TO THE EDITOR
Laminin-332 is a large extracellular basement membrane zone protein that is critical for dermal–epidermal cohesion. The laminin-332 heterotrimer (α3/β3/γ2) is believed to link keratinocytes with the basement membrane zone by simultaneously binding epidermal integrin receptors via the C-terminal globular domain of its α3 chain, and type VII collagen through domains on the short arm of its β3 chain (Chen et al., 1997; Rousselle et al., 1997). Antibody-induced inhibition of laminin-332’s integrin-binding domains produces extensive skin blistering (Rousselle et al., 1991; Kirtschig et al., 1995); however, the in vivo significance of the laminin β3 short arm in dermal–epidermal cohesion has not been tested directly.
To further study the laminin β3 short arm in dermal–epidermal cohesion, we produced two deletion mutants of the laminin β3 cDNA. One (ΔVI) contained a deletion of domain VI (LN) but left the type VII collagen-binding domain intact. The other contained a deletion of the entire β3 short arm I comprising domains VI and V-III (LN, LE, LF), which includes the collagen-binding region (ΔVI-III). Mutant and wild-type (WT) β3 chain cDNAs were retrovirally expressed in laminin β3 null junctional epidermolysis bullosa (JEB Null) primary keratinocytes (Waterman et al., 2007).
ΔVI, ΔVI-III, and WT keratinocytes were cultured atop the devitalized dermis as described (Ortiz-Urda et al., 2003) and the resulting skin equivalents were examined 3–4 weeks after grafting to severe combined immunodeficiency mice (Figure 1a). WT grafts showed no clinical or microscopic blistering, where as ΔVI and ΔVI-III grafts showed significant subepidermal blistering and erosions. Laminin-332 null grafts uniformly failed, with no evidence of overlying human epidermis (not shown).
Figure 1. Clinical, histological, and immunofluorescent microscopic analysis of primary human xenografts expressing engineered laminin mutants.
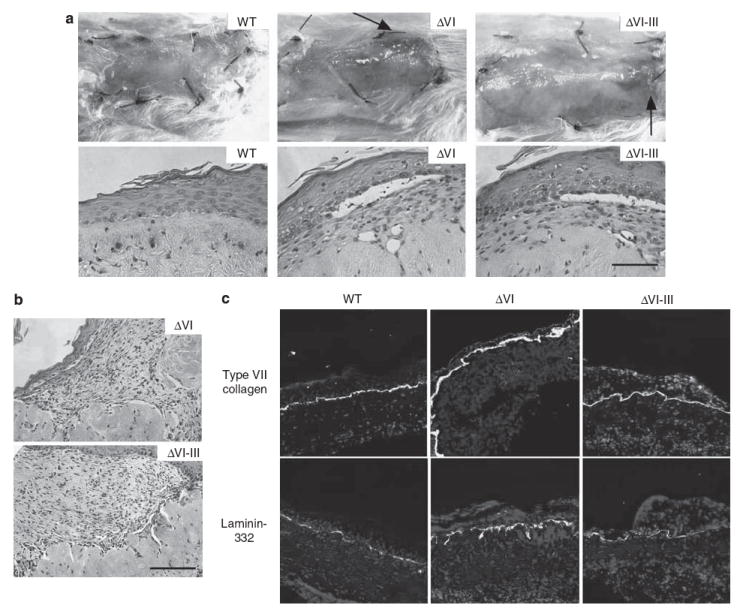
(a) Clinical (upper panels) and microscopic (lower panels) appearance of human skin equivalents 3 weeks after xenografting to severe combined immunodeficiency (SCID) mice. Grafts expressing wild-type (WT) and mutant (ΔVI, ΔVI–III) laminin-332 are shown as indicated (the arrows depict foci of granulation tissue in mutant grafts). (b) Microscopic appearance of granulation tissue arising in mutant laminin xenografts. (c) Laminin-332 and type VII collagen expression in xenografted skin as shown by immunofluorescent microscopy using the indicated antibodies. Bar = 100 μm.
Increased granulation tissue has long been recognized as a characteristic feature of patients with lethal and non-lethal JEB (Marinkovich and Bauer, 2008). Interestingly, ΔVI and ΔVI-III grafts also showed prominent granulation tissue (Figure 1b), confirming an association of granulation tissue with laminin-332 defects. Non-blistered areas of mutant grafts showed a linear deposition of laminin-332 and type VII collagen at the dermal–epidermal junction, similar to WT cells (Figure 1c), suggesting that blistering/granulation tissues in mutant skin equivalents werenot due to reduced laminin-332 or type VII collagen deposition at the dermal–epidermal junction. As these studies were performed in immunodeficient mice, it is likely that laminin-332 defects promoted the development of granulation tissue without the involvement of the memory immune system.
Scanning and transmission electron microscopic analysis (Figure 2a) of JEB Null keratinocytes before grafting showed marked rounding and poor association with the culture surface, compared with the flat and well-attached WT keratinocytes. Both ΔVI and ΔVI-III cells showed degrees of flattening and culture substrate apposition intermediate between JEB Null and WT cells.
Figure 2. Electron microscopic analysis of primary keratinocytes expressing engineered laminin mutants.
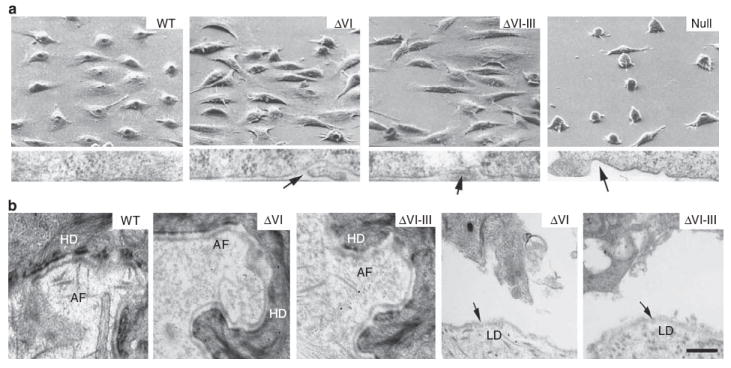
(a) Scanning electron micrscopy (upper panels) and transmission electron microscopy of the cell-culture surface interface (lower panels) of primary human junctional epidermolysis bullosa (JEB) laminin β3 null keratinocytes, retrovirally transduced with WT mutant ΔVI, mutant ΔVI-III, or vector control (Null) cDNA. Arrows in the lower panels point to areas of poor apposition of keratinocyte plasma membrane with the culture surface. (b) Transmission electron microscopic analysis of the indicated intact (left three panels) or separated (right two panels) graft areas. Arrows point to the lamina densa (LM). Abbreviations: AF, anchoring fibril; HD, hemidesmosome. Bar = 1 μm.
Mutant ΔVI and ΔVI-III skin analyzed by transmission electron microscopy 4 weeks after grafting (Figure 2b) showed rudimentary hemidesmosomes (HDs) compared with WT grafts. Anchoring fibrils in ΔVI grafts were hypoplastic compared with WT control, whereas little or no anchoring fibrils were seen in ΔVI-III skin equivalents. Separation of both ΔVI and ΔVI-III grafts occurred in the lamina lucida, with a continuous lamina densa (LD, arrows) on the dermal side of the split.
ΔVI-III skin grafts were noted to contain both HD defects associated with lack of domain VI and type VII collagen-binding defects associated with lack of laminin β3 domain V-III. However, despite these dual defects, mutant grafts only showed lamina lucida, as opposed to sub-lamina densa separation. This suggests that the HD defect in ΔVI-III grafts was more significant and more easily disrupted by external forces than the lack of type VII collagen/laminin-332 binding associated with the absence of laminin β3 domain V-III. As type VII collagen is known to bind to other molecules, including collagen IV in the lamina densa (Burgeson et al., 1985) and collagen I in the papillary dermis (Villone et al., 2008), it is possible that these interactions can partially stabilize type VII collagen and provide a cohesive force even in the absence of laminin-332.
We previously noted that the ΔVI mutant showed slightly decreased laminin γ2 chain processing (Waterman et al., 2007); however, as inhibition of γ2 chain processing increases rather than decreases adhesion (Gagnoux-Palacios et al., 2001), γ2 chain processing is not the likely reason for the decreased ΔVI cell adhesion. The laminin β3 short arm lies distant from the integrin-binding α3 domain on the laminin-332 molecule, and, as would be expected, deletion of β3 domain VI does not alter the binding of either α3β1 or α6β4 integrins (Waterman et al., 2007), ruling out integrin binding as a possible cause of deficient ΔVI adhesion.
In summary, this study introduces a previously unreported animal model for the study of the function of individual domains of basement membrane zone molecules in the process of dermal–epidermal cohesion. This approach of mutational structure–function analysis has the potential to be used with other null recessive epidermolysis bullosa subtypes (for example, LAMC2, LAMA3, COL7A1, COL17A1, ITGB4, and ITGA6), which would further extend our understanding of dermal–epidermal cohesion.
We have also shown here that the adhesion deficiencies associated with deletion of laminin β3 domain IV are associated with defects of HD formation. In vitro, normal keratinocytes show central clustering of HD components, distinct from peripheral focal adhesions (Jones et al., 1998). HD components in ΔVI cells, on the other hand, localized to the cell periphery, in close association with focal adhesions (Waterman et al., 2007). In the current in vivo study, HDs in ΔVI skin grafts were poorly formed, compared with control grafts. In addition, ΔVI skin grafts separated at the intra-lamina lucida level, exactly where a defect of laminin-332/HD association would occur. Thus, in total, these findings suggest that, through its promotion of HD assembly, domain VI of the laminin β3 chain has a significant role in promoting dermal–epidermal cohesion in the skin.
Also noteworthy is the inter-relationship between granulation tissue formation and laminin-332 defects seen in our studies. This confirms the clinical observations made in JEB patients with laminin-332 defects. Thus, future applications of this model may also help us to understand better how epidermal cells communicate with their associated stromal and innate immune cells to modulate wound healing.
Acknowledgments
We gratefully acknowledge the assistance of the Stanford University Department of Surgical Pathology. The US Veterans Affairs Office of Research and Development, and the National Institutes of Health grant R01 AR047223 supported this work.
Abbreviations
- HD
hemidesmosome
- JEB Null
null junctional epidermolysis bullosa
- WT
wild type
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
References
- Burgeson RE, Morris NP, Murray LW, et al. The structure of type VII collagen. Ann N Y Acad Sci. 1985;460:47–57. doi: 10.1111/j.1749-6632.1985.tb51156.x. [DOI] [PubMed] [Google Scholar]
- Chen M, Marinkovich M, Veis A, et al. Interactions of the amino-terminal noncollagenous (NC1) domain of type VII collagen with extracellular matrix components. A potential role in epidermal-dermal adherence in human skin. J Biol Chem. 1997;272:14516–22. doi: 10.1074/jbc.272.23.14516. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios L, Allegra M, Spirito F, et al. The short arm of the laminin gamma2 chain plays a pivotal role in the incorporation of laminin 5 into the extracellular matrix and in cell adhesion. J Cell Biol. 2001;153:835–50. doi: 10.1083/jcb.153.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–94. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kirtschig G, Marinkovich MP, Burgeson RE, et al. Anti-basement membrane autoantibodies in patients with anti-epiligrin cicatricial pemphigoid bind the a subunit of laminin 5. J Invest Dermatol. 1995;105:543–8. doi: 10.1111/1523-1747.ep12323431. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Bauer EA. Inherited epidermolysis bullosa. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. 7. New York: McGraw-Hill; 2008. pp. 505–16. [Google Scholar]
- Ortiz-Urda S, Lin Q, Green CL, et al. Injection of genetically engineered fibroblasts corrects regenerated human epidermolysis bullosa skin tissue. J Clin Invest. 2003;111:251–5. doi: 10.1172/JCI17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Keene DR, Ruggiero F, et al. Laminin 5 binds the NC-1 domain of type VII collagen. J Cell Biol. 1997;138:719–28. doi: 10.1083/jcb.138.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, et al. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–76. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villone D, Fritsch A, Koch M, et al. Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J Biol Chem. 2008;283:24506–13. doi: 10.1074/jbc.M802415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman EA, Sakai N, Nguyen NT, et al. A laminin-collagen complex drives human epidermal carcinogenesis through phosphoinositol- 3-kinase activation. Cancer Res. 2007;67:4264–70. doi: 10.1158/0008-5472.CAN-06-4141. [DOI] [PubMed] [Google Scholar]


