Abstract
Background
Left ventricular pacing (LVP) to induce cardiac memory (CM) in dogs results in a decreased transient outward K current (Ito) and reduced mRNA and protein of the Ito channel accessory subunit, KChIP2. The KChIP2 decrease is attributed to a decrease in its transcription factor CREB (cAMP response element binding protein).
Objective
To determine the mechanisms responsible for the CREB decrease that is initiated by LVP.
Methods
CM was quantified as T wave vector displacement in 18 LVP dogs. In 5 dogs, Ang II receptor blocker, saralasin, was infused before and during pacing. In 3 dogs, proteasomal inhibitor, lactacystin, was injected into the left anterior descending artery before LVP. Epicardial biopsies were taken before and after LVP. Neonatal rat cardiomyocytes (NRCM) were incubated with H2O2 (50 μmol/L) for 1h with or without lactacystin.
Results
LVP significantly displaced the T wave vector and was associated with increased lipid peroxidation and increased tissue Ang II levels. Saralasin prevented T vector displacement and lipid peroxidation. CREB was significantly decreased after 2h of LVP and was comparably decreased in H2O2–treated NRM. Lactacystin inhibited the CREB decrease in LVP dogs and H2O2 -treated NRM. LVP and H2O2 both induced CREB ubiquitination and the H2O2-induced CREB decrease was prevented by knocking down ubiquitin.
Conclusion
LVP initiates myocardial Ang II production and ROS synthesis leading to CREB ubiquitination and its proteasomal degradation. This sequence of events would explain the pacing-induced reduction in KChIP2, and contribute to altered repolarization and the T wave changes of cardiac memory.
Keywords: Cardiac Memory, cAMP response element binding protein, Angiotensin II, Reactive Oxygen Species, Ubiquitination and proteasomal degradation
1. Introduction
The T wave changes of cardiac memory are a long-recognized phenomenon (1). The persistent memory following long periods of ventricular pacing depends on a transcriptional process, the outcome of which is reduction of the transient outward current, Ito (2) and an altered ventricular repolarization gradient (3,4). Ito is carried via a channel whose pore-forming unit in dog and man is Kv4.3 (5). The accessory protein KChIP2 influences both the magnitude and kinetics of Ito (6).
The cyclic AMP response element binding protein, CREB, binds in the KChIP2 promoter region and appears necessary for baseline Ito expression (7). For example, in regions of hearts treated with CREB antisense (7), nuclear CREB is reduced and neither an action potential notch nor Ito is seen. During ventricular pacing of normal hearts for 1-2h or longer, nuclear CREB is also reduced and this reduction accompanies the T wave changes of long-term memory. However, the mechanism for CREB reduction has not been determined in the setting of cardiac pacing.
In this study we hypothesized that ubiquitination and proteasomal degradation are mechanisms whereby CREB reduction occurs, and that these processes are initiated by pacing-induced angiotensin II production and synthesis of reactive oxygen species (ROS). Because the CREB reduction commences within 2h of onset of pacing, we used a 2h pacing protocol to test our hypothesis.
2. Methods
Experiments were performed using protocols approved by Columbia University’s Institutional Animal Care and Use Committee and conform to the Guide for Care and Use of Laboratory Animals (NIH Publication NO. 85-23, revised 1996).
Intact Animal Studies
We used previously described procedures (8) to isoflurane-anesthetize 10 adult male mongrel dogs (22-25 kg, Chestnut Ridge Kennels, Chippensburg, PA) and implant bipolar pacing electrodes to left atrium (LA) and anterior wall of left ventricle (LV). After 45 min LA pacing, an epicardial reference biopsy was obtained 1-2 cm from the LV pacing electrode. We used a programmable stimulator (Bloom Associates, Reading PA) to AV sequentially pace (basic cycle length =400 ms, AV delay =50 ms), ensuring 100% ventricular capture for 2h. Additional LV epicardial biopsies were then taken 1-2 cm from electrode. All biopsies were immediately frozen in liquid nitrogen.
In five additional dogs, we infused saralasin, a competitive angiotensin II inhibitor(8,9), (0.5 μg/Kg/min, IV) starting 30 min before LV pacing and continuing throughout 2h of LV pacing. Biopsies were taken as above.
In three additional dogs, we catheterized the left femoral artery with 7 Fr introducer sheath, and engaged left coronary artery ostium with 6 Fr Amplatz Left 0.75 guiding catheter (Cordis, Warren NJ) under fluoroscopy. We infused the selective proteasomal inhibitor, lactacystin (10) (40 μmol/L, Boston Biochem, Cambridge, MA), via a transit microcatheter 2.8/2.5 Fr (Cordis, Warren NJ) with the tip just distal to first diagonal branch. LAD infusion began after 15 minutes of atrial pacing and continued for 30 minutes (total volume =15 mL). After the infusion a reference biopsy was taken and AV sequential pacing was initiated. Two hours later, epicardial biopsies were taken, one from the LAD distribution within 2 cm of the ventricular pacing electrode, and the other from remote site.
Western Blotting
Western blotting was performed as described previously (11) using CREB, ubiquitin (Cell Signaling Technology, Danvers, MA), PCNA (Abcam, Cambridge, MA) and Cyclophilin A (Upstate Biotech, Billerica, Massachusetts) antibodies.
Immunochemistry
Immunochemistry of sections from LV epicardial tissues before and after LV pacing or from neonatal rat ventricular cardiomyocytes (NRCM) treated with or without H2O2 was performed as previously described (12) using CREB antibody.
Angiotensin II Measurement
Tissue angiotensin II levels were measured by enzyme-linked immunoassay (Peninsula Labs, Torrance, CA) according to the manufacturer’s instructions using a modified extraction procedure (13,14). Angiotensin II was extracted from LV tissue that had been homogenized with a Polytron in 4 mol/L guanidine thiocyanate/1% trifluoroacetic acid (TFA) (250 mg of tissue/10 ml buffer) containing PMSF (0.1 mmol/L), aprotinin (1 ng/ml), leupeptin (1 ng/ml), pepstatin (0.1 μmol/L) and benzamidine (1μg/ml). The homogenate was clarified (10,000 × g ×20 min) before application by gravity flow to a pre-conditioned Sep-Pak C18 column (Varian; pre-washed with methanol 3 ml followed by 1 % TFA 10 ml). Then the column was washed 1 % TFA/1 % NaCl (5 ml) ×2, and MeOH/H2O/TFA (30/69/1, v/v; 2 ml) ×2. The sample was eluted with MeOH/H2O/TFA (80/19/1, v/v; in two portions 1 ml followed by 0.5 ml), evaporated to dryness and reconstituted in assay buffer. Recovery of [3H]angiotensin II was 75 ± 2 %.
Lipid Peroxidation Measurement
Lipid peroxidation was measured in left ventricular tissues using a colorimetric assay kit LPO-586 (BIOXYTECH, Portland, OR) that detects malondialdehyde and 4-hydroxyalkenal.
Intracellular ROS Measurements
NRCM were loaded with dihydroethidium (10 μmol/L), a non-fluorescent membrane-permeant probe that interacts with O2− to liberate membrane-impermeant ethidium cations that fluoresce upon intercalating with nuclear DNA, in presence of vehicle, angiotensin II (1 μmol/L) or H2O2 (50 μmol/L). Cell images were viewed every 3-5 min using a Zeiss LSM 510 NLO confocal microscope (excitation 470–490 nm, emission 510–550 nm).
Cardiomyocyte culture
NRCM were prepared as previously (11) with a density of 5 × 106 cells/100-mm dishes, and used in 4 different experiments: 1) Treatment with 50 μmol/L H2O2 for different time intervals followed by Western blotting of whole cell lysates for CREB and cyclophilin A; 2) Treatment with 50 μmol/L H2O2 for different time intervals followed by Western blotting of cytosolic and nuclear fractions for CREB and PCNA; 3) Treatment with angiotensin II for measurement of intracellular ROS; 4) Treatment with lactacystin (10 μmol/L) ×30 min and 50 μmol/L H2O2 ×1h. Whole cell lysates were then Western blotted for CREB;
Ubiquitin siRNA transfection
Ubiquitin siRNA knockdown was performed per manufacturer’s instructions. NRCM were transiently transfected with ubiquitin siRNA (Santa Cruz Biotech, Santa Cruz, CA). A scramble siRNA was used as a control. Silencing efficiency was evaluated by Western blotting using ubiquitin antibody (Stratagene, La Jolla, CA). Twenty four hours after transfection, cells were treated with H2O2 ×1h and whole cell lysates were Western blotted.
Statistical analysis
Results are expressed as means ± S.E.M. Comparisons between group means were made by student’s t-test except for angiotensin II, for which the Mann-Whitney U test was used. P<0.05 was considered significant.
3. Results
LV pacing-induced cardiac memory is associated with a post-translational CREB decrease in canine heart
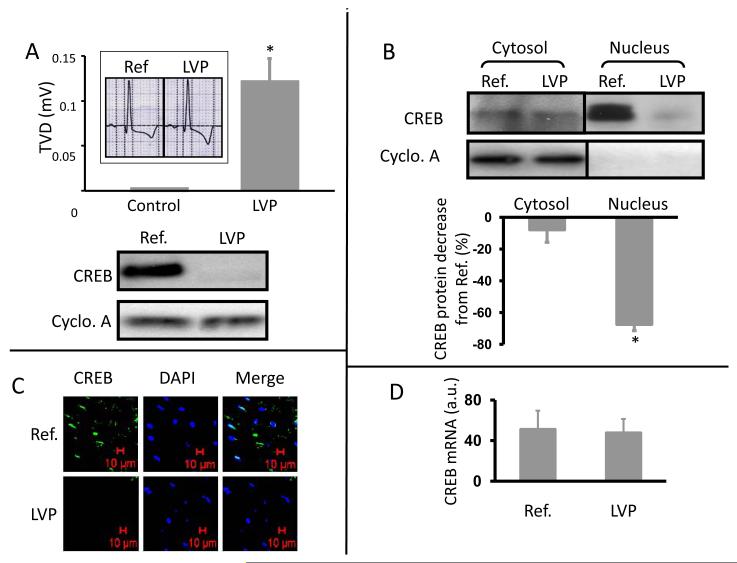
LV pacing led to a significant T-wave vector displacement (Figure 1A, upper) and a decrease of CREB in whole cell lysates of epicardial myocardium (Figure 1A, lower). These outcomes are comparable to the changes in T-wave configuration and CREB abundance previously described in cardiac memory (8). We now asked whether the LV pacing-induced CREB decrease results from translocation of CREB from nucleus to cytosol or from a decrease in the total CREB level in cells. Figure 1B shows that cytosolic CREB was not significantly altered by 2h ventricular pacing while there was a 65% decrease in nuclear CREB (P<0.05). Immunohistology (Figure 1C) shows that nuclear CREB is decreased after LV pacing without an increase in cytosolic distribution. These findings suggest LV pacing may induce 1) decreased CREB transcription and/or 2) a posttranslational CREB decrease. To test the first possibility, we performed quantitative real-time PCR using total RNA from biopsies before and after 2h of LV pacing. CREB transcription was unchanged by 2h of LV pacing (Figure 1D), suggesting the LV pacing-induced CREB decrease is posttranslational.
Figure 1. Two hours of LV pacing induce a significant T wave vector displacement and a significant decrease of CREB in canine heart.
Panel A (upper): Representative ECG (insert) and summary of T-wave vector displacement (TVD) before and after LV pacing (LVP). *, p<0.05 vs control, n=10. Panel A (lower): Whole cell lysate of cardiac biopsies from one dog before (Ref.) and after (LVP) 2 hours of LV pacing, Western blotted for CREB. Panel B: Representative images and summary data of Western blotting for CREB in cytosolic and nuclear fractions of cardiac biopsies before (Ref.) and after (LVP) 2 hours of LV pacing. Cyclophilin A (Cyclo. A) =loading control. *, p<0.05 vs Ref., n=8. Panel C: Images of immunohistochemical studies of biopsies before (Ref.) and after (LVP) 2 hours of LV pacing (n=3). DAPI =nuclear marker. Panel D: Summary data of real-time PCR for CREB before (Ref) and after (LVP) 2 hours of LV pacing (n=8). See text for discussion.
LV pacing increases myocardial angiotensin II and products of reactive oxygen species (ROS) synthesis
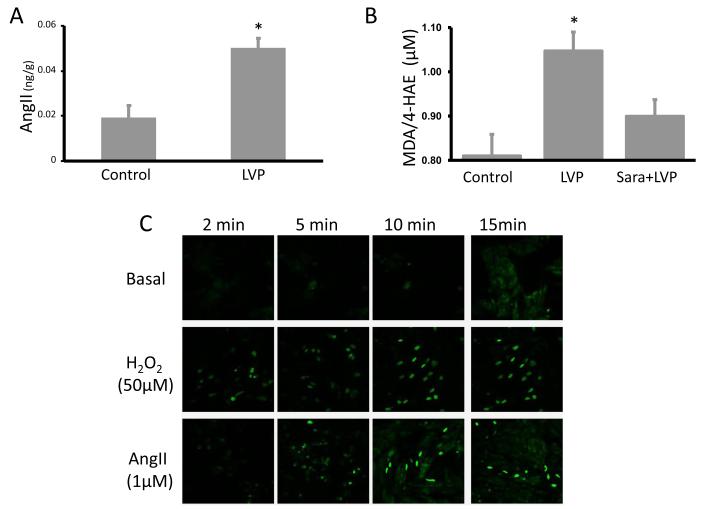
Given the role of angiotensin II in initiating short-term cardiac memory (8,15), we hypothesized that tissue angiotensin II levels might increase between onset of pacing and ECG recording of cardiac memory. Figure 2A shows that by 2h of LV pacing a significant increase of tissue angiotensin II has occurred. We did not determine the time course of this evolution within the 2h.
Figure 2. Induction of cardiac memory is associated with a significant increase in angiotensin II and lipid peroxidation in canine hearts.
Panel A: Angiotensin II (AngII) level measured in left ventricular tissue homogenates from control (n=6) and 2 hours of LV paced (LVP, n=6) dogs. Panel B: Malondialdehyde and 4-hydroxyalkenals measured in Control group (n=10), LV paced group (LVP, n=14) and LV-paced in the presence of Saralasin (Sara + LVP, n=4). Panel C: The ROS levels in neonatal rat cardiomyocytes were measured with a redox-sensitive indicator dihydroethidium (Dye, 5uM) before (Basal) and 2, 5, 10 and 15 min after onset of H2O2 (50 μmol/L) or angiotensin II treatment (1 μmol/L, n=3 *, p<0.05 vs control). Each line in each panel represents sequential measurements from one dish. See text for discussion.
Angiotensin II‘s effects on cardiomyocytes can be mediated in part by ROS (16) and our previous results demonstrate that low H2O2 concentrations (50 μmol/L) significantly decrease CREB abundance in NRCM in culture(11). Because lipid peroxidation is an outcome of ROS activity we measured myocardial lipid peroxidation during the LV pacing protocol with and without saralasin. Figure 2B shows that LV tissue homogenates from dogs with cardiac memory manifested significantly greater lipid peroxidation than control groups. Saralasin infusion during pacing prevented this increase in lipid peroxidation, indicating angiotensin II is a key factor in the LV pacing-induced increase in ROS in the heart.
We used dihydroethidium, a superoxide indicator, to further test the role of angiotensin II in the increased intracellular ROS production in NRCM. Figure 2C shows that treating cells with 1 μmol/L angiotensin II increased intracellular ROS abundance within 15 min, an effect similar to that of 50 μmol/L H2O2. These results support our hypotheses that LV pacing-induced increase in angiotensin II leads to generation of ROS and provides a rationale for considering a role for ROS as a mediator of the electrical remodeling characteristic of cardiac memory.
Oxidative stress induces comparable changes in CREB in a cardiac myocyte culture system as does cardiac memory in the canine model
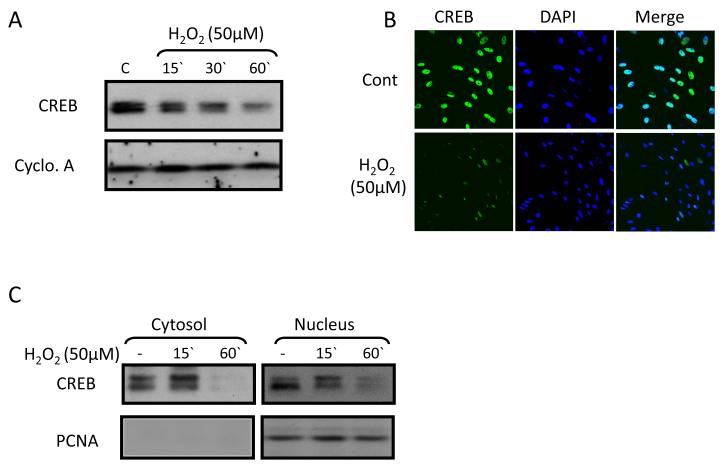
H2O2 (50 μmol/L) was the source of oxidative stress in NRCM cultures. Western blotting and immunocytochemistry show that H2O2 induced time-dependent decrease in total CREB (Figure 3 A,B) as we reported previously (11). The CREB reduction occurs in nucleus and cytosol (Figure 3C). This cytosolic CREB decrease is of interest in light of the results in the intact heart. Note that in the heart in situ there is a 10% CREB decrease in cytosol after 2h LVP (Figure 1B). That the cytosolic CREB decrease is non-significant in situ and is significant in culture (Figure 3) may reflect differences in the cell type (canine adult vs. rat neonate), the setting (in vivo vs. in culture) and/or the intervention (pacing vs. ROS). These questions remain to be resolved.
Figure 3. H2O2 treatment induces a decrease in CREB in neonatal rat cardiomyocytes.
Panels A and B: H2O2 (50 μmol/L, 1 hour) treatment induced a decrease of CREB in Western blot and immunocytochemistry studies (n=4). Cyclophilin A (Cyclo. A) = loading control. DAPI (blue) =nuclear marker. Panel C: Cytosolic and nuclear fractions of neonatal rat cardiomyocytes treated with or without H2O2 (50 μmol/L, 1 hour) were subjected to Western blotting for CREB (n=3). The quality of the fractionation was confirmed by Western blotting for PCNA, a nuclear marker which is seen in nuclear but not cytosolic fractions. See text for discussion.
LV pacing- and ROS-induced CREB decreases are prevented by proteasomal inhibition in vivo and in vitro
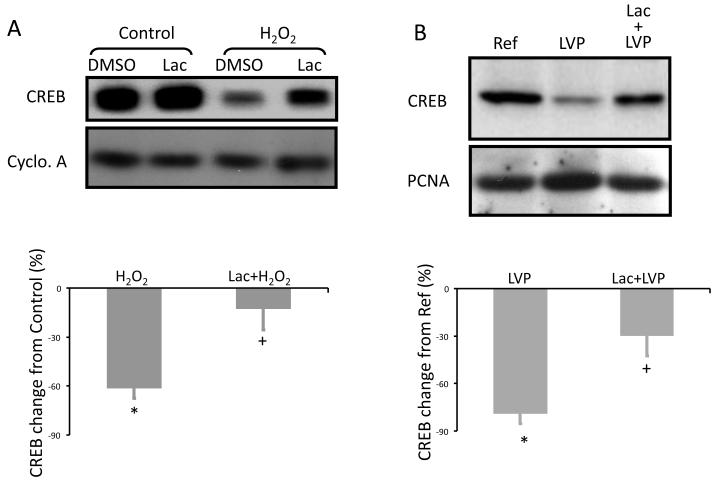
Previous studies have noted the role of the proteasome in hypoxia-induced CREB degradation (17). Here we tested the hypothesis that the LV pacing-induced CREB decrease and the ROS-induced CREB decrease result from its proteasomal degradation. Treating cultured NRCM with the proteasomal inhibitor lactacystin prior to H2O2 treatment prevented the H2O2-induced CREB decrease (Figure 4A). Pre-injecting lactacystin in canine heart prevented the LV pacing-induced CREB decrease in the LAD-fed area but not at a remote site (Figure 4B).
Figure 4. The proteasomal inhibitor lactacystin prevents LV pacing- induced CREB decreases in vivo and or H2O2-dependent CREB decreases in vitro.
(A) Neonatal rat cardiomyocytes were treated with DMSO (diluent) plus lactacystin (Lac, 10 μmol/L, 30 min) alone or is followed by H2O2 (50 μmol/L, 1 hour). Whole cell lysates were then Western blotted for CREB. Pre-treating cardiomyocytes with Lac prevented H2O2-induced CREB decrease (n=3). *, p<0.05 vs. Control; +, p<0.05 vs. H2O2 alone. (B) Lac was infused via the canine LAD artery before LV pacing. A reference biopsy was taken before LV pacing (Ref) and additional biopsies were taken after 2 hours of LV pacing from the LAD-fed area (Lac-LVP) and an LAD-non-fed area (LVP). Whole cell lysates of the biopsies were Western blotted for CREB (n=3). *, p<0.05 vs. Ref.; +, p<0.05 vs. LVP alone. See text for discussion.
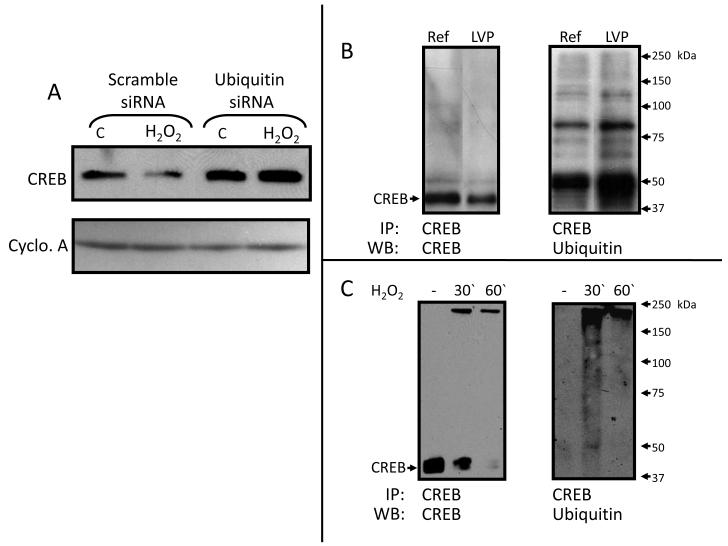
Ubiquitin plays a role in the ROS-induced CREB decrease
To investigate a possible role for ubiquitin in the ROS-induced CREB decrease we first used siRNA to knock down ubiquitin in NRCM. This prevented ROS-induced CREB reduction (Figure 5A). We then used immunoprecipitation with CREB antibody followed by Western blotting for ubiquitin to test whether there is an actual increase in CREB ubiquitination after LV pacing or H2O2 treatment. These experiments demonstrated an increase in CREB ubiquitination, evidenced by the increase of the smear above 43 kDa after LV pacing of canine heart for 1h (Figure 5B) or treating cardiomyocytes with H2O2 (50 μmol/L) for 30 min (Figure 5C).
Figure 5. Ubiquitin plays a role in LV pacing- or H2O2 -dependent CREB decrease.
Panel A: Neonatal rat cardiomyocytes were treated with Ubiquitin siRNA and a control group was treated with scramble siRNA. Whole cell lysates treated with or without H2O2 (50 μmol/L, 1 hour) were subjected to Western blotting for CREB (n=2). Panel B: CREB was immunoprecipitated from whole cell lysates of biopsies taken before (Ref) and after an hour of LV pacing (LVP). Precipitated proteins were subjected to Western blotting for CREB (left) and ubiquitin (right) (n=3). Panel C: CREB was immunoprecipitated from whole cell lysates of neonatal rat cardiomyocytes that were treated with or without H2O2 (50 μmol/L for indicated time intervals). Precipitates were subjected to Western blotting for CREB (left) and ubiquitin (right) (n=3). See text for discussion.
Discussion
Cardiac memory has been recognized for over 25 years (1). However, the molecular/biochemical determinants of the action potential changes that contribute to the T wave alterations in memory are only partly understood. This study we focused on just one type of action potential, that of ventricular epicardium; on one characteristic of that action potential, the phase 1 notch; and on one component of the ion channel contributing current to the notch, the accessory subunit KChIP2. Hence, our results are reduced to a universe delimited by the pacing-induced T wave change, CREB reduction during the first 1-2h of pacing and the events determining the fate of CREB in this setting.
We previously have reported the cyclic AMP response element near the promoter region of KChIP2, the parallel reductions in CREB and KChIP2 protein and the altered transmural repolarization gradient that follows chronic pacing and CREB reduction (7). We also have shown that the relationship between CREB and KChIP2 is obligatory: CREB antisense injection into myocardium during sinus rhythm and normal cardiac activation leads to reduced CREB and reduced transient outward potassium current, Ito, and action potential notch in the injected region. These findings led us to ask how pacing initiates CREB reduction and what the responsible mechanism is. Our results suggest the initiator is ventricular pacing-induced increases in angiotensin II and ROS, while the reduced CREB levels reflect degradation via an ubiquitin-proteasome pathway.
Bringing these elements together is the demonstration that angiotensin II promotes ROS synthesis (16) and that low H2O2 concentrations significantly decrease CREB abundance in cultured NRCM (15). This outcome is mediated by protein kinase C (PKC), extracellular signal-regulated kinase, ribosomal protein S6 kinase, and protein kinase D (11). We have now found that ventricular pacing of canine heart induces a significant increase in tissue angiotensin II levels as well as an increase in lipid peroxidation, a marker of increased ROS activity. Infusion of the angiotensin II receptor blocker, saralasin, inhibits this pacing-induced increase in lipid peroxidation. Also supporting the relationship between angiotensin II and lipid peroxidation is our observation that exposing NRCM to angiotensin II induces an increase in intracellular ROS. Other investigators have found that binding of angiotensin II to the AT-1 receptor activates NAD(P)H oxidase, a major source of ROS production in cells (16). In rat vascular smooth muscle cells angiotensin II activation of NAD(P)H oxidase is biphasic, showing an initial peak of H2O2 production at 30 seconds via PKC-mediated activation of NAD(P)H oxidase. The second phase of NAD(P)H oxidase activation is mediated by signaling pathways including p47phox, c-src, EGF receptor, PIP3 and GTPase Rac-1 (18). This second phase leads to increased H2O2 production at 30 minutes. Our current study is consistent with this cause and effect relationship of angiotensin II and ROS in cardiomyocytes. Together, these findings strongly support the hypothesis that the ventricular pacing-induced CREB decrease is initiated by an angiotensin II-ROS pathway.
In the setting of ventricular pacing, CREB reduction is localized: at reference sites distant from the pacing electrode CREB shows no change (8). This localized change in CREB probably contributes to a gradient of change in ion channel transcription. We have already mentioned the critical role of KChIP2 to the function of Ito. Yet recent data indicate it may have a role in other ion currents as well, including INa (19) and ICa,L (20). In earlier studies, we noted the alteration in ICa,L accompanying chronic pacing to induce cardiac memory (21), and recently Thomsen et al documented that KChIP2 contributes to ICa,L in mouse myocardium (20). Hence a role for CREB as a transcription factor important to the function of multiple channels is a distinct possibility. Studies in other tissues have shown that regulation of CREB depends in part on its ubiquitination and proteasomal degradation under stimuli such as hypoxia or high-glucose (17,22). However, this is the first report that this degradation process is responsible for the ventricular pacing-induced CREB decrease and the downstream ion channel changes attendant upon CREB levels (7).
The changes we report reflect those responsible for the onset of long term memory, but do not impact on short-term memory in heart. Regarding the latter, Doronin et al have shown that angiotensin II binding to a macromolecular complex consisting of the AT-1 receptor, Kv4.3 and KChIP2, results in internalization of the channel and loss of Ito (23). This process requires minutes for its occurrence [36] and - in epicardial myocytes - is unassociated with transcriptional changes in Kv4.3 (23,24). Hence, angiotensin II appears to play at least two roles in the evolution of cardiac memory. The first role is in short term memory where it appears essential for initiating the trafficking changes that occur. The second role is in initiating ROS synthesis and a subset of processes that result in altered CREB levels and the onset of long-term memory. Complicating the picture is that chronic administration of ACE inhibitors or AT-1 receptor blockers delay the onset of long-term memory but long-term expression with undiminished magnitude eventually does occur (21). This outcome suggests that processes additional to those described here contribute to the long-term expression of memory. Biophysical experiments have shown that not only Ito but also ICa,L and IKr are altered in long-term memory (2,21,25). These currents not only affect repolarization but their channel subunits are controlled by transcription factors other than CREB.
Clinical Implications
Cardiac pacing has been a commonplace intervention for over half a century (26). More recently the occurrence of pacing-induced remodeling has come to attention (27,28), and one of the most benign expressions of remodeling is cardiac memory. The initiation of cardiac memory incorporates altered activation (29), altered stretch (30) and angiotensin II production. Given the role of angiotensin II to effect a variety of physiologic and pathologic outcomes, including ROS synthesis, it may be that the benign T wave change of cardiac memory is an initial marker in a pacing-induced sequence that can eventually result in cardiac failure (31). If this is the case, then monitoring of cardiac memory evolution may provide early clues regarding pathology yet to come.
Limitations
We previously showed that CREB levels are reduced within 2h of onset of LV pacing and that 3 weeks later there are reduced binding of nuclear proteins to CRE and reduced CRE binding activity in the promoter region of Kv4.3 (8). We chose the 2h time point for the present study as a convenience for considering the initiation of CREB reduction and the beginnings of downstream transcriptional changes. As alluded to above, the outcomes here should not be extrapolated to explain all the changes seen in long-term memory. These await discovery.
Another limitation is that direct application of angiotensin II to cultured cardiomyocytes does not change CREB levels (unpublished data). This might be because of differences in the cells studied (NRCM in culture versus adult canine myocytes in situ), because cardiomyocytes in culture often respond differently to outside stimuli than cardiomyocytes in vivo (32), and/or because angiotensin II does not result in sufficient accumulation of superoxide in cultured cardiomyocytes to induce a CREB decrease. This possibility is supported by our previous finding that H2O2 concentrations lower than 50 μmol/L do not reduce CREB (11).
Finally, while our experiments have added an additional layer to our understanding of of repolarization and its modulation, we recognize that what we now understand is only a fraction of the control mechanisms determining the repolarization process.
Acknowledgement
This work was supported by USPHS/NHLBI grant HL-67101.
Sources of Funding This work was supported by USPHS/NHLBI grant HL-67101.
Glossary of terms
- cAMP response element binding protein (CREB)
CREB is a transcription factor which binds to the DNA sequence at the promoter region of certain proteins and regulates their transcription.
- Saralasin
an octapeptide analog of AngII with amino acids 1 and 8 are replaced with sarcosine and alanine, respectively; a nonselective AngII receptor antagonist.
- Ubiquitination
covalent binding of one or more ubiquitins (a small protein) to a protein to be proteasomally degraded.
- Proteasomal degradation
a mechanism by which cells regulate the quantities of certain proteins and degrade misfolded proteins.
- Proteasome
an intracellular organelle, is a large protein complex that recognizes ubiquitinated (unneeded or damaged) proteins and degrades them.
- Lactacystin
a small molecule which binds to the catalytic subunit of proteasome with high specificity and inhibits its activity..
- Lipid Peroxidation
the oxidative degradation of lipids.
Footnotes
Disclosures None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Confilict of interest: none declared
References
- 1.Rosenbaum MB, Blanco HH, Elizari MV, Lazzari JO, Davidenko JM. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50:213–22. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, McKinnon D, Dixon JE, et al. Transient outward current, Ito1, is altered in cardiac memory. Circulation. 1999;99:1898–905. doi: 10.1161/01.cir.99.14.1898. [DOI] [PubMed] [Google Scholar]
- 3.Coronel R, Opthof T, Plotnikov AN, et al. Long-term cardiac memory in canine heart is associated with the evolution of a transmural repolarization gradient. Cardiovasc Res. 2007;74:416–25. doi: 10.1016/j.cardiores.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Jeyaraj D, Wilson LD, Zhong J, et al. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation. 2007;115:3145–55. doi: 10.1161/CIRCULATIONAHA.107.688317. [DOI] [PubMed] [Google Scholar]
- 5.Kong W, Po S, Yamagishi T, Ashen MD, Stetten G, Tomaselli GF. Isolation and characterization of the human gene encoding Ito: further diversity by alternative mRNA splicing. Am J Physiol. 1998;275:H1963–70. doi: 10.1152/ajpheart.1998.275.6.H1963. [DOI] [PubMed] [Google Scholar]
- 6.Rosati B, Pan Z, Lypen S, et al. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001;533:119–25. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patberg KW, Obreztchikova MN, Giardina SF, et al. The cAMP response element binding protein modulates expression of the transient outward current: implications for cardiac memory. Cardiovasc Res. 2005;68:259–67. doi: 10.1016/j.cardiores.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Patberg KW, Plotnikov AN, Quamina A, et al. Cardiac memory is associated with decreased levels of the transcriptional factor CREB modulated by angiotensin II and calcium. Circ Res. 2003;93:472–8. doi: 10.1161/01.RES.0000088785.24381.2F. [DOI] [PubMed] [Google Scholar]
- 9.Castellion AW, Fulton RW. Preclinical pharmacology of saralasin. Kidney Int Suppl. 1979:S11–9. [PubMed] [Google Scholar]
- 10.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 11.Ozgen N, Guo J, Gertsberg Z, Danilo P, Jr., Rosen MR, Steinberg SF. Reactive oxygen species decrease cAMP response element binding protein expression in cardiomyocytes via a protein kinase D1-dependent mechanism that does not require Ser133 phosphorylation. Mol Pharmacol. 2009;76:896–902. doi: 10.1124/mol.109.056473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozgen N, Dun W, Sosunov EA, et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res. 2007;75:758–69. doi: 10.1016/j.cardiores.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell DJ, Kladis A, Duncan AM. Nephrectomy, converting enzyme inhibition, and angiotensin peptides. Hypertension. 1993;22:513–22. doi: 10.1161/01.hyp.22.4.513. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence AC, Evin G, Kladis A, Campbell DJ. An alternative strategy for the radioimmunoassay of angiotensin peptides using amino-terminal-directed antisera: measurement of eight angiotensin peptides in human plasma. J Hypertens. 1990;8:715–24. doi: 10.1097/00004872-199008000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Ricard P, Danilo P, Jr., Cohen IS, Burkhoff D, Rosen MR. A role for the renin-angiotensin system in the evolution of cardiac memory. J Cardiovasc Electrophysiol. 1999;10:545–51. doi: 10.1111/j.1540-8167.1999.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 17.Taylor CT, Furuta GT, Synnestvedt K, Colgan SP. Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia. Proc Natl Acad Sci U S A. 2000;97:12091–6. doi: 10.1073/pnas.220211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Touyz RM, Chen X, Tabet F, et al. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–13. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 19.Deschenes I, Armoundas AA, Jones SP, Tomaselli GF. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J Mol Cell Cardiol. 2008;45:336–46. doi: 10.1016/j.yjmcc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomsen MB, Wang C, Ozgen N, Wang HG, Rosen MR, Pitt GS. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ Res. 2009;104:1382–9. doi: 10.1161/CIRCRESAHA.109.196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plotnikov AN, Yu H, Geller JC, et al. Role of L-type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107:2844–9. doi: 10.1161/01.CIR.0000068376.88600.41. [DOI] [PubMed] [Google Scholar]
- 22.Costes S, Vandewalle B, Tourrel-Cuzin C, et al. Degradation of cAMP-responsive element-binding protein by the ubiquitin-proteasome pathway contributes to glucotoxicity in beta-cells and human pancreatic islets. Diabetes. 2009;58:1105–15. doi: 10.2337/db08-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doronin SV, Potapova IA, Lu Z, Cohen IS. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. J Biol Chem. 2004;279:48231–7. doi: 10.1074/jbc.M405789200. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Gao J, Wang H, et al. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–8. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 25.Obreztchikova MN, Patberg KW, Plotnikov AN, et al. I(Kr) contributes to the altered ventricular repolarization that determines long-term cardiac memory. Cardiovasc Res. 2006;71:88–96. doi: 10.1016/j.cardiores.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Siddons AH. Long-term artificial cardiac pacing: experience in adults with heart block. Ann R Coll Surg Engl. 1963;32:22–41. [PMC free article] [PubMed] [Google Scholar]
- 27.Ozgen N, Rosen MR. Cardiac memory: a work in progress. Heart Rhythm. 2009;6:564–70. doi: 10.1016/j.hrthm.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji Y, Zicha S, Qi XY, Kodama I, Nattel S. Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia: discrete arrhythmogenic consequences related to differential delayed-rectifier changes. Circulation. 2006;113:345–55. doi: 10.1161/CIRCULATIONAHA.105.552968. [DOI] [PubMed] [Google Scholar]
- 29.Geller JC, Rosen MR. Persistent T-wave changes after alteration of the ventricular activation sequence. New insights into cellular mechanisms of ‘cardiac memory’. Circulation. 1993;88:1811–9. doi: 10.1161/01.cir.88.4.1811. [DOI] [PubMed] [Google Scholar]
- 30.Sosunov EA, Anyukhovsky EP, Rosen MR. Altered ventricular stretch contributes to initiation of cardiac memory. Heart Rhythm. 2007 doi: 10.1016/j.hrthm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Kaab S, Nuss HB, Chiamvimonvat N, et al. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–73. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- 32.Mortensen A, Sorensen IK, Wilde C, et al. Biological models for phytochemical research: from cell to human organism. Br J Nutr. 2008;99(E Suppl 1):ES118–26. doi: 10.1017/S0007114508965806. [DOI] [PubMed] [Google Scholar]