the location of the red (and white) circles was the same, while in the other half of the trials, the location of one white and one red circle was swapped. Participants, who sat with their hands on the table in front of them, were asked to decided whether the location of the red circles was the same or different as the initial screen, by raising the index finger on their left or right hand, respectively. “Same” and “different” trials were in the same random order for all participants. Participants were awarded one point for each trial that they correctly identified as the “same” or “different.” There was no difference in the number of trials that were correctly identified as same or different by the participants between time 1 (M = 17.89, range = 16–20) and time 2 (M = 17.82, range = 14–19), t = .24, p = .82. This procedure was approved by the Central University Research Ethics Committee of Oxford University, and informed consent was obtained from each participant.
Data were analyzed off-line using the software, AVERAGE, version 1.85 (Deppe et al., 1997). Information on individual trials could then be extracted from each participant's electronic output file using a Matlab script (Mathworks Inc., Sherborn, MA) written by one of the authors (NB). A laterality index (LI) was computed as the dependent variable in both testing sessions. First, a difference plot for each individual was created by subtracting the percent cerebral blood flow velocity measured by the right probe averaged across all accepted trials, from that measured by the left probe. The LI is a measurement that is extracted from difference curve, calculated as the mean blood flow velocity difference in a 2 s window centerd on the peak value during the recognition phase of the spatial memory task (22–32 s after the start of each trial). A positive LI indicated greater left than right hemisphere activation, with a negative index signifying the reverse.
RESULTS
Figure 1 plots the difference curves, averaged across all participants, for time 1 and time 2. The intra-class correlation (ICC) was used to calculate the similarity between the two difference curves. The ICC is conceptually similar to the Pearson correlation coefficient, but is sensitive to the position, as well as shape, of the two curves. The ICC between the two curves was strongly positive, r = .97, p < .001.
Fig. 1.
Mean difference waves for Time 1 and Time 2. This figure also presents critical points in the task and the period for laterality index (LI) calculation (i.e., the period in which the peak difference is measured).
We then sought to determine whether there were differences in the mean activation recorded by the two Doppler probes between time 1 and time 2. Paired t tests found no difference between sessions in measurements taken by the left probe, M difference = 0.49, SD of difference = 3.04, t(29) = 0.89, p = .38, or right probe, M difference = 0.43, SD of difference = 3.26, t(29) = 0.72, p = .48, during the time-window of interest (22–32 s after the start of each trial).
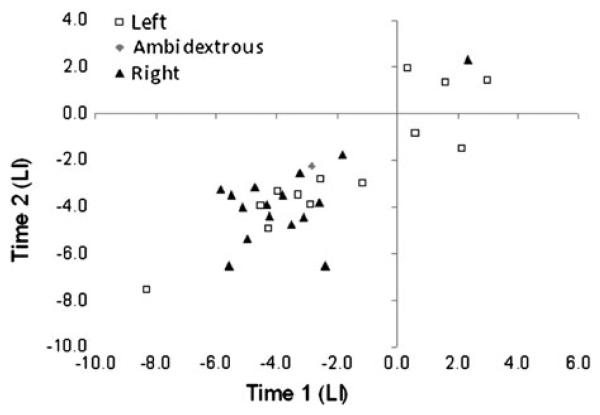
Participants' LI for the spatial memory task in the second session were then computed. A paired-samples t test found no difference between the mean LI for the first (M = −2.81, SD = 2.69) and second sessions in this participant sample (M = −3.05, SD = 2.4), t(29) = 0.92, p = .37. Figure 2 shows a scatter plot of each participant's LI at the two time points as a function of handedness. Visual inspection suggests a high-level of congruence between the LIs for the two sessions, and this was confirmed using Pearson's correlation coefficient, r(30) = .84, p < .001.
Fig. 2.
Association between participants' laterality indexes (LIs) for Time 1 and Time 2 as a function of handedness.
We then sought to determine whether reliability varied as a function of the time between testing sessions. A “reliability index” was calculated by subtracting the LI of time 2 from the LI of time 1. The mean reliability index did not significantly differ from zero, M = −.25; SD = 1.48, t(29) = .92, p = .27. No correlation was observed between the reliability index and the number of days between testing sessions, r = .01, p = .94.
Analyses then concentrated on whether there was habituation in cerebral blood perfusion across the trials. For each person, separate LIs were calculated for each accepted trial (using the same method outlined previously) in the second session. Two mean LIs were then computed for each person: one averaged across the LIs for the first 10 trials (trials 1 to 10) and the other averaged across the LIs for the second 10 trials (trials 11 to 20). There was no difference in the number of accepted trials between the first-half (n = 9.87; SD = .43) and second-half of the task (n = 9.57; SD = 1.33), t(29) = 1.14, p = .26. An independent t test found no difference in the average LI between the first 10 and the second 10 trials, M difference = 0.59, SD of difference = 2.43, t(29) = 1.32, p = .2.
In the first testing session, participants completed a word generation task, which provided a measure of cerebral lateralization for language production (based on a LI). For a full description of this task, please refer to Whitehouse and Bishop (2009). A final analysis examined the association between cerebral lateralization for spatial memory (measured at time 2) and language (measured at time 1). Figure 3 shows that the majority of individuals were right hemisphere-lateralized for spatial memory and left hemisphere-lateralized for language production. A minority of individuals had both functions lateralized to either the left-hemisphere (n = 7) or right-hemisphere (n = 5), while one participant only showed the reverse pattern of typical asymmetry (i.e., left hemisphere dominant for spatial memory, and right-hemisphere dominant for language production).
Fig. 3.
Laterality for spatial memory and language production in right-handed, left-handed, and ambidextrous participants. A positive index indicates greater left-hemisphere than right-hemisphere activation, with a negative index signifying the reverse.
DISCUSSION
Visuospatial skills are subserved by a large-scale cognitive system spanning both cerebral hemispheres, but focused in the right hemisphere in the majority of the population. Whitehouse and Bishop (2009) presented a task of spatial memory that can be used with fTCD to determine hemispheric dominance of these skills, and the current study examined the reproducibility of this paradigm. Figure 1 shows that the difference in mean activation of the two cerebral hemispheres when completing the task was highly similar between the two sessions. Figure 2 shows that this pattern was observed at the individual level also. Further analyses found no evidence for habituation in blood perfusion across trials, and that time between sessions had no effect on reproducibility. These data suggest that the spatial memory paradigm is a reliable method for examining lateralization of visuospatial skills.
It is important to note that although reliability between the two sessions was good (r = .84), it was not perfect. Two participants exhibited greater left- than right-hemisphere activation in the first session (Participant 11 = 0.62; Participant 29 = 1.12), but the reverse pattern in the second session (Participant 11 = −0.86; Participant 29 = −1.5). Importantly, the difference in LIs between sessions for these two participants were of a similar magnitude to that observed among other participants in the current study, as well as for participants retested on the word generation paradigm (Knecht et al., 1998).
For over a decade, the silent word generation has been considered the gold standard method for determining language lateralization using fTCD. However, consensus on a comparable paradigm for examining lateralization of visuospatial skills has yet to be achieved. One of the impediments to research in this area is that visuospatial ability encompasses a range of cognitive functions, and the genetic, biological, and/or environmental factors influencing lateralization may differ between skills. Spatial orientation has been examined with fTCD using a variety of mental rotation paradigms. Although the majority of these tasks have elicited greater right- than left-hemisphere activation, this has often been found to be unstable at the individual level (Bulla-Wellwig, Vollmer, Gotzen, Skreczek, & Hartje, 2005 ; Hartje, Ringelstein, Kistinger, Fabianek, & Willmes, 1994 ; Serrati et al., 2000). Dorst et al. (2008) suggested that the lack of reproducibility may be a result of limitations in task design, such as the requirement of a verbal response from participants. More consistent right-hemisphere activation has been elicited by tasks of spatial attention, in particular the Landmark task, in which participants are required to judge the point at which a vertical line bisects a horizontal line (Flöel, Buyx, Brietenstein, Lohmann, & Knecht, 2005). Using fTCD, Flöel et al. (2002) found the reproducibility of hemispheric activation to be high in 20 healthy adult participants, r = .9. However, visuospatial attention is likely to be a necessary precursor to visuospatial processing, even though it engages somewhat different cerebral mechanisms (for a review, see Awh & Jonides, 2001).
Hemispheric activation for spatial memory has been examined by Smith, Jonides, and Koeppe (1996) using positron emission tomography (PET). Participants (n = 11) completed a dot-recognition task, similar to that used in the current study, in which they were required to determine whether a probe encircled the location of one of an array of previously presented dots. All four areas of significant activation during task performance were found to be in the right hemisphere (areas in the ventrolateral frontal cortex, occipital cortex, parietal cortex, and premotor cortex). The current study provides cross validation for these findings using fTCD, suggesting that spatial memory may be a reliable and valid way to determine laterality of visuospatial skills.
Developing rigorous methods for determining functional cortical organization of cognitive skills is of wide-ranging importance. Indentifying the neural areas that may mediate cognitive functions can help in minimizing cognitive impairments following neurosurgery (Dorst et al., 2008), and provide information regarding shifts in cerebral activation during the course of learning paradigms or recovery from neurological deficits (Frackowiak, 1997). These techniques also have the potential to provide insights into the cause(s) or correlates of neurodevelopmental disorders for which atypical functional lateralization has been implicated, such as schizophrenia (Dragovic & Hammond, 2005), specific language impairment (Whitehouse & Bishop, 2008), and dyslexia (Illingworth & Bishop, in press). By demonstrating that lateralization of a spatial memory task can be reliably assessed using fTCD, the current study has presented a quick, noninvasive, and relatively low-cost method for assessing visuospatial lateralization in a clinical or research setting.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust. We thank Emma Whitehouse for her helpful comments on a previous version of this manuscript.
REFERENCES
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Science. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Bryden M, Hécaen M, DeAgostini M. Patterns of cerebral organization. Brain and Language. 1983;20:249–262. doi: 10.1016/0093-934x(83)90044-5. [DOI] [PubMed] [Google Scholar]
- Bulla-Hellwig M, Vollmer J, Gotzen A, Skreczek W, Hartje W. Hemispheric asymmetry of arterial blood flow velocity changes during verbal and visuospatial tasks. Neuropsychologia. 2005;34:987–991. doi: 10.1016/0028-3932(96)00021-8. [DOI] [PubMed] [Google Scholar]
- Deppe M, Knecht S, Henningsen H, Ringelstein EB. AVERAGE: A Windows program for automated analysis of event-related cerebral blood flow. Journal of Neuroscience Methods. 1997;75:147–154. doi: 10.1016/s0165-0270(97)00067-8. [DOI] [PubMed] [Google Scholar]
- Deppe M, Knecht S, Papke K, Lohmann H, Fleischer H, Heindel W, et al. Assessment of hemispheric language lateralization: A comparison between fMRI and fTCD. Journal of Cerebral Blood Flow and Metabolism. 2000;20:263–268. doi: 10.1097/00004647-200002000-00006. [DOI] [PubMed] [Google Scholar]
- Dorst J, Haag A, Knake S, Oertel WH, Hamer HM, Rosenow F. Functional transcranial Doppler sonography and a spatial orientation paradigm identify the non-dominant hemisphere. Brain and Cognition. 2008;68:53–58. doi: 10.1016/j.bandc.2008.02.123. [DOI] [PubMed] [Google Scholar]
- Dragovic M, Hammond G. Handedness in schizophrenia: A quantitative review of evidence. Acta Psychiatrica Scandinavica. 2005;111:410–419. doi: 10.1111/j.1600-0447.2005.00519.x. [DOI] [PubMed] [Google Scholar]
- Flöel A, Buyx A, Brietenstein C, Lohmann H, Knecht S. Hemispheric lateralization of spatial attention in right-and left-hemispheric language dominance. Behavioral Brain Research. 2005;158:269–275. doi: 10.1016/j.bbr.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Flöel A, Lohmann H, Breitenstein C, Dräger B, Buyx A, Henningsen H, et al. Reproducibility of hemispheric blood flow increases during line bisectioning. Clinical Neurophysiology. 2002;113:917–924. doi: 10.1016/s1388-2457(02)00077-9. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ. The cerebral basis of functional recovery. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. Academic Press; San Diego, CA: 1997. pp. 275–299. [Google Scholar]
- Hartje W, Ringelstein EB, Kistinger B, Fabianek D, Willmes K. Transcranial Doppler ultrasonic assessment of middle cerebral artery blood flow velocity changes during verbal and visuospatial cognitive tasks. Neuropsychologia. 1994;32:1443–1452. doi: 10.1016/0028-3932(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Illingworth S, Bishop DVM. Atypical cerebral lateralisation in adults with developmental dyslexia demonstrated using functional transcranial Doppler ultrasound. Brain and Language. doi: 10.1016/j.bandl.2009.05.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Ebner A, Henningsen H, Huber T, Jokeit H, et al. Noninvasive determination of language lateralization by functional transcranial Doppler sonography: A comparison with the Wada test. Stroke. 1998;29:82–86. doi: 10.1161/01.str.29.1.82. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Ringelstein E-B, Wirtz M, Lohmann H, Dräger B, et al. Reproducibility of functional transcranial Doppler sonography in determining hemispheric language lateralization. Stroke. 1998;29:1155–1159. doi: 10.1161/01.str.29.6.1155. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, King DW. Amobarbital effects and lateralized brain function: The Wada Test. Springer-Verlag; New York: 1992. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. British Journal of Psychology. 1971;66:53–59. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Serrati C, Finocchi C, Calautti C, Bruzzone GL, Colucci M, Gandolfo C, et al. Absence of hemispheric dominance for mental rotation ability: A transcranial Doppler study. Cortex. 2000;36:415–425. doi: 10.1016/s0010-9452(08)70850-5. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating spatial and verbal working memory using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. Journal of Neurosurgery. 1960;17:266–282. [Google Scholar]
- Whitehouse AJO, Bishop DVM. Cerebral dominance for language function in adults with specific language impairment or autism. Brain. 2008;131:3193–3200. doi: 10.1093/brain/awn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJO, Bishop DVM. Hemispheric division of function is the result of independent probabilistic biases. Neuropsychologia. 2009;47:1938–1943. doi: 10.1016/j.neuropsychologia.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]