Abstract
Background
Understanding the pathways regulating mesenchymal progenitor cell fate during hepatogenesis may provide insight into postnatal liver injury or liver bioengineering. While β-Catenin has been implicated in the proliferation of fetal hepatic epithelial progenitor cells, its role in mesenchymal precursors during hepatogenesis has not been established.
Materials and Methods
We used a murine model of conditional deletion of β-Catenin in mesenchyme using the Dermo1 locus (β-CateninDermo1) to characterize the role of β-Catenin in liver mesenchyme during hepatogenesis.
Results
Lineage tracing using a LacZ reporter indicates that both hepatic stellate cells and pericytes derive from mesenchymal Dermo1 expressing precursor cells. Compared to control littermate livers, β-CateninDermo1 embryonic livers are smaller and filled with dilated sinusoids. While the fraction of mesenchymally-derived cells in β-CateninDermo1 embryos is unchanged compared to littermate controls, there is an increase in the expression of the mesenchymal markers, DESMIN, α-SMA, and extracellular deposition of COLLAGEN type I, particularly concentrated around dilated sinusoids. Analysis of the endothelial cell compartment in β-CateninDermo1/Flk1lacZ embryos revealed a marked reorganization of the intrahepatic vasculature. Analysis of various markers for the endodermally-derived hepatoblast population revealed marked alterations in the spatial expression pattern of pan-CYTOKERATIN but not E-CADHERIN, or ALBUMIN. β-CateninDermo1 phenocopies mesenchymal deletion of Pitx2, a known regulator of hepatic mesenchymal differentiation both during both organogenesis and postnatal injury.
Conclusions
Our data implicate Mesenchymal β-Catenin signaling pathway in the differentiation of liver mesenchymal progenitor cells during organogenesis, possibly via Pitx2. Hepatic Mesenchymal β-Catenin signaling, in turn, modulates the development of both endothelium and endodermally-derived hepatoblasts, presumably via other downstream paracrine pathways.
Keywords: hepatogenesis, Wnt, β-Catenin, stellate cell, Pitx2, Dermo1
INTRODUCTION
In the adult liver, mesenchymally-derived Hepatic Stellate Cells (HSC) are located in the space of Disse, just beneath the fenestrated endothelium that lines and separates the vascular sinusoids from hepatocytes. Under normal conditions, HSC serve as a reservoir for vitamin A and lipid storage and can be identified by their expression of DESMIN. During liver injury, HSCs transdifferentiate toward a myofibroblastic phenotype producing extra-cellular matrix (ECM) which contribute to the fibrogenic response to injury, which is also referred to as an activated state (1). These cells can be identified with a loss of vitamin A storage and DESMIN expression, as well as induced expression of α-SMOOTH MUSCLE ACTIN (αSMA) and ECM proteins such as COLLAGEN Type I. Understanding the underlying molecular mechanisms for how differentiation of HSCs occur is, therefore, of great importance in the treatment and prevention of hepatic fibrosis after liver injury. The specific role for Wnt/β-Catenin signaling in transdifferentiation of HSC is unclear. Kordes et al showed that Wnt signaling maintains HSC in a quiescent state, whereas Cheng et al showed that antagonism of Wnt signaling inhibits HSC activation in culture (2, 3). Since tissue repair and regeneration commonly recapitulate ontogeny, studying the role of β-Catenin signaling in mesenchymal cells during hepatogenesis may provide insight into how HSC are regulated postnatally during injury.
Mesenchymal-to-epithelial instructions are a critical component of hepatogenesis. A close interaction between endodermal progenitor cells from the foregut endoderm, as well as instructive signals from surrounding mesenchymal septum transversum, are all essential components of proper liver induction and formation. Lineage tracing experiments using the MesP1-Cre+/−/Rosa26+/− (herein called MesP1LacZ) mice to specifically drive LacZ expression in the mesenchyme surrounding the foregut endoderm, indicate that the mesoderm surrounding the liver bud give rise to liver mesenchymal cells, such as submesothelial cells and their derivates, hepatic stellate cells (HSC) and perivascular smooth muscle cells (pericytes). These mesenchymal cells all co-express DESMIN and αSMA so that the only histologic distinction between HSC and pericytes during hepatogenesis is the proximity of perivascular mesenchymal cells to the sinusoids (4). However, relatively little is known about the molecular mechanisms controlling mesenchymal progenitor cell regulation and differentiation during liver organogenesis (5).
The Wnt family of ligands regulate stem cell renewal and differentiation through the stabilization and nuclear translocation of the transcription factor β-Catenin. Delanghe et al showed that knocking out β-Catenin specifically in the mesenchyme leads to defects in multiple organ systems with severe cardiac and vasculogenesis-related defect (6). These conditional knockouts also largely phenocopy null mutation of the homeobox gene Pitx2 in terms of arrest in turning of the body axis and defective body wall closure, partial right pulmonary isomerism, cardiac tract abnormalities, and facial abnormalities such as defective development of the mandibular and maxillary facial prominences and regression of the stomodeum (7). Furthermore, loss of β-Catenin in the lung leads to reduced number of mesenchymal parabronchial smooth muscle progenitor cells and impaired differentiation of endothelial cells. Together these observations indicate a role for β-Catenin in the amplification and differentiation of mesenchymal progenitor cells during organogenesis, possibly through the Pitx family of transcription factors.
Wnt/β-Catenin signaling is critical for hepatogenesis. The lack of β-Catenin during hepatogenesis results in increased hepatocyte cell death and decreased proliferation as well as decreased expression of the transcription factors CCAAT-Enhancer Binding Protein-α and Hepatocyte Nuclear Factor-4α, both of which are important for the function of mature hepatocytes (8). Additionally, organ cultures with knockdown of β-Catenin utilizing antisense methodology leads to loss of biliary epithelial markers (9). Conversely, stabilization of β-Catenin inhibits hepatoblast expansion and hepatocyte differentiation while promoting biliary differentiation (10). Together these studies indicate an important role for β-Catenin in proliferation as well as differentiation of hepatoblasts. The role of β-Catenin signaling in mesenchymal precursor cells during liver organogenesis, however, is less clear.
Herein, we investigate the role of β-Catenin signaling in differentiation of mesenchymal cells in the developing liver by using transgenic mice expressing Cre recombinase under the control of the mesenchyme-specific Dermo1 promoter to establish a conditional knock out β-Catenin in the mesenchyme of embryonic liver.
MATERIALS AND METHODS
Mutant embryos
β-Cateninf/f,CmvCre/+, Rosa26R+/+, and Flk1lacZ/+ mice were obtained from Jackson Laboratory (USA). Dermo1Cre/+ were a gift from Dr. David Ornitz (11). Utilizing Cre recombinase genetics, β-Catenin+/− mice were obtained by crossing β-Cateninf/f mice with CmvCre/+ mice, wherein Cre is expressed in the germ line resulting in knockout of β-Catenin. Next, β-Catenin+/− mice were crossed with Dermo1Cre/+ mice to obtain double heterozygous males (Dermo1Cre/+/β-Catenin+/−), which were then crossed with β-Cateninf/f females to generate mesenchymal specific abrogation of β-Catenin (herein called β-CateninDermo1). Analysis of 327 embryos from F1 intercrosses revealed that the Dermo1Cre/+/β-cateninf/− conditional knockout (herein called β-CateninDermo1) is lethal at embryonic day E13.5-E14.5 due to the severe cardiac and vasculogenesis-related defects as previously published (6). Littermates not expressing Cre were used as controls.
In addition, β-Cateninf/f mice were intercrossed with Rosa26R+/+ to generate β-Cateninf/f/Rosa26R+/+ homozygous mice. β-Cateninf/f/Rosa26R+/+ mice were then crossed with Dermo1Cre/+/β-Catenin+/− mice to generate β-CateninDermo1/Dermo1LacZ/+ mice. Littermates were used as controls. To generate β-CateninDermo1/Flk1Lacz/+ mice, β-Cateninf/f mice were intercrossed with Flk1Lacz/+ mice. Progeny were then intercrossed with Dermo1Cre/+/β-Catenin+/− mice.
Finally, Pitx2Dermo1 were generated in a similar fashion described above as with the β-CateninDermo1 mice using Pitx2f/f, Pitx2+/−, and Dermo1Cre/+mice. Four conditional knockouts (Pitx2Dermo1) from two litters were analyzed for each experiment. Similar to β-CateninDermo1, mesenchymal deletion of Pitx2 in Pitx2Dermo1 mice is embryonic lethal by E14.5. Littermates not expressing Cre were used as controls.
PCR was performed to validate genotype. All animal experiments were performed in accordance NIH guidelines.
Localization of β-galactosidase (LacZ) activity
Livers from E11.5-P1 embryos were fixed with 4% paraformaldehyde, and LacZ expression was characterized by β-galactosidase activity using X-gal solution as described by Kelly et al (12). For histology, E13.5-P1 livers were further embedded in 3% low melting Agarose and cut into 500 μM sections using Vibrotome (Leica VT 1000S) from Leica, IL, USA. Samples were then stained with X-gal solution, embedded in paraffin, and sectioned at 5μm thickness as previously described (13). Liver samples were then stained overnight with X-gal solution at 37°C, washed in PBS, fixed for 1h with 4% PFA at 4°C and washed in PBS. Samples were dehydrated and embedded in paraffin for immunohistochemistry or immunofluorescence as described below.
Histology, immunohistochemistry, and histological analysis
Embryos were isolated from the embryonic stages described. After fixation in 4% PFA, dehydration and paraffin embedding, 5 μm sections were cut and samples deparaffinated in Histochoice (Sigma-Aldrich, MO, USA) followed by rehydration through ethanol to water. Antigen retrieval was performed by microwaving the slides in Unmasking solution (Vector Laboratories, CA, USA) for 4 minutes x3. Immunohistochemistry was carried out by using EnVision+ Dual Link System HRP (DAB+) (DacoSytomation, CA, USA) according to instructions from the manufacturer. For immunofluorescence, tissues were treated identically as for immunohistochemistry but after incubated with the primary antibody, the slides were incubated with secondary antibodies for 1.5 hours at room temperature conjugated with FITC or Cy3 from Jackson laboratories as indicated. Slides were then mounted with Vectashield containing DAPI (Vector Laboratories, CA, USA) and photomicrographs were taken. Primary antibodies used were α-Albumin (1:200) (DakoCytomation, CA, USA), α-Desmin (1:100) (DakoCytomation, CA, USA), α-Pan Cytokeratin (1:100) (Sigma-Aldrich, MO, USA)., α-Smooth Muscle Actin (1:100) (Sigma-Aldrich, MO, USA), α-Collagen (Karlan, AZ, USA). Overnight incubation at 4°C was used for all primary antibodies. Controls excluding the primary antibody were in all cases negative.
The number of cells staining positive for DESMIN, E-CADHERIN, ALBUMIN, pan-CYTOKERATIN and LacZ were quantified and normalized to high powered field or the total number of nuclei in a high powered field. Quantification was performed on 3 nonsequential sections and averaged for each animal. Areas positive for LacZ staining were quantified using ImageJ (NIH) and normalized to the total area analyzed per section. Data were expressed as mean ± standard deviation. Student's t-test was used to assess statistical significant differences between groups. Significant difference was defined as P < 0.05.
RESULTS
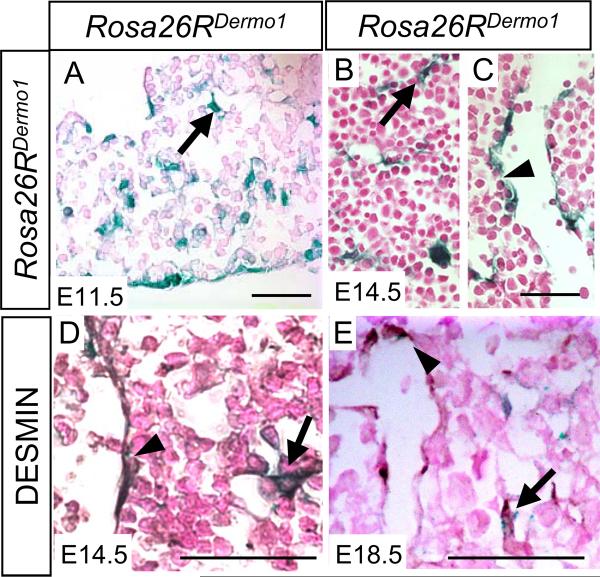
Dermo1cre/+ mice, expressing Cre recombinase under the control of Dermo1 (Twist2) promoter, have been previously used to conditionally knock out genes in the mesenchyme (9, 10). Liver mesenchymal specificity of Dermo1cre/+ was tested in a cross with Rosa26R mice to generate Dermo1LacZ mice, which express LacZ after driver line specific Cre-mediated excision of the loxP flanked nontranscribed neo cassette (14). X-gal staining for LacZ expression (or β-galactosidase activity) confirmed activity of the Dermo1cre construct as early as embryonic day (E) 11.5 during hepatogenesis (Figure 1A–C). From E11.5 through E18.5, LacZ expression was detected scattered throughout the liver parenchyma in fibroblast-like cells (arrows) as well as in cells adjacent to blood vessels (arrowhead). Mesenchymal specificity of the Dermo1 driver line was confirmed by co-localization of LacZ expression with DESMIN, a marker for mesenchymally-derived cells (1). Double positive fibroblast-like cells represent hepatic stellate cells, and double positive perivascular cells represent pericytes (Figure 1D and E, arrow and arrowhead respectively). Co-expression of LacZ and DESMIN in Dermo1LacZ mice indicates that both hepatic stellate cells and pericytes derive from a common Dermo1 expressing precursor cell, similar to published observations with MesP1LacZ (4).
Figure 1.
Spatial and temporal expression pattern of LacZ in Rosa26Dermo1 livers embryos during hepatogenesis at E11.5 (A) and E14.5 (B and C). Co-localization with the mesenchymal marker DESMIN (brown) and β-galactosidase activity (blue) at E14.5 (D) and E18.5 (E). Arrows indicates fibroblast-like cells with β-galactosidase activity distributed throughout the liver parenchyma and arrowheads indicate cells with β-galactosidase activity lining the sinusoids. Bars denote 25 µm.
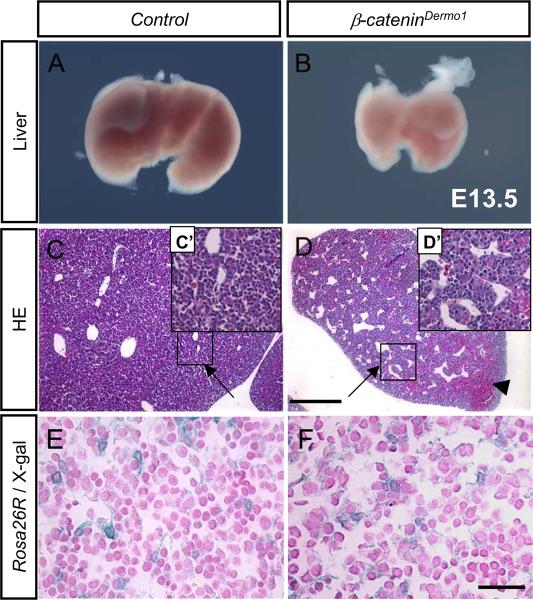
To investigate the role of β-Catenin in the mesenchymal compartment in the liver during liver organogenesis, we generated conditional knockout mice lacking β-Catenin expression in the mesenchyme (β-CateninDermo1). As previously reported, these conditional knockout embryos are lethal by E13.5–14.5 due to cardiac and vasculogenesis-related defects (6). We consistently observed that fetal livers from β-CateninDermo1 at E13.5 and E14.E were smaller than livers from littermate controls (Figure 2A, B). Histologic analysis revealed larger and more numerous vessels in the β-CateninDermo1 livers compared to controls (Figure 2C, C', D and D'). This defect in the β-CateninDermo1 liver is consistent with the previously described defect in maturation of endothelial cells, which results in leaky blood vessels throughout the embryo (10). Pools of blood could also be detected within the livers of conditional knockout mice at E13.5 (Figure 2D, arrowhead). X-gal staining of β-CateninDermo1/Dermo1LacZ/+ revealed in no change in the fraction of total cells that are LacZ expressing compared to littermate controls (0.250±0.077 in control mice vs. 0.300±0.125 positive cells per total number of cells in β-CateninDermo1 mice; p-value not significant (NS); Figure 2E, F).
Figure 2.
Phenotype analysis of mesenchymal β-Catenin deletion in E13.5 β-CateninDermo1 embryo livers. β-CateninDermo1 livers are smaller than those of control littermates (A, B). Hematoxilin/Eosin staining shows disturbed architechture in the β-CateninDermo1 with dilated sinusoids (arrow) and abnormally pooled blood (arrowhead) (C, D, and insets C', D'bars denote 200 µm.). LacZ expression in β-CateninDermo1;Rosa26R and littermate controls (E and F, bars denote 50 µm).
To further analyze the effect of β-Catenin knockout in the mesenchyme on mesenchymal cellular differentiation, immunohistological analyses were performed for different mesenchymal markers. Expression of DESMIN was increased in the β-CateninDermo1 livers compared to that of littermate controls (17.8±3.6 vs. 13.6±4.5 DESMIN+ cells/hpf, n=13, p < 0.05, Figure 3A–B, arrows). This was primarily due to increased DESMIN expression in perivascular cells adjacent to larger and more numerous intraparenchymal blood vessels. When DESMIN expressing cells were normalized to total cells instead of area in order to account for more area in β-Catenin Dermo1 mouse livers occupied by larger and more numerous blood vessels, the increase in DESMIN positive cells was still noted in the conditional knockout mice compared to wildtype littermates (0.17±0.06 vs. 0.10±0.04, n=13, p<0.05). Qualitative assessment of increased α-SMA expression paralleled DESMIN expression detected along the intraparenchymal blood vessels of the conditional knockout livers of β-CateninDermo1 mice (Figure 3C–D). Increased expression of both DESMIN and α-SMA, together with increased deposition of Collagen type I (Figure 3E–F), indicate a possible defect in mesenchymal differentiation in the β-CateninDermo1 conditional knockout livers.
Figure 3.
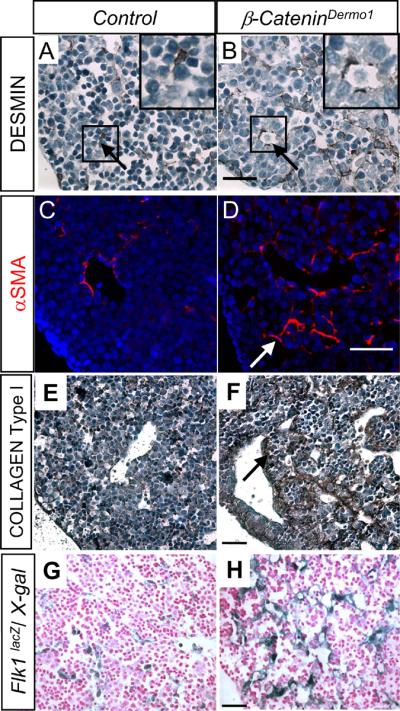
Immunohistochemical analysis of mesenchymal markers in E14.5 β-CateninDermo1 embryo livers. Increased staining for DESMIN (A, B and insets A', B') αSMA (C, D arrow) along dilated sinusoids in β-CateninDermo1 livers compared to control littermates. Increased deposition of COLLAGEN Type I in β-CateninDermo1 livers compared to control littermate (E, F arrow). Increased LacZ expression in E14.5 β-CateninDermo1;Flk1LacZ livers outlining the dilated blood vessels in β-CateninDermo1 livers compared to control livers (G, H). Bars denote 50μm.
The defect in vascular development identified histologically was further analyzed in β-CateninDermo1/Flk1LacZ mice. Flk1 encodes the receptor of Vascular Endothelial Growth Factor-2, which is expressed in relatively immature vasculature (14). β-CateninDermo1/Flk1LacZ mice, therefore, enable labeling of the fetal hepatic vasculature in the setting of mesenchymal deletion of β-Catenin. Staining for LacZ expression indicated an alteration in the spatial expression pattern of Flk1 in the β-Catenin conditional knockout mice compared to their control littermates (Figure 3G–H) with increased expression of Flk1 in regions of dilated blood vessels. However, morphometric analysis of total LacZ expression for the entire liver was unchanged (21.8±3.4 vs. 19.3±15.1, p-value NS) indicating a redistribution of Flk1 expression β-CateninDermo1 livers. From these data, we infer that knockout of β-Catenin in hepatic mesenchymal cells leads to the dilated fetal intrahepatic vasculature potentially through altered perivascular signaling.
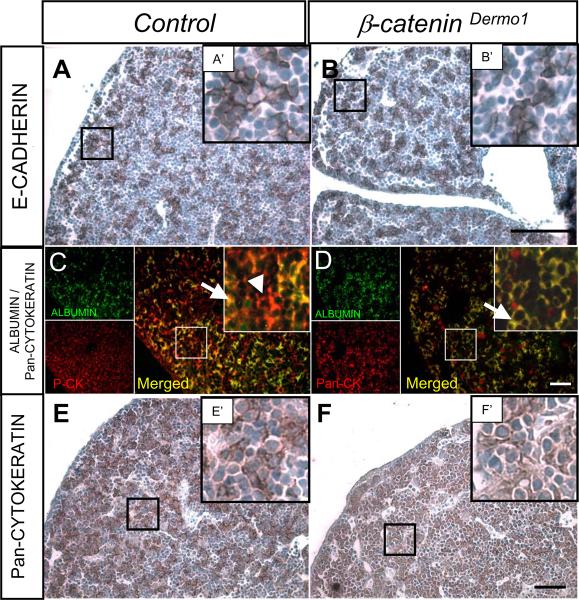
We then sought to determine if inactivation of β-Catenin in the mesenchyme affects the cells of endodermal origin. Immunohistochemistry for E-CADHERIN, a marker of cells of endodermal lineage, revealed no qualitative difference in spatial distribution of expression (Figure 4A, B and insets A', B'). Quantitative analysis of number of E-CADHERIN positive cells per area did not show any difference between control littermates and β-Catenin conditional knockout mice (201±26 vs. 224±29 E-CADHERIN positive cells/hpf, respectively; n=18; p-value NS). These data imply that conditional deletion of β-Catenin in the mesenchyme does not affect the quantity of endodermally-derived cells.
Figure 4.
Immunohistochemical analysis of epithelial markers E14.5 β-CateninDermo1 embryo livers. Staining for E-CADHERIN in the E14.5 β-CateninDermo1 livers (B, B') and littermate control (A, A'). Immunofluorescent staining for ALBUMIN (green), Pan-CYTOKERATIN (red) and merge (arrow, yellow) (C, D). Arrowhead denotes Pan-CYTOKERATIN only positive cells in the control compared to β-CateninDermo1. Immunohistochemical staining for pan-CYTOKERATIN mark bile duct epithelial cells and hepatoblasts (brown) (E, F' and insets E', F'). Bars denote 50 µm
To further investigate differentiation of cells of the endodermal compartment, we performed immunofluorescence against ALBUMIN (marker of hepatocytes) and Pan-CYTOKERATIN (marker of bile duct epithelium). Co-localization of ALBUMIN and Pan-CYTOKERATIN identifies hepatic epithelial progenitor cells, or hepatoblasts, with the capacity to differentiate into hepatocytes or cholangiocytes. Hepatoblasts co-expressing ALBUMIN and Pan-CYTOKERATIN were identified in both control mice (Figure 4C, arrow) and β-CateninDermo1 embryos (Figure 4D, arrow). There were no significant differences in the total number of cells positive for either ALBUMIN or pan-CYTOKERATIN (Control vs. β-CateninDermo1: 74.6±17.5 vs. 74.7±20.0 ALBUMIN+ cells/hpf respectively, p-value NS; 153±29 vs. 158±27 Pan-CYTOKERATIN+ cells/hpf, respectively, p-value NS, n=11). There was a trend toward an increase in the number of ALBUMIN+Pan-CYTOKERATIN+ cells in β-CateninDermo1 livers although this was not significant (60.8±19.0 vs. 66.5±6.4 cells/hpf, p-value NS, n=6). While the total number of Pan-CYTOKERATIN+ cells (yellow hepatoblasts plus red cholangiocytes) per high-powered field was not reduced, the number of Pan-CYTOKERATIN only positive cells (red stained cells in the merged images, cholangiocyte differentiated) was reduced in β-CateninDermo1 embryo livers compared to littermate controls (Control vs. β-CateninDermo1: 84.9±21.2 vs. 41.1±21.2 Pan-CYTOKERATIN positive cells/hpf, respectively, p<0.05, n=6). Furthermore, there was a more clustered appearance to the distribution of all Pan-CYTOKERATIN positive cells (cholangiocytes plus hepatoblasts) in control mice compared to β-CateninDermo1 littermates (Figure 4E–F and insets). Collectively, these data may indicate a possible delay in cholangiocyte differentiation in the β-CateninDermo1 livers.
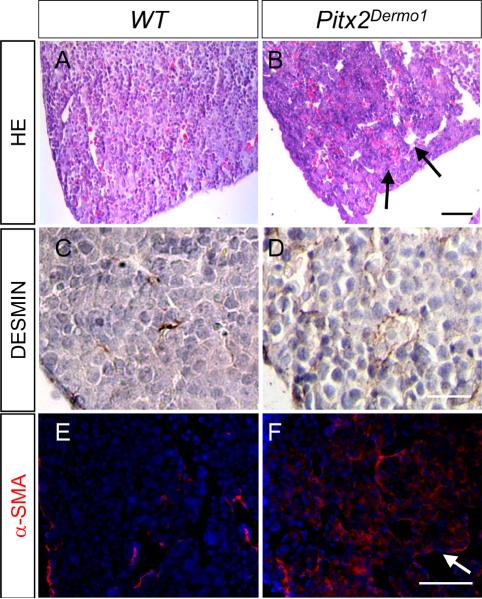
Since β-CateninDermo1 mice grossly phenocopy Pitx2-/- mice and the conditional deletion of β-Catenin in the mesenchyme leads to progressive loss of PITX2 during lung organogenesis (6), we wanted to investigate the relationship between mesenchymal β-Catenin and Pitx2 during hepatogenesis. Since Pitx2-/- mice exhibit a phenotype consisting of smaller livers with dilated sinusoidal spaces (15) very similar to our observed β-CateninDermo1 phenotype, we sought to determine if expression of Pitx2 specifically expressed in the mesenchyme is critical during hepatogenesis. We, therefore, bred Pitx2Dermo1 mice, which lack Pitx2 expression in the Dermo1-expressing mesenchyme. Littermates lacking the Dermo1-Cre transgene were used as controls. A total of four conditional knockouts at E14.5 and their littermate controls were obtained from two different litters. We observed that in comparison to wildtype livers, E14.5 Pitx2Dermo1 livers were smaller and were filled with dilated blood vessels similar to β-CateninDermo1 livers (Figure 5A and B). The similarity in dilated vasculature of the Pitx2Dermo1 and that of the previously reported Pitx2-/- livers (15) indicates that it is specifically the mesenchymal expression of Pitx2 that is responsible for the vascular phenotype. Similar to β-cateninDermo1 mice, Pitx2Dermo1 livers also exhibit increased expression of DESMIN and αSMA (Figure 5C–F). Similar to β-cateninDermo1 mice, Pitx2Dermo1 mice demonstrate no obvious changes in epithelial differentiation in terms of ALBUMIN and Pan-CYTOKERATIN expression (data not shown). These data indicate that β-Catenin activation may signal through downstream Pitx2 in the mesenchyme during hepatogenesis as it does during lung organogenesis.
Figure 5.
Phenotype analysis of E14.5 Pitx2Dermo1. Hematoxylin and Eosin staining of Pitx2Dermo1 mice (B) and control littermates (A). Bar indicates 50 µm. Arrow indicates dilated sinusoids in the conditional knockout. Immunohistochemical staining for mesenchymal DESMIN (brown) (C, D) Bar denotes 25 µm. Immunofluorescent staining for mesenchymal αSMA (red) (E, F) show increased staining in conjunction with dilated blood vessels in Pitx2Dermo1 livers compared to control littermates (E, F arrow). Bar denotes 50 µm (C, E).
DISCUSSION
Although Wnt/β-Catenin signaling plays a role in adult HSC, its role in embryonic hepatic mesenchyme is less clear. In this study, we identified a role for β-Catenin in the differentiation of the liver mesenchymal cells using transgenic mice expressing Cre recombinase under the mesenchyme-specific promoter Dermo1 to specifically knock out β-Catenin in the mesenchymally-derived cells. Delanghe et al previously showed that conditional deletion of β-Catenin in the Dermo1-expressing mesenchyme during lung organogenesis blocks differentiation of mesenchymal progenitor cells into parabronchial smooth muscle cells and also inhibits vascular endothelial maturation (7). The authors also show that mesenchymal deletion of β-Catenin coincides leads to the loss of Pitx2 expression. Furthermore, the overall phenotype of β-CateninDermo1 mice also phenocopies Pitx2 null mice, further indicating a link between β-Catenin and Pitx2 signaling. Indeed, other studies show that the β-Catenin signaling pathway both induces Pitx2 expression and also directly activates PITX2 (6, 7).
We show that β-CateninDermo1 embryos develop smaller livers filled with peripherally-located, dilated and leaky sinusoids, identical to the phenotype previously described for Pitx2 null embryos (15, 16). Moreover, the histologic phenotype of the previously described complete knockout of Pitx2 is recapitulated with the mesenchymal deletion of Pitx2 in our study, indicating that expression of Pitx2, specifically in the mesenchyme in particular, is important during hepatogenesis. Additionally, increased expression of DESMIN, α-SMA, and Type I COLLAGEN, particularly in the region of aberrant hepatic vasculogenesis in both the conditional knockout of β-Catenin and Pitx2 indicates a similar defect in mesenchymal differentiation. We, thus, conclude that Pitx2 is downstream of β–Catenin activation in the mesenchyme during hepatogenesis.
We hypothesized that altered mesenchymal-β–Catenin signaling would affect hepatic vasculogenesis. Semela et al detailed a link between pericyte signaling and endothelium by showing in vitro that hepatic pericytes promote tubular formation of endothelial cells through Platelet-derived Growth Factor (PDGF) and Ephrin-B2 pathways (17). In this study, we show disrupted intrahepatic vasculogenesis with abrogation of mesenchymal β–Catenin signaling through the formation of dilated and leaky sinusoids. We further showed marked increases in expression of Flk1 in endothelial cells surround these aberrantly formed sinusoids. It is unclear, however, whether loss of mesenchymal-β–Catenin signaling directly affects either PDGF or Ephrin-B2 pathways.
There are numerous examples of mesenchymal-epithelial interactions. During lung organogenesis, parabronchial smooth muscle cells promote the branching morphogenesis of the adjacent epithelial lining through paracrine pathways (18). We previously showed that embryonic HSCs secrete Fibroblast Growth Factor-10 to promote proliferation and survival of adjacent hepatoblasts (13). In this study, we show that mesenchymal deletion of β-Catenin results in a significant reduction in Pan-CYTOKERATIN-positive cholangiocytes. Since hepatoblasts as well as both cholangiocytes and hepatocytes can still be identified in β-cateninDermo1 mice, it is clear that bilineage differentiation of hepatoblasts still occurs. One plausible explanation for the observation that cholangiocytes are diminished and disorganized is that mesenchymal inactivation of β–Catenin signaling results in a delay or defect in cholangiocyte differentiation (Figure 6). A number of mesenchymally-derived growth factors have been identified as affecting hepatoblast proliferation and differentiation. In particular, the Fibroblast Growth Factors secreted by hepatic stellate cells affect both proliferation and differentiation toward a cholangiocyte cell fate (13, 19). The mechanism involved here warrant further investigation.
Figure 6.
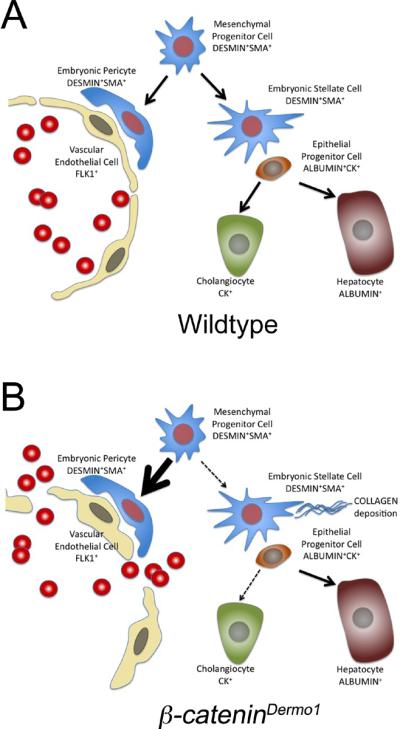
Schematic for hypothesis model. (A) Wildtype and (B) β-CateninDermo1 livers.
In summary, we show that mesenchymal β-Catenin activation is not essential for the formation of hepatic stellate cells and pericytes as both cell types are present with inactivation of β-Catenin. However, mesenchymal β-Catenin activation is necessary for proper mesenchymal differentiation as inactivation of β-Catenin in the mesenchyme leads to increased collagen deposition, as well as increased DESMIN and α-SMA expression near sinusoids and blood vessels. Furthermore, absence of β-Catenin in the mesenchyme during liver organogenesis leads leaky and dilated sinusoids characterized by altered expression of the endothelial marker Flk1. Similarities between the livers of conditional β-Catenin knockout mice and conditional Pitx2 knockout mice is consistent with previous findings that mesenchymal β-Catenin signals through Pitx2 in the lung mesenchyme suggesting that β-Catenin/Pitx2 signaling might be important for maintenance of hepatic mesenchymal progenitor cells. We speculate that absence of β-Catenin or Pitx2 in the hepatic mesenchyme might lead to a switch in mesenchymal differentiation towards a perivascular or a myofibroblast like phenotype thus affecting the blood vessel differentiation and formation as well as endoderm differentiation toward a cholangiocyte cell fate.
Acknowledgments
Financial support: This study was supported in part by the Research Center for Alcoholic Liver and Pancreatic Diseases (P50 AA11999) funded by the National Institute on Alcohol Abuse and Alcoholism (KSW). This work was also supported by National Institutes of Health grants K08 AA016290 (KSW) as well as grants from the Saban Research Institute Career Development (KSW).
Abbreviations
- CKO
Conditional Knock Out
- ECM
Extra-Cellular Matrix
- Pitx2
paired-like homeodomain 2
- HSC
Hepatic Stellate Cells
- FGF10
Fibroblast Growth Factor 10
- FGFR2b
Fibroblast Growth Factor Receptor 2 IIIb
- E11.5
embryonic day 11.5
- Hnf4α
Hepatocyte nuclear factor 4α
- DAPI
4',6-Diamidino-2-phenylindole
- β-galactoside
- SMA
Smooth muscle actin
- CK19
Cytokeratin 19
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kordes C, Sawitza I, Haussinger D. Canonical Wnt signaling maintains the quiescent stage of hepatic stellate cells. Biochem Biophys Res Commun. 2008;367:116–123. doi: 10.1016/j.bbrc.2007.12.085. [DOI] [PubMed] [Google Scholar]
- 3.Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, Tsukamoto H. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G39–49. doi: 10.1152/ajpgi.00263.2007. [DOI] [PubMed] [Google Scholar]
- 4.Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Jr., Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2009;49:998–1011. doi: 10.1002/hep.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan SA. Mechanisms controlling early development of the liver. Mech Dev. 2003;120:19–33. doi: 10.1016/s0925-4773(02)00328-3. [DOI] [PubMed] [Google Scholar]
- 6.De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, et al. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS ONE. 2008;3:e1516. doi: 10.1371/journal.pone.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai D, Liu W, Ma L, Dong F, Lu MF, Wang D, Verzi MP, et al. Pitx2 regulates cardiac left-right asymmetry by patterning second cardiac lineage-derived myocardium. Dev Biol. 2006;296:437–449. doi: 10.1016/j.ydbio.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, Stolz DB, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monga SP, Monga HK, Tan X, Mule K, Pediaditakis P, Michalopoulos GK. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124:202–216. doi: 10.1053/gast.2003.50000. [DOI] [PubMed] [Google Scholar]
- 10.Decaens T, Godard C, de Reynies A, Rickman DS, Tronche F, Couty JP, Perret C, et al. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology. 2008;47:247–258. doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- 11.Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- 12.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 13.Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, Veltmaat JM, et al. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology. 2007;46:1187–1197. doi: 10.1002/hep.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HZ, Degar BA, Rogoulina S, Resor C, Booth CJ, Sinning J, Gage PJ, et al. Hematopoiesis following disruption of the Pitx2 homeodomain gene. Exp Hematol. 2006;34:167–178. doi: 10.1016/j.exphem.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Kieusseian A, Chagraoui J, Kerdudo C, Mangeot PE, Gage PJ, Navarro N, Izac B, et al. Expression of Pitx2 in stromal cells is required for normal hematopoiesis. Blood. 2006;107:492–500. doi: 10.1182/blood-2005-02-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135:671–679. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 19.Sekhon SS, Tan X, Micsenyi A, Bowen WC, Monga SP. Fibroblast growth factor enriches the embryonic liver cultures for hepatic progenitors. Am J Pathol. 2004;164:2229–2240. doi: 10.1016/S0002-9440(10)63779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]