Abstract
Purpose
To enhance real-time magnetic resonance guided catheter navigation by overlaying colorized multiphase MR angiography (MRA) and cholangiopancreatography (MRCP) roadmaps for anatomic context.
Materials and Methods
Time-resolved MRA and respiratory-gated MRCP were acquired prior to real-time imaging in a pig model. MRA and MRCP data were loaded into a custom real-time MRI reconstruction and visualization workstation where they were displayed as Maximum Intensity Projections (MIPs) in distinct colors. The MIPs were rendered in three dimensions (3D) together with real-time multi-slice imaging data using alpha blending. Interactive rotation allowed different views of the combined data.
Results
Fused display of the previously acquired MIP angiography data with real-time imaging added anatomical context during endovascular interventions in swine. The use of multiple MIPs rendered in different colors facilitated differentiation of vascular structures, improving visual feedback during device navigation.
Conclusion
Interventional real-time MR imaging may be enhanced by combining with previously acquired multiphase angiograms. Rendered as 3D MIPs together with 2D slice data, this technique provided useful anatomical context that enhanced MRI guided interventional applications.
Keywords: Image-guided interventions, real-time MRI, 3D roadmaps, MRA, MRCP, maximum intensity projection (MIP)
INTRODUCTION
Radiation-free imaging, short, wide bore magnets, high-performance gradient systems, phased-array receivers (1) and arrival of MR compatible instruments, such as needles, active guidewires and catheters make real-time MRI guided interventions compelling (2-9). In addition to these hardware developments, real-time pulse sequence improvements and new imaging techniques have been developed specifically for real-time MRI guided interventional procedures (10-13).
Efficient real-time pulse sequences allow faster acquisition of k-space data and improve imaging frame rate, which is necessary for real-time feedback during the intervention. Frame rate could be further improved using parallel MRI (1), which employs multiple receiver coils to produce images from partial sets of k-space data. Coil sensitivity profiles are used to either suppress the image domain artifacts (10,11) or to estimate the missing k-space samples (12,13). These processes require significant additional computational load to provide the necessary reconstruction speed with reasonable image quality. Recent technological advances have allowed parallel MRI reconstruction in real-time on a single memory space multi-processor workstation (10, 13).
Interventional procedures have inherent risks; a small deviation from the desired device trajectory may result in complication. Active (i.e. MR signal receiving) guidewires (2-4) facilitate tracking of device position in real-time. Additionally, unique color assignment to each active device channel dramatically improves the device tracking during interventions (5,14). However, real-time pulse sequences are usually restricted to single or multiple 2D slices for higher frame rate. Often the need arises to see structures outside of the imaging plane, such as different portions of or different vascular trees. Contrast-enhanced MR angiography offers excellent depiction of such structures but cannot be performed in real-time and repeated at will. Nevertheless, it may be beneficial to import these data sets into a real-time MR imaging environment and display them in conjunction with live 2D images. In this work, we demonstrate an implementation of this ‘real-time fusion’ capability and present our initial experimental results of its use in vivo.
The proposed method expands on previous work in that angiograms of different phases or vascular structures may be displayed in an integrated fashion in 3D with real-time imaging planes. Our custom MR data reconstruction and visualization environment dynamically renders multiple colorized 3D Maximum Intensity Projections (MIPs) in real-time using previously acquired magnetic resonance angiography (MRA) and magnetic resonance cholangiopancreatography (MRCP) data, and displays them with multiple slice real-time MR images using alpha-blending in a 3D rendering. This implementation also colorizes image data from active invasive devices for blending with surface coil images. At the end, positions of multiple active invasive devices can be visualized in the additional context of multiple vascular structures, thereby combining advantages of real-time and non-real-time imaging.
MATERIALS AND METHODS
MR Data Acquisition
Animal protocols were approved by the institutional animal care and use committee in accordance with NIH guidelines. Pigs underwent ketamine and/or telezol/xylazine induction followed by endotracheal intubation, mechanical ventilation, and 1-2% isoflurane. Femoral artery and venous access used percutaneous techniques.
Time-resolved MRA of the great vessels and respiratory-gated MRCP images of a pig were acquired prior to the real-time MRI guided procedure, using a short, wide bore Siemens Espree 1.5 T MRI scanner (Siemens Medical Solutions, Erlangen, Germany). During a breath hold, the MRA was acquired with a 3D FLASH sequence with the following parameters: TR = 2.8 ms, TE = 1.2 ms, flip angle = 24°, FOV = 327×327 mm, and voxel size = 2.3 mm × 1.3 mm × 2 mm. For MRCP imaging, a respiratory navigator gated fast spin echo sequence (HASTE) was used with the following parameters: TR = 2773 ms, TE = 620 ms, flip angle = 180°, FOV = 380×380 mm, and voxel size = 1.0 mm × 1.0 mm × 1.5 mm. Real-time Steady-State Free-Precession (SSFP) images were acquired with TR = 3 ms, flip angle = 45°, FOV = 340×255 mm with acquisition matrix of 192×108. 18 receiver coils were used during real-time imaging, and HTGRAPPA (13) was employed as a parallel imaging technique with acceleration rates of 2 and 3. Image reconstruction and display were performed on a general purpose Linux workstation with two dual-core AMD Opteron 2216 processors and with a NVIDIA Quadro FX graphics card.
Generating 3D MIPs
Previously acquired multi-phase MRA and MRCP data were manually cropped using Siemens Syngo 3D tools (Leonardo, Siemens) to show only the structures of interest. DICOM images were then exported and loaded onto the real-time MRI workstation at the beginning of the intervention. 3D colorized MIPs were rendered in real-time using SGI’s Volumizer library (http://www.sgi.com/products/software/volumizer). These MIPs were alpha-blended with real-time reconstructed multi-slice images and colorized active guidewire images and rendered together in 3D during the intervention.
This capability was added to a interactive real-time MRI scan control and display system that includes a volume rendered display of multiple 2D slices (14). The 3D display window is interactive; the MIPs are dynamically re-rendered from its new vantage point as the user rotates the scene. When rendering multiple angiographic data sets, each one can be individually turned on or off. They could also be grouped for joint visualization. With these capabilities, our improved real-time visualization interface can show only what is needed at a particular stage of a procedure to avoid extra clutter in the rendering (see Fig. 1).
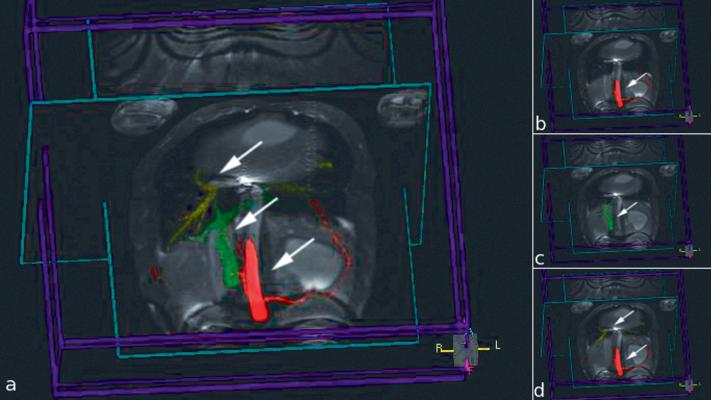
Figure 1.
Image captures from the 3D rendering for use in MRI-guided TIPS experiment showing multiple colorized MIPs rendered with two orthogonal real-time image slices. Each MIP is indicated by an arrow. a) All MIPs are visualized. b) Only hepatic artery (red) is shown c) Only portal vein (green) is shown. d) Hepatic artery (red) and hepatic vein (yellow) are grouped and shown.
Experiments
The 3D colorized MRA/MRCP roadmaps capability was deployed during two experimental MRI-guided real-time interventions: (i) pulmonary thrombectomy, and (ii) transjugular intrahepatic portosystemic shunt (TIPS).
Pulmonary Thrombectomy
Venous thromboembolism occurring in the pulmonary arteries is a common cause of death (15). Massive pulmonary thromboembolism causes hemodynamic compromise or collapse, and mechanical treatment options are limited (15). We are developing real-time MRI techniques to relieve vascular obstruction and rescue patients suffering massive pulmonary thromboembolism. Distinction between different venous structures and visualization of the boundaries with respect to 3D anatomical locations are crucial during this procedure. Combining multiple angiographic data sets with real-time imaging may improve visual feedback and efficacy of this treatment.
TIPS
Percutaneous transjugular intrahepatic portosystemic shunting (16) relieves portal venous congestion due to cirrhosis. In this procedure, a catheter traverses hepatic parenchyma to connect a portal vein to a hepatic vein. Absent suitable image guidance, the procedure requires multiple “blind” attempts to traverse the liver from a vein using a sharp needle, and risks life-threatening non-target puncture as well as significant patient discomfort. TIPS has several critical stages: initial venous access, angioplasty, and stent-graft insertion. During the procedure, it is important to distinguish different venous and arterial and biliary structures and their 3D relationships which can be difficult under X-ray fluoroscopy. The biliary tree and hepatic artery must be avoided while placing a shunt between the portal and hepatic veins. This procedure may also benefit from complimentary display of multiple angiographic data sets while guiding invasive devices under real-time MRI. TIPS is typically performed using X-ray fluoroscopic guidance but has been reported in humans using a combined “double-doughnut” MRI and X-ray system (17).
In both experiments, angiographic data are acquired prior to the invasive portion of the experiment for the generation of related 3D MIPs.
RESULTS
Pulmonary Thrombectomy
Figure 2 shows the angiography MIPs of pulmonary veins (in yellow) and pulmonary arteries (in light blue) with colored active catheter image (arrow) blended with the real-time multi-slice images. The active catheter contains separate receiver channels: a small coil at the tip (red) and a longer coil down the shaft (green). The pulmonary arteries and veins were clearly visible and differentiable during the experiment using separately colored MIPs. Pulmonary artery branches were accessed via femoral veins under the guidance of 3D MRA MIPs.
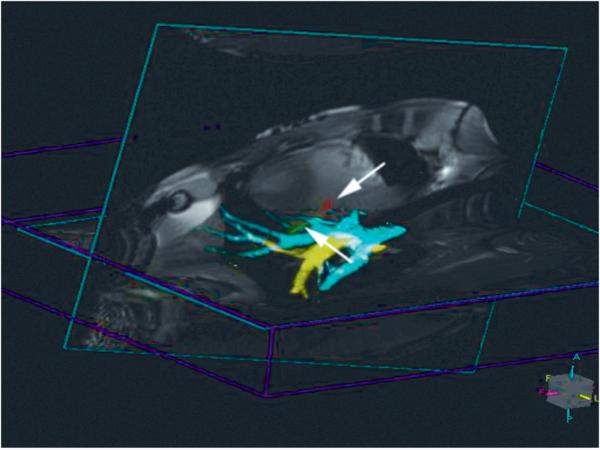
Figure 2.
Colorized 3D MIP of pulmonary veins (yellow) and arteries (light blue) shown in conjunction with colorized active device (marked with arrows: tip shown in red, shaft shown in green) and two real-time image slices.
TIPS
Figure 3 shows dynamically rendered 3D jugular vein MIP (in yellow) overlaid on real-time multi-slice images. In Figure 4, dynamically updated 3D MIPs of biliary tree (in yellow), active guidewire (in blue), hepatic artery (in red), hepatic vein (in yellow), and portal vein (in green) are displayed in conjunction with real-time reconstructed multi-slice images. These MIPs guided the physician during the entire TIPS procedure; (i) jugular access, (ii) safe traversal to the liver avoiding biliary tree and hepatic artery, and (iii) percutaneously connecting hepatic and portal vein.
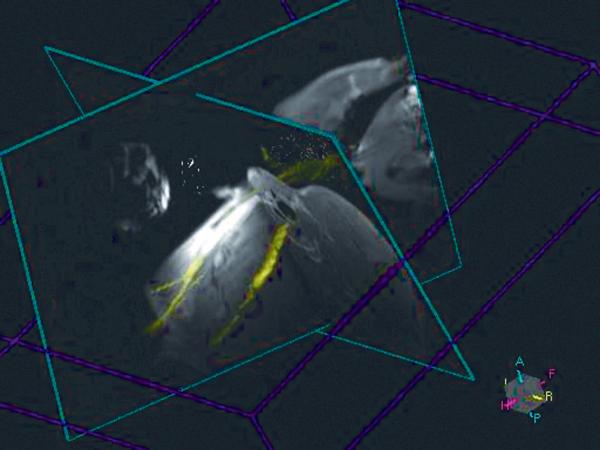
Figure 3.
3D colored MIP of jugular vein displayed with real-time images for guidance during TIPS procedure for jugular access.
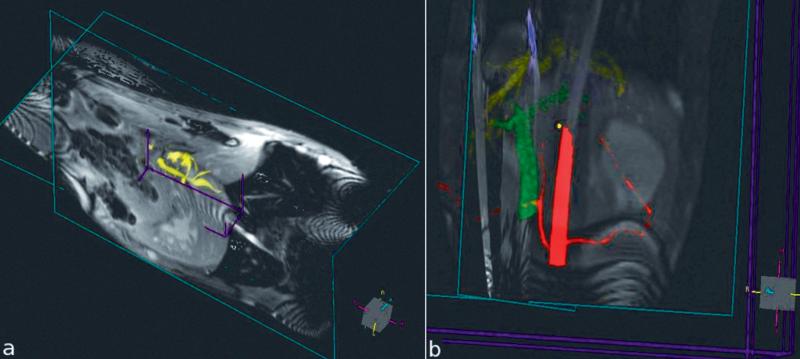
Figure 4.
3D colored dynamically updated MIPs displayed in conjunction with real-time multi-slice images during MRI guided TIPS procedure. a) Biliary tree in yellow. b) Active guidewire in blue, hepatic artery in red, hepatic vein in yellow, and portal vein in green.
DISCUSSION
Real-time MRI guided vascular interventional procedures generally acquire a limited number of 2D slices to achieve adequate temporal resolution. Previously acquired, non-real-time data might enhance these limited data by providing additional anatomical context to improve visual feedback during interventions.
We hereby demonstrate a system for dynamically updating 3D MIPs of different vascular structures in real-time using previously acquired time-resolved MRA and MRCP data. These MIPs are colorized and visualized in an interactive manner with multi-slice real-time images and colorized active devices. Partial image transparency is achieved using alpha blending and indicates multiple distinct data sets together for improved image guidance. All angiographic datasets are loaded when real-time imaging is started. The user can choose which datasets are displayed for a particular portion of the procedure.
Other groups have proposed the use of previously acquired images as roadmaps in X-ray/CT and XMR systems (18-20), and this was commercially implemented by Siemens (Syngo iPilot tool, Siemens Medical Solutions, Germany) and by Philips Medical Systems (Koninklijke Philips Electronics N.V.). Even though some of these methods permit using MRI data to create roadmaps, the procedures still require X-ray/CT imaging, and thus patient radiation exposure. Our method not only avoids radiation but avoids registration issues, since the roadmaps and real-time images share the same coordinate system and they are acquired without moving the patient in the scanner.
We showed preliminary results of our newly developed 3D multiple roadmap utility which may become an important tool in certain MRI guided interventional procedures. Usage of the technique was illustrated in early phase experimental pulmonary thrombectomy and TIPS procedures. Since these procedures are not yet stabilized, there is no gold standard allowing us to report improvements in procedure time or success rate when multiple MR angiographic roadmaps are used in conjunction with real-time MR imaging. Once the active device design and procedural protocols mature to a certain extent, the benefits of the proposed technique will be quantified during follow-up experiments.
Roadmap data have inherent limitations. They may become outdated. They are static and may misalign with “live” images because of cardiac, respiratory, or other patient motion. Breath-holding, ECG-gating, more sophisticated motion correction techniques, or periodic intraprocedural updates, might enhance roadmap utility.
In conclusion, integrated interactive colorized MIP display of multiple angiographic data sets allows 3D visualization of vascular structures during real-time MRI guided procedures. This additional information could facilitate navigation of invasive devices through and between complex vascular spaces, and has the potential of improving patient safety, reducing procedure time and increasing efficacy. This capability could enhance several MRI-guided applications, such as TIPS, angioplasty, biopsy, stent placement, and electrophysiology procedures. Previously acquired Computerized Tomography (CT) data, or similar volumetric data from any other imaging modality (e.g. 3D ultrasound), could also be combined as colorized 3D MIPs for deployment during real-time MRI guided interventions after an initial registration process.
ACKNOWLEDGEMENTS
We thank Victor Wright and Kathy Lucas for assistance in experiments. NHLBI and Siemens Medical Systems have a Cooperative Research and Development Agreement that includes real-time MRI scan control and display.
Grant Support: Supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health, USA, Z01-HL005062-07 (RJL)
REFERENCES
- 1.Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16:192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- 2.Ocali O, Atalar E. Intravascular magnetic resonance imaging using a loopless catheter antenna. Magn Reson Med. 1997;37:112–118. doi: 10.1002/mrm.1910370116. [DOI] [PubMed] [Google Scholar]
- 3.Kocaturk O, Saikus CE, Guttman MA, et al. Whole shaft visibility and mechanical performance for active MR catheters using copper-nitinol braided polymer tubes. J Cardiovasc Magn Reson. 2009;11:29. doi: 10.1186/1532-429X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kocaturk O, Kim AH, Saikus CE, et al. Active two-channel 0.035″ guidewire for interventional cardiovascular MRI. J Magn Reson Imag. 2009;30:461–5. doi: 10.1002/jmri.21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lederman RJ. Cardiovascular interventional magnetic resonance imaging. Circulation. 2005;112:3009–3017. doi: 10.1161/CIRCULATIONAHA.104.531368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolandaivelu A, Lardo AC, Halperin H. Cardiovascular magnetic resonance guided electrophysiology studies. J Cardiovasc Magn Reson. 2009;11:21. doi: 10.1186/1532-429X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hushek SG, Martin AJ, Steckner M, Bosak E, Debbins J, Kucharzyk W. MR systems for MRI-guided interventions. J Magn Reson Imag. 2008;27:253–266. doi: 10.1002/jmri.21269. [DOI] [PubMed] [Google Scholar]
- 8.Ratnayaka K, Faranesh AZ, Guttman MA, Kocaturk O, Saikus CE, Lederman RJ. Interventional cardiovascular magnetic resonance: still tantalizing. J Cardiovasc Magn Reson. 2008;10:62. doi: 10.1186/1532-429X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JA, Starr PA, Larson PS. Software requirements for interventional MR in restorative and functional neurosurgery. Neurosurg Clin N Am. 2009;20:179–86. doi: 10.1016/j.nec.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Guttman MA, Kelmman P, Dick AJ, Lederman RJ, McVeigh ER. Real-time accelerated interactive MRI with adaptive TSENSE and UNFOLD. Magn Reson Med. 2003;50:315–321. doi: 10.1002/mrm.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellman P, Epstein FH, McVeigh ER. Adaptive sensitivity encoding incorporating temporal filtering (TSENSE) Magn Reson Med. 2001;45:846–852. doi: 10.1002/mrm.1113. [DOI] [PubMed] [Google Scholar]
- 12.Breuer FA, Kellman P, Griswold MA, Jakob PM. Dynamic autocalibrated parallel imaging using temporal grappa (TGRAPPA) Magn Reson Med. 2005;53:981–985. doi: 10.1002/mrm.20430. [DOI] [PubMed] [Google Scholar]
- 13.Saybasili H, Kellman P, Griswold MA, Derbyshire JA, Guttman MA. HTGRAPPA: Real-time B1-weighted image domain TGRAPPA reconstruction. Magn Reson Med. 2009;61:1425–1433. doi: 10.1002/mrm.21922. [DOI] [PubMed] [Google Scholar]
- 14.Guttman MA, Ozturk C, Raval AN, et al. Interventional cardiovascular procedures guided by real-time imaging: an interactive interface using multiple slices, adaptive projection modes and live 3D renderings. J Magn Reson Imaging. 2007;26:1429–1435. doi: 10.1002/jmri.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelage J, El Hajjam M, Lagrange C, et al. Pulmonary artery interventions: an overview. Radiographics. 2005;25:1653–1667. doi: 10.1148/rg.256055516. [DOI] [PubMed] [Google Scholar]
- 16.Roessle M, Haag K, Ochs A, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. The New England Journal of Medicine. 1994;330:165–171. doi: 10.1056/NEJM199401203300303. [DOI] [PubMed] [Google Scholar]
- 17.Kee ST, Ganguly A, Daniel BL, et al. MR-guided transjugular intrahepatic portosystemic shunt creation with use of a hybrid radiography/MR system. J Vasc Interv Radiol. 2005;16:227–234. doi: 10.1097/01.RVI.0000143766.08029.6E. [DOI] [PubMed] [Google Scholar]
- 18.Soederman M, Babic D, Homan R, Andersson T. 3D roadmap in neuroangiography: technique and clinical interest. Neuroradiology. 2005;47:735–740. doi: 10.1007/s00234-005-1417-1. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez LF, Silva R, Ozturk C, et al. Technology preview: x-ray fused with magnetic resonance during invasive cardiovascular procedures. Catheter Cardiovasc Interv. 2007;70:773–82. doi: 10.1002/ccd.21352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruijters D, Babic D, Homan R, Mielekamp P, ter Haar Romeny BM, Suetens P. Real-time integration of 3-D multimodality data in interventional neuroangiography. J Electron Imag. 2009;18:033014. [Google Scholar]