Abstract
Successful management of epithelial skin cancers with imiquimod 5% cream (Aldara®), an immunomodulatory agent, led to speculation that it may promote an immune response against melanoma. Studies, mostly case reports, have assessed the value of imiquimod as a topical treatment for dermal melanoma metastases that prove difficult to manage surgically. The precise value of imiquimod, however, in treatment of dermal and subcutaneous metastases remains unclear. A case at our institution elucidates histopathologically that subcutaneous metastases may progress despite excellent treatment of superficial dermis in the same location. In preparation for a clinical trial using imiquimod to treat patients with dermal melanoma metastases, we have treated several patients off protocol. We present a case report in which the observed changes are documented photographically and histologically. The patient experienced dramatic improvement in the locally treated dermis with concurrent regional treatment failure in the subcutaneous space. Our experience supports growing evidence that imiquimod for some provides an effective option for dermal disease. The unique histological documentation we provide regarding the differential effectiveness of imiquimod in treating various tissue components may help guide future investigations regarding optimal clinical application of imiquimod therapy for melanoma metastases.
Imiquimod 5% cream (Aldara®), a topical immunomodulatory agent, is currently an accepted therapy for HPV-induced genital warts, actinic keratoses, superficial basal cell carcinoma and squamous cell carcinoma.1,2 Successful management of epithelial skin cancers with imiquimod led clinicians to speculate that this agent may promote an immune response against melanoma cells.3,4 This hypothesis has been explored as a treatment for lentigo maligna. Multiple case studies and four open-label studies suggest that imiquimod may eradicate lentigo maligna in some cases.5-8 Surgery remains the recommended treatment for lentigo maligna, but topical imiquimod therapy may be appropriate in cases where comorbid conditions or anatomic constraints limit the appropriateness of surgery.
Furthermore, several studies have assessed the potential value of imiquimod as a topical treatment for dermal metastases of melanoma that are not easily managed with surgery. Most of these are case reports, which demonstrate a therapeutic effect of imiquimod on dermal melanoma metastases1,4,9,10. Some of these reports included other therapeutic modalities, which complicate a pure assessment of the efficacy of imiquimod. Also, a clinical trial of 10 patients treated with a combination of topical imiquimod with intralesional interleukin-2 for combinations of dermal and subcutaneous metastases led to regression of some lesions, but failed to induce complete regressions of treated lesions in any of the 10 patients. Thus, the value of imiquimod in topical treatment of dermal and subcutaneous metastases remains unclear.
In preparation for beginning a clinical trial at our institution for use of imiquimod in patients with dermal melanoma metastases, we have treated several patients off protocol. Results in one patient are of particular interest because she had a complete regression of extensive superficial dermal metastases but had coincident progression of alarge subcutaneous metastases underlying the treated skin. These changes have been documented photographically and histologically. We present this case because it highlights, on one hand, the dramatic effectiveness locally in treated dermis, but also points out concurrent regional failure in the subcutaneous space. Considering this observation, in the context of prior limited reports, may be helpful in guiding future investigations of the optimal clinical application of imiquimod therapy for melanoma metastases.
Case
At our institution, we have treated six patients with metastatic melanoma using imiquimod. Patients are instructed to apply the cream daily for 5 days each week with adjustments made for frequency of application based on individual response and toxicities. Three of the patients did not use the treatment as prescribed, one patient had progressive dermal and subcutaneous disease in spite of diligent use, and two have had significant regression of dermal metastases. The patient who is the focus of this report was our best responder. This patient is a 58-year-old white female who presented, in January 2003, with a malignant melanoma (superficial spreading type; Clark level III; Breslow thickness 0.75 mm; with regression) on her right anterior scalp, which was treated with a wide local excision followed by a flap reconstruction from the posterior lateral scalp. In March 2004, several bluish-black macules and papules were noted on the right anterior forehead in a 4 × 5 cm region located anterior to the previous excision. These were treated with a wide local excision followed by split-thickness skin graft repair. On histopathologic review, multifocal areas of metastatic melanoma were found, extending from the junctional zone into the dermis and in some cases reaching the reticular dermis. There was a 2.1 × 1.2 cm area of coalescent dark black to blue macules and papules near the center of the skin resection surface. In addition, several macules extended radially toward the margins of the specimen, especially at the anterior and right lateral borders. Nonetheless, margins of resection were clear.
In August 2004, the patient enrolled in a melanoma peptide vaccine trial (UVA MEL-43) for adjuvant therapy. Unfortunately, in September 2004, she again presented with metastatic lesions on her scalp, requiring withdrawal from the trial. These recurrences were followed clinically from September 2004 to May 2005, without treatment. Surgical resection was not performed, as the disease process demonstrated an ability to recur despite adequate surgical resection. However, the lesions progressed; thus, additional therapies were used (May 2005–May 2006), including high dose interleukin-2, Temozolamide, and another melanoma peptide vaccine trial (UVA MEL-41). Unfortunately, the patient progressed regionally at the scalp (Fig. 1A,B).
Fig. 1.
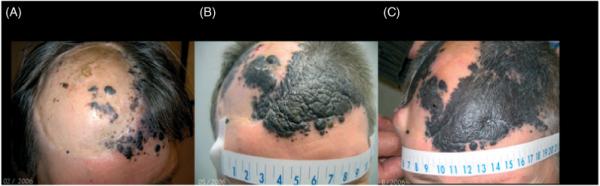
This figure demonstrates the patient’s scalp melanoma that initially progressed in size between February 2006 (Fig. 1A) and May 2006 (Fig. 1B). During this time, various treatments had been attempted, including high dose interleukin-2, Temozolamide, and a melanoma peptide vaccine trial (UVA MEL-41). With the initiation of Aldara between May 2006 and November 2006, clinical evidence of regression is evident. The regression is evident in that the multipapular melanoma shown in Fig. 1B was replaced by the flatter black discoloration of Fig. 1C. Comparison of Fig. 1C to 1B also shows arrest of radial extension of the dermal disease, which had been rapid prior to May 2006.
In May 2006, the patient was offered imiquimod 5% cream topical treatments to her scalp with the understanding that this was not a standard indicated use of imiquimod. She applied imiquimod five evenings a week to the pigmented area, washed it off each morning and documented her treatment regimen. After 6 months of treatment, her multipapular dermal scalp melanoma metastases changed, and were replaced by a flat black discoloration where the tumor had been (Fig. 1C). Clinically, these changes were consistent with regression of the melanoma, with residual pigmentation. During this 6 month interval on imiquimod, there was no significant lateral extension of the melanoma. She did, however, develop a palpable nodule 16 × 12 mm deep to the dermis on the right forehead, clinically suspicious for a new subcutaneous metastasis (Fig. 1C). The subcutaneous location of the low T1, bright T2 enhancing nodule is especially apparent on MRI (Fig. 2).
Fig. 2.
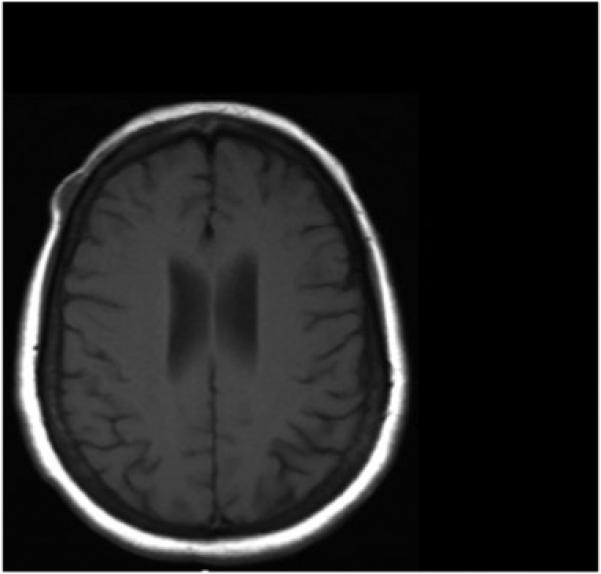
This figure shows an MRI image demonstrating the subcutaneous location of the low T1, bright T2 metastatic melanoma nodule.
The subcutaneous right forehead nodule was excised, along with some of the overlying skin, which contained the residual dermal pigmentation. These were evaluated histologically. The subcutaneous nodule was a large tumor nodule of melanoma, with focal loss of cohesion. The adjacent and overlying skin contained tumoral melanosis and inflammation, without any residual melanoma evident in the skin. We cannot completely exclude the possibility that some foci of viable melanoma may have persisted in the dermis, as only a representative area was biopsied, however, the rapid radial growth of the dermal melanoma, shown in the comparison of Fig. 1B to Fig. 1A, did not continue once imiquimod was applied, (Fig. 1C), and there was notable flattening of the dermal melanoma throughout the lesion, which is typical for regressions of dark pigmented melanoma. A skin biopsy taken prior to imiquimod therapy is shown in Fig. 3A,B, which may be compared to a tissue section taken after imiquimod therapy (Fig. 3C,D,E). After imiquimod therapy, melanoma cells had been replaced by pigment-laden macrophages and fibrosis, though the dyscohesive melanocytes in the deep subcutis suggest residual tumor. Figure 4 further shows a scanning magnification of subcutaneous metastasis located in the deep subcutis that persisted in spite of treatment.
Fig. 3.
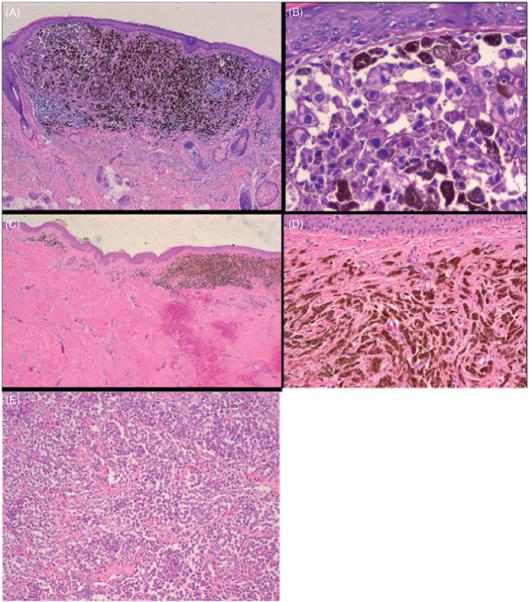
(A) Total magnification (40×, H&E stained) shows a skin biopsy taken prior to beginning Aldara treatment. (B) The atypical melanocytes are readily apparent in nests, (total magnification 200×, H&E stained). (C) (total magnification 20×, H&E stained) shows significant fibrosis and a focus of tumoral melanosis, or residual pigment within macrophages in a treated focus, in the more superficial portion of the treated dermis, consistent with Aldara therapy. (D) (total magnification 100×, H&E stained) shows in higher magnification tumoral melanosis also indicating treatment-associated changes whereas (E) (total magnification 100×, H&E stained) shows dyscohesive melanocytes in the deep subcutis, suggesting persistent subcutaneous tumor.
Fig. 4.
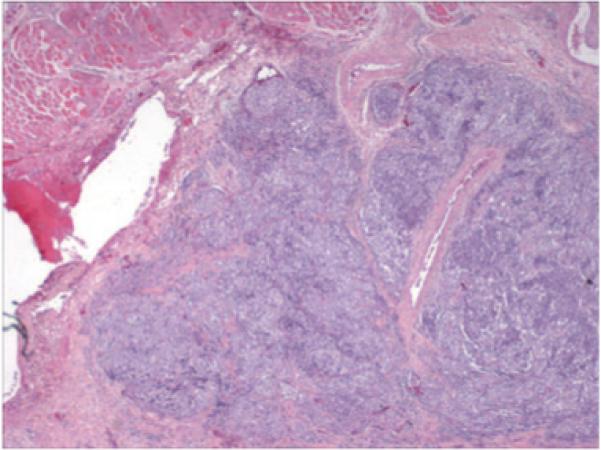
Total magnification (20×, H&E stained) reveals a low power (scanning) magnification of metastatic melanoma in the deep subcutis. Note that the metastasis lies deep to the layer of skeletal muscle.
Discussion
Our work demonstrates histopathologically-evident regression of dermal melanoma, with persistence of subcutaneous disease, after treatment with imiquimod. Dermal melanoma metastases certainly present a management challenge, as widespread cutaneous metastases do not lend themselves well to surgical management. Even excision in well defined disease does not prevent all recurrent local-regional dermal metastases.9 Other treatment alternatives with varying degrees of success and toxicity have included radiotherapy, isolated limb perfusion, regional hyperthermia, cryotherapy, electrosurgery and curettage, CO2 laser ablation, intralesional chemotherapy, systemic chemotherapy and systemic immunotherapy.10-14 There is currently a need for a standardized safe and effective treatment option for dermal melanoma metastases.
Imiquimod is an immunomodulating, heterocyclic imidazoquinoline amide, with apparent antitumoral properties. It stimulates Toll-like receptor 7 on dendritic cells, macrophages, and monocytes that in turn trigger proinflammatory cytokine release and enhancement of cell-mediated immunity. Cytokines induced include interferon-alpha, tumor necrosis factor alpha, IL-6 and IL-8. Haematoxylin and eosin stains reveal intense lymphocytic infiltration of the tumor, and in one report following treatment, no viable tumor cells were evident after 10 weeks of imiquimod therapy.2 Additionally, imiquimod might theoretically protect against tumor metastases by enhancing not only migration of epidermal Langerhans cells to local lymph nodes but also tumor antigen presentation to T lymphocytes. Optimally, this may stimulate a specific antitumor immune response with systemic impact; however, little evidence exists in support of this claim.
A phase I/II clinical trial suggests that imiquimod effectively treats many superficial dermal and subcutaneous metastases. Thirteen patients were recruited, of whom ten completed 12 weeks or more of treatment. Group one consisted of five patients with dermal and subcutaneous disease, whereas group two included five patients with isolated subcutaneous disease.15 Imiquimod combined with intralesional IL-2 induced regressions of some lesions in patients with mixed dermal and subcutaneous disease. The impression of the authors was that imiquimod alone controlled dermal disease, but subcutaneous disease was better treated in combination with intralesional IL-2. In only one patient of those 10, there was complete regression of all dermal metastases. The present report is consistent with the impression that dermal metastases may be controlled with topical imiquimod therapy, but that deeper subcutaneous disease may not be controlled.
Our experience lends support to the growing evidence that imiquimod provides an effective option for dermal disease in a subset of patients, especially those who may not be candidates for surgery. Regardless, its use alone may not be sufficient in combating subcutaneous disease. The present report includes histopathology of lesions before and after imiquimod treatment that correlates well with clinical findings. Our experience and a review of the literature support the need for clinical trials that are large enough to define the response rate of dermal metastases to topical imiquimod therapy. It appears likely that imiquimod alone may be insufficient for treatment of subcutaneous metastases, but this premise deserves more rigorous testing, likely with testing of combination therapies for those deeper lesions.
References
- 1.Woodmansee C, Pillow J, et al. The role of topical immune response modifiers in skin cancer. Drugs. 2006;66:1657. doi: 10.2165/00003495-200666130-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ugurel S, Wagner A, et al. Topical imiquimod eradicates skin metastases of malignant melanoma but fails to prevent rapid lymphogenous metastatic spread. Br J Dermatol. 2002;147:621. doi: 10.1046/j.1365-2133.2002.488811.x. [DOI] [PubMed] [Google Scholar]
- 3.Bong AB, Bonnekoh B, et al. Imiquimod, a topical immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology. 2002;205:135. doi: 10.1159/000063904. [DOI] [PubMed] [Google Scholar]
- 4.Steinmann A, Funk JO, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555. JO. [PubMed] [Google Scholar]
- 5.Cotter MA, McKenna JK, et al. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147. doi: 10.1111/j.1524-4725.2007.34031.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajpar SF, Marsden JR. Imiquimod in the treatment of lentigo maligna. Br J Dermatol. 2006;155:653. doi: 10.1111/j.1365-2133.2006.07476.x. [DOI] [PubMed] [Google Scholar]
- 7.Spenny ML, Walford J, et al. Lentigo maligna (melanoma in situ) treated with imiquimod cream 5%: 12 case reports. Cutis. 2007;79:149. [PubMed] [Google Scholar]
- 8.du Plessis PJ. Lentigo maligna successfully treated with imiquimod. S Afr J Surg. 2007;45:72. [PubMed] [Google Scholar]
- 9.Vereecken P, Mathieu A, et al. Management of cutaneous locoregional recurrences of melanoma: a new therapeutic perspective with imiquimod. Dermatology. 2003;206:279. doi: 10.1159/000068901. [DOI] [PubMed] [Google Scholar]
- 10.Wolf IH, Smolle J, et al. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol. 2003;139:273. doi: 10.1001/archderm.139.3.273. [DOI] [PubMed] [Google Scholar]
- 11.Fraker DL, Alexander HR, et al. Palliation of regional symptoms of advanced extremity melanoma by isolated limb perfusion with melphalan and high-dose tumor necrosis factor. Cancer J Sci Am. 1995;1:122. [PubMed] [Google Scholar]
- 12.Fraker DL, Alexander HR, et al. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: results of a tumor necrosis factor dose-escalation study. J Clin Oncol. 1996;14:479. doi: 10.1200/JCO.1996.14.2.479. [DOI] [PubMed] [Google Scholar]
- 13.Feldman AL, Alexander HR, Jr., et al. Management of extremity recurrences after complete responses to isolated limb perfusion in patients with melanoma. Ann Surg Oncol. 1999;6:562. doi: 10.1007/s10434-999-0562-x. [DOI] [PubMed] [Google Scholar]
- 14.Utikal J, Zimpfer A, et al. Complete remission of multiple satellite and in-transit melanoma metastases after sequential treatment with isolated limb perfusion and topical imiquimod. Br J Dermatol. 2006;155:488. doi: 10.1111/j.1365-2133.2006.07333.x. [DOI] [PubMed] [Google Scholar]
- 15.Green DS, Bodman-Smith MD, et al. Phase I/II study of topical imiquimod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol. 2007;156:337. doi: 10.1111/j.1365-2133.2006.07664.x. [DOI] [PubMed] [Google Scholar]


