Abstract
Despite its common use in nonmyeloablative preparative regimens, the pharmacokinetics of fludarabine are poorly characterized in hematopoietic cell transplantation (HCT) recipients and exposure-response relationships remain undefined. Our objective of this study was to evaluate the association between plasma F-ara-A exposure, the systemically circulating moiety of fludarabine, and engraftment, acute graft vs host disease (GVHD), treatment-related mortality (TRM) and survival after HCT. The preparative regimen consisted of cyclophosphamide 50 mg/kg/day i.v. day −6; plus fludarabine 30-40 mg/m2/day i.v. on days −6 to −2 and TBI 200 cGy on day −1. F-ara-A pharmacokinetics were performed with the first dose of fludarabine in 87 adult patients. Median (range) F-ara-A AUC (0-∞) was 5.0 ug*hr/mL (2.0-11.0), clearance 15.3 L/hour (6.2-36.6), Cmin 55 ng/mL (17-166), and concentration on dayzero 16.0 ng/mL (0.1-144.1). Despite dose reductions, patients with renal insufficiency had higher F-ara-A exposures. There was strong association between high plasma concentrations of F-ara-A and increased risk of TRM and reduced overall survival. Patients with an AUC(0-∞) greater than 6.5 ug*hr/mL had 4.56 greater risk of TRM and significantly lower survival. These data suggest that clinical strategies are needed to optimize dosing of fludarabine to prevent overexposure and toxicity in HCT.
Keywords: fludarabine, f-ara-a, nonmyeloablative, treatment related mortality, pharmacokinetics
Introduction
Conventional myeloablative HCT is associated with significant treatment-related morbidity and mortality, and therefore often limited to younger patients with good clinical status and organ function. Nonmyeloablative or reduced-intensity preparative regimens reduce toxicity and risk of TRM, allowing more patients to undergo allogeneic HCT that would otherwise not be suitable for traditional transplantation.
Fludarabine (9-β-D-arabinofuranosyl-2-fluoroadenine monophosphate), a purine analog with potent antitumor and immunosuppressive activity, is a common component of conditioning regimens prior to allogeneic nonmyeloablative HCT.1-3 Its immunosuppressive properties promote engraftment, development of mixed chimerism, and graft-vs-leukemia effects, however, whether fludarabine exposure levels influence engraftment, GVHD, or TRM remains unclear.4-6 Fludarabine phosphate is a prodrug that undergoes rapid dephosphorylation to the systemically circulating compound, F-ara-A. F-ara-A is then phosphorylated intracellularly by several kinases to the active 9-beta-D-arabinofuranosyl-2-fluoroadenine triphosphate, which is ultimately responsible for inhibition of DNA synthesis and RNA production, ultimately resulting in apoptosis.2-3, 7
The pharmacokinetics of fludarabine have been studied extensively in several diseases states but limited data exists in HCT.8-18 Fludarabine pharmacokinetics in nonmyeloablative HCT was evaluated when combined with busulfan at doses of 30 mg/m2 given daily over 4 days.19-20 In these reports, sample sizes were limited and the relationship between F-ara-A pharmacokinetics and clinical outcomes such as engraftment and toxicity were not evaluated.19-20 Therefore, the goal of our study was to evaluate the relationship between fludarabine pharmacokinetics, and engraftment, acute GVHD, TRM and survival.
Methods
Patients
This was a single center, pharmacokinetic-pharmacodynamic study of fludarabine in 87 patients who underwent allogeneic nonmyeloablative HCT. Patients were eligible to participate if they met protocol eligibility criteria for transplantation and were to undergo a related or unrelated nonmyeloablative allogeneic HCT containing fludarabine, cyclophosphamide and TBI along with cyclosporine and mycophenolate mofetil (MMF) for posttransplant immunosuppression. This study was approved by the Institutional Review Board and the Cancer Protocol Review Committee. All patients provided written informed consent.
Preparative Regimen and Immunosuppression
The preparative regimen consisted of cyclophosphamide 50mg/kg/day i.v. day −6 (total dose 50mg/kg); fludarabine 40mg/m2/day i.v. on days −6 to −2 (total dose 200mg/m2); and TBI 200cGy single fraction on day −1 (total dose 200cGy). Fludarabine was dose-reduced to 30-35mg/m2/day in 9 patients with pre-existing mild to moderate renal impairment at the discretion of the clinical team. Patients without intensive chemotherapy in the prior 6 months received equine antithymocyte globulin 15mg/kg i.v. every 12 hours for 3 days (total dose 90 mg/kg), with methylprednisolone 1mg/kg i.v. every 12 hours.
Intravenous MMF and cyclosporine was started on day -3 and converted to oral as tolerated. Cyclosporine doses were adjusted to maintain whole blood troughs of 200-400ng/ml.
Fludarabine Pharmacokinetics
F-ara-A pharmacokinetic sampling was prospectively performed with the first dose of fludarabine. Fludarabine was administered intravenously over one hour at a constant rate and samples were obtained at times 0, 1.6 (100min), 2, 3, 4, 6, 8, 12, and 24 hours after start of infusion. On day of transplant a sample was obtained prior to stem cell infusion. Five mL of blood was collected at each sampling time and placed in a heparinized green-top tube for F-ara-A analysis. All samples, within 60 minutes of collection, were centrifuged at 3400 rpm for 10 minutes at 4 degrees C, and the plasma removed and stored at -80 degrees C until analysis. Plasma serum creatinine (SCr) was obtained on day of pharmacokinetics and creatinine clearance (CrCl) estimated by the Cockcroft and Gault equation using ideal body weight.21
Bioanalysis
Detection and quantification of plasma F-ara-A was performed with HPLC-UV at 267nm (Agilent Technologies, Wilmington, DE). The chromatographic separation was performed with a Phenomenex Polar-RP, 4.6 × 250mm, reversed phase column with a 4-micron particle size (Phenomenex, Torrance, CA). The mobile phase consisted 50 mM sodium phosphate and acetonitrile (90%, 10% v/v) at a pH of 1.6., with a flow-gradient of 0.75 ml/min for 0-8 min, 1.2 ml/min for 9-22 min and 0.75 ml/min for 0.5 min. Total run time was 23 minutes. Following the addition of internal standard (20 ng of 5, 6 dimethylbenzimidazole) plasma samples (0.75 ml) were extracted using a 3cc Waters Oasis MAX solid phase extraction cartridge. The plasma samples were pretreated with 0.8 ml of 4% phosphoric acid and loaded on to the conditioned SPE cartridge and rinsed with 2 ml of 4% NH4OH. The final elution was performed with 2 ml of 4% NH4OH in methanol. The eluant was dried with a stream of nitrogen at 37 degrees C and reconstituted with 50 ul of mobile phase. F-ara-A standard and the 5, 6 dimethylbenzimidazole were obtained from Sigma-Aldrich (Milwaukee, WI). The assay was linear in the range of 10-3000 ng/mL. Assay accuracy, intraday and interday variability were 93.5-100.1%, 1.6-3.6% and 1.3-3.3%, respectively.
Pharmacokinetic Analysis
For each patient, F-ara-A plasma concentration-time data was analyzed using noncompartmental methods (WinNonLin Professional 5.2). Area-under-the-curve AUC(0-∞) was estimated by the log/linear trapezoidal method as AUC (0-t*) + C(t*)/Ke where C(t*) was the last concentration and Ke is the terminal first order elimination rate constant. Ke was calculated from the slope of the log-linear portion of the plasma-concentration time curve using linear regression analysis. The terminal half-life was 0.693/Ke. Clearance was determined by dose/AUC(0-∞). Volume of distribution was estimated from terminal phase of the time-concentration data. Cmax was as the largest observed concentration. Cmin(trough) was the 24 post dose concentration.
Statistical Analysis
The primary objective of this study was to determine the relationship between F-ara-A systemic exposure and neutrophil engraftment. Secondary objectives were to evaluate the relationship between F-ara-A exposure and incidence of acute GVHD, TRM, and overall survival. Neutrophil engraftment, acute GVHD, TRM, and survival data were collected through the transplant database. GVHD was staged and graded according to the standard GVHD criteria based on clinical and pathological criteria.22-23 Day of neutrophil engraftment was defined as the first of 3 consecutive measures of an absolute neutrophil count (ANC) >500 cells/uL in association with partial or complete chimerism by day 42 after transplantation. Graft failure was defined as survival without an ANC >500 cells/uL at day 42 or autologous reconstitution. TRM was death without disease progression or relapse. For the survival analysis, death due to any cause was considered an event. Pharmacokinetic data was summarized by descriptive statistics. Normal renal function was defined as a CrCl >70ml/min. Renal impairment was defined as CrCl< 70ml/min.24
Probabilities and 95% confidence intervals (CI) of acute GVHD and TRM were calculated using the cumulative incidence function while neutrophil engraftment and overall survival were calculated using the Kaplan-Meier method. Recursive partitioning regression analysis was used to determine optimal cutpoints for F-ara-A pharmacokinetic parameters (AUC(0-∞), Cmin, clearance, and Dayzero) towards clinical endpoint. Univariate analysis was performed evaluating each pharmacokinetic parameter towards neutrophil engraftment, TRM at 30 days, 100 days, 6 and 12 months, and overall survival at 6 and 12 months, and time to acute GVHD to day 100. Univariate analysis was also performed to evaluate clinical factors towards engraftment, TRM, overall survival and acute GVHD.
Cox proportional hazards regression was used to model independent predictors of TRM at 6 months posttransplantation and included the following: F-ara-A AUC(0-∞), age, use of ATG in preparative regimen, recipient CMV status, donor source, comorbidity score25-26, CrCl, and acute GVHD grades II-IV.
Results
Patient characteristics are described in Table 1. A total of 87 adult patients had fludarabine pharmacokinetic sampling performed. Nine patients received dose reductions of fludarabine due to pre-existing renal impairment [30mg/m2/day (n=1), 32mg/m2/day (n=5) and 35mg/m2/day (n=3)].
Table 1. Patient Demographics.
| Median (range) / N (%) | |
|---|---|
| N | 87 |
| Age (years) | 55(20-69) |
| Fludarabine dose (mg/m2) | 40(30-40) |
| Weight (kg) | 82.6(41.5-139.5) |
| Male | 56 (64%) |
| Female | 31(36%) |
| Serum creatinine (mg/dL) 1 | 0.9(0.4-1.5) |
| Creatinine clearance (mL/min) 1 | 82.1(49.5-153.2) |
| Recipient CMV positive | 53(61%) |
| Comorbidity Score 2 | |
| 0 | 9 (10%) |
| 1-2 | 32 (37%) |
| ≥3 | 46 (53%) |
| Disease | |
| Acute lymphoblastic leukemia | 6(7%) |
| Acute myelogenous leukemia | 26(30%) |
| Chronic myeloid leukemia | 1(1%) |
| Other leukemia | 6(7%) |
| Myelodysplastic syndrome | 14(16%) |
| Non-Hodgkin's lymphoma | 17(20%) |
| Hodgkin's lymphoma | 8(9%) |
| Other | 9(10%) |
| Graft Source | |
| Bone marrow | 2(2%) |
| Peripheral blood stem cell | 21(24% |
| Cord Blood | 64(74%) |
| 1 umbilical cord | 3(5%) |
| 2 umbilical cords | 61(95%) |
| Donor | |
| HLA Matched-related | 22(25%) |
| Unrelated | 65(75%) |
| Disease Risk3 | |
| Standard risk | 28(32%) |
| High risk | 59(68%) |
| ATG in the conditioning regimen | 40(46%) |
obtained on day of pharmacokinetic sampling,
Sorror et al (ref 25),
Standard risk is defined as acute leukemia in first or second remission and CML in chronic phase, all other malignancies were classified as high risk.
F-ara-A Pharmacokinetics
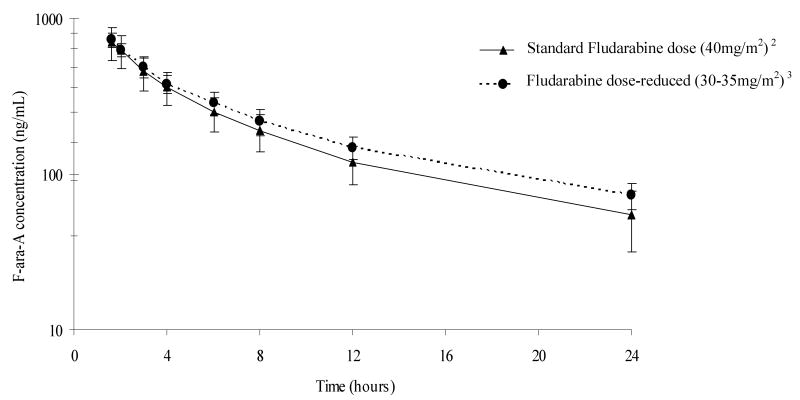
F-ara-A pharmacokinetics parameters are shown in Table 2. The concentration-time profiles for patients receiving the standard dose of fludarabine (40mg/m2) and modified doses of fludarabine (30-35mg/m2) are shown in Figure 1. F-ara-A exposure was variable with up to a 5.7 fold difference in AUC(0-∞) among patients receiving the standard fludarabine dose of 40mg/m2/day. Individuals with preexisting mild to moderate renal impairment, despite dose reductions of 20-25%, had higher F-ara-A exposures including AUC(0-∞) and trough concentrations (Table 2). F-ara-A clearance was reduced by ∼25% in patients with decreased renal function. F-ara-A was detectable on day of transplant in all patients. F-ara-A dose adjusted AUC(0-∞) was poorly correlated with CrCl (r2=0.22, p< 0.01) and SCr (r2=0.02, p=0.18).
Table 2. F-ara-A pharmacokinetic parameters with first dose of fludarabine1.
| Standard Dose | Dose-Reduced | |
|---|---|---|
| N | 78 | 9 |
| Fludarabine dose | 40mg/m2 | 30-35mg/m2 |
| Creatinine Clearance, mL/min | 85.9(49.5-153.2) | 57.1(50.5-65.1) |
| Cmax, ng/mL | 958 (384-2046) | 798 (694-1073) |
| Cmin trough, ng/mL | 52 (17-166) | 69 (39-93) |
| AUC(0-∞), ug*hr/mL | 4.9 (2.0-11.5) | 5.5 (4.3-7.0) |
| Volume of distribution, L/kg | 1.95 (0.89-4.78) | 1.72 (1.40-2.51) |
| Clearance, L/hr | 16.0 (6.2-36.6) | 11.5 (6.9-15.2) |
| Half-life, hours | 8.53 (3.75-22.18) | 10.32 (7.81-14.48) |
| Dayzero2, ng/mL | 15.0 (0.1-144.1) | 19.0 (4.0-63.6) |
data are median (range),
single concentration obtained on day of transplant
Figure 1. F-ara-A Time vs Concentration Profile with first dose of fludarabine1.
1data are mean (standard deviation), 2patients receiving standard doses of fludarabine with CrCl median (range) of 85.9ml/min (49.5-153.2), 3patients receiving dose modifications of fludarabine based on pre-existing mild to moderate renal insufficiency with CrCl median (range) of 57.1ml/min (50.5-65.1).
Relationship between F-ara-A Exposure and Clinical Outcomes
Engraftment of Donor Cells
Primary neutrophil recovery occurred in 86% of patients at a median (range) of 11 days (1-38) following HCT. Graft failure occurred in 14 (16%) patients. In univariate analysis, no F-ara-A pharmacokinetic parameters were associated with engraftment.
Acute Graft vs Host Disease
Cumulative incidences of grades II-IV and III-IV acute GVHD at day 100 were 50% and 17%, respectively. In univariate analysis, patients with an F-ara-A AUC(0-∞) >6.5ug*hr/mL had a significantly lower incidence of acute GVHD grades II-IV (14%) compared to those ≤6.5ug*hr/mL (52%), p=0.04. No other F-ara-A pharmacokinetic parameters were associated with development of grades II-IV or III-IV acute GVHD.
Treatment-Related Mortality
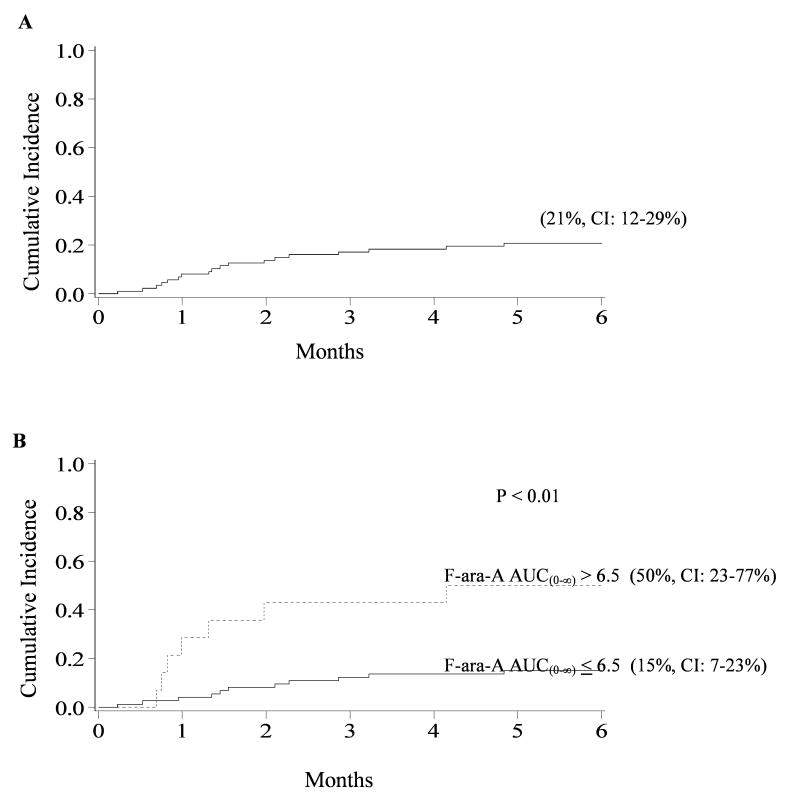
The incidence of TRM at 6 months was 21% (95% CI, 12-29%), occurring at a median of 1.5 months (range, 0.2-6.1) posttransplant (Figure 2, panel A). A total of 18 treatment-related deaths occurred with multi-organ failure (n=8) the most common cause. Pulmonary and/or cardiac involvement was present in the majority of multi-organ failure deaths. The other causes of TRM included encephalopathy (n=3), infection (n=3), bleeding/thrombosis (n=3), and acute GVHD (n=1). In univariate analysis, patients with F-ara-A AUC(0-∞) >6.5ug*hr/mL had significantly higher cumulative incidence of TRM at 6 months (50%) compared to patients with F-ara-A AUC(0-∞) ≤6.5ug*hr/mL (15%), p<0.01 (Figure 2, panel B). The cause of death in the 7 patients with TRM and F-ara-A AUC(0-∞) >6.5ug*hr/mL included multi-organ failure (n=4), encephalopathy (n=1), infection (n=1) and bleeding/thrombosis (n=1). TRM at 6 months was also significantly higher in patients with F-ara-A clearance ≤12.5L/h when compared to patients with an F-ara-A clearance >12.5L/h (45% vs 12%, p<0.01). F-ara-A Cmin>80ng/mL was associated with higher cumulative incidence of TRM at 6 months vs F-ara-A Cmin ≤80ng/mL (64% vs 15%, p<0.01). F-ara-A concentrations on day of transplant ≤30ng/mL were also associated with reduced TRM (12%) compared to patients with F-ara-A >30ng/mL (53%), p<0.01. Of the 87 patients, 14 patients (16.1%) had F-ara-A AUC(0-∞) >6.5ug*hr/mL, 65 patients (74.7%) had troughs >80ng/mL, and 12 patients (10.44%) had an F-ara-A clearance <12.5L/h. Seventeen (14%) had F-ara-A concentrations ≥30ng/mL on the day of stem cell infusion. F-ara-A pharmacokinetic parameters were also significant (p<0.01) towards TRM at 30 days, 100 days and 12 months (data not shown). In univariate analysis other clinical factors associated with higher risk of TRM at 6 months were ATG in the preparative regimen (30% vs 11%, p=0.02) and CrCl <70ml/min compared to ≥70ml/min (22% vs 2%, p<0.01).
Figure 2. Cumulative incidence of TRM for patients 6 months after nonmyeloablative HCT.
(A) Overall TRM. (B) TRM for patient with F-ara-A AUC(0-∞) ≤6.5ug*hr/mL compared to patients with AUC(0-∞) >6.5ug*hr/mL.
The Cox regression analysis for F-ara-A AUC(0-∞) is given in Table 3. At 6 months following transplantation, the only independent predictor of TRM was F-ara-A AUC(0-∞). Patients with an F-ara-A AUC(0-∞) >6.5ug*hr/mL had a 4.56 greater risk of TRM than individuals with an AUC(0-∞) ≤6.5ug*hr/mL (p=0.02).
Table 3. Multiple regression analysis of TRM at 6 months following nonmyeloablative HCT.
| Factor | Relative Risk of TRM (95% CI) | P-value |
|---|---|---|
| F-ara-A AUC(0-∞) | .02 | |
| ≤ 6.5 ug hr/mL* | 1.00 | |
| > 6.5 ug hr/mL | 4.56 (1.22-17.14) | |
| Age (yrs) | .89 | |
| < 55* | 1.00 | |
| ≥ 55 | 0.92 (0.26-3.20) | |
| ATG in conditioning regimen | .09 | |
| Yes* | 1.00 | |
| No | 0.36 (0.11-1.19) | |
| Recipient CMV status | .93 | |
| Negative* | 1.00 | |
| Positive | 0.94 (0.27-3.34) | |
| Donor Source | .97 | |
| Matched-related* | 1.00 | |
| Unrelated | 1.03 (0.31-3.40) | |
| Comorbidity Score | .18 | |
| 0* | 1.00 | |
| 1-2 | 0.22 (0.04-1.25) | |
| ≥3 | 0.60 (0.12-2.98) | |
| Creatinine clearance | .41 | |
| ≥ 70 ml/min* | 1.00 | |
| < 70 ml/min | 1.63 (0.52-5.13) | |
| aGVHD grades III-IV | .15 | |
| No* | 1.00 | |
| Yes | 2.91 (0.67-12.60) |
reference group
Overall Survival
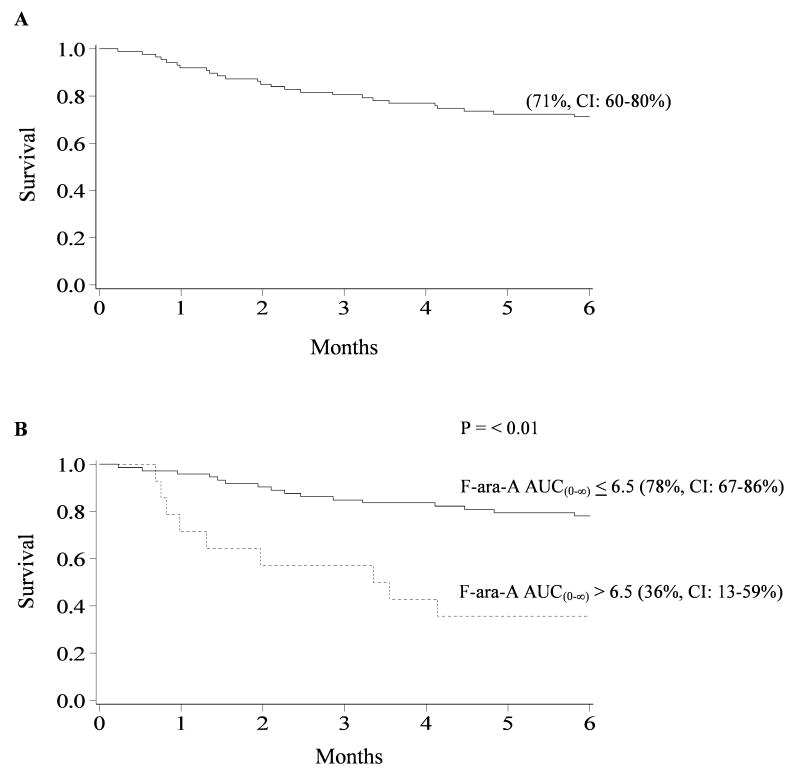
Six month overall survival was (71%, 95% CI: 60-80%) with 47 patients alive at a median of 12 months (range, 0.2-29.5) (Figure 3, panel A). The most frequent causes of death were disease relapse or progression (n=7), multi-organ failure (n=8), encephalopathy (n=3), infection (n=3), bleeding/thrombosis (n=3), and acute GVHD (n=1). In univariate analysis, patients with F-ara-A AUC(0-∞) >6.5ug*hr/mL had significantly lower overall survival (36%) at 6 months compared to patients with F-ara-A AUC(0-∞) ≤6.5ug*hr/mL (78%), p<0.01 (Figure 3, panel B). Similarly, overall survival at 6 months was lower in patients with F-ara-A clearance ≤12.5L/h compared to clearance >12.5L/h, 41% vs 81%, p<0.01, respectively. Elevated F-ara-A trough >80ng/mL was associated with reduced overall survival 6 months posttransplantation (27% vs 76%), p<0.01. On the day of transplant, patients with F-ara-A concentrations >30ng/mL had significantly lower overall 6 month survival compared to patients with concentrations ≤30ng/mL (41 vs 80%), p<0.01. Pharmacokinetic parameters were also significant (p<0.01) towards overall survival at 12 month (data not shown).
Figure 3. Cumulative proportion of overall survival for patients 6 months after nonmyeloablative HCT.
(A) Overall survival. (B) Overall survival for patient with F-ara-A AUC(0-∞) ≤6.5ug*hr/mL compared to patients with AUC(0-∞) >6.5ug*hr/mL.
Discussion
This is the first study to evaluate F-ara-A exposure and its relationship to stem cell engraftment, GVHD, TRM, and survival in nonmyeloablative allogeneic HCT. We showed that F-ara-A exposure is highly variable with 5.7 fold difference in AUC in patients receiving fludarabine at 40mg/m2. Our data also demonstrates that higher F-ara-A concentrations are associated with a greater risk of TRM and decreased survival. Finally, in preparative regimens that use 40mg/m2 of fludarabine, dose reductions greater than 20-25% should be considered in patients presenting for transplant with renal impairment.
The pharmacokinetic parameters in our study were consistent to those previously described in HCT patients.19-20 Studies outside the setting of HCT demonstrate F-ara-A clearance correlates with CrCl, with F-ara-A urinary excretion as high as 60% of the total administered dose.13 In phase I trials of fludarabine a decline in renal function was associated with a decrease in total body clearance and volume of distribution.18 As as result, fludarabine dose reductions of 20-25% are recommended by the manufacturer in patients with CrCl< 70 ml/min.27 In our study, the nine patients with pre-existing mild to moderate renal impairment (median CrCl 57.1ml/min), despite receiving a 20-25% dose reduction, had higher plasma concentrations, reduced clearance of F-ara-A, and longer half-life compared to patients not receiving dose reductions (median CrCl 85.9ml/min). Therefore, dose reductions >20-25% may be required in HCT patients with mild to moderate renal impairment in preparative regimens that use fludarabine 40mg/m2.
Our analyses showed a strong relationship between high plasma F-ara-A exposure, TRM and overall survival. This is not surprising since higher exposure may result in greater tissue damage, and more potent and longer immunosuppression. A high comorbidity score and poor renal function may also be associated with greater TRM, however, they had no independent effect in the multivariate analyses. Patients also received cyclophosphamide and TBI. The metabolism and clearance of cyclophosphamide and its metabolites have been shown to be influenced by renal function and increased concentrations are associated increased risk of toxicity in HCT.28-29 Given that multiple factors influence TRM, accumulation of F-ara-A plasma concentrations may be a surrogate marker of overall preparative regimen intensity.
Higher doses of fludarabine (40-100 mg/m2) have been associated with delayed onset severe neurotoxicity including progressive multifocal leukoencephalopathy (PML), coma and death. Early phase I/II trials conducted in relapsed leukemia estimated an 18% incidence of severe fludarabine neurotoxicity at these doses.30-31 In allogeneic HCT the incidence of neurotoxicity and risk factors are not well defined. Fludarabine has been suggested as a potential risk factor for neurotoxicity following umbilical cord transplantation.32 Patients with elevated systemic plasma concentrations of F-ara-A may experience higher drug concentrations in the central nervous system. However, several confounding variables must be considered. PML resulting from opportunistic infection with JC virus has been reported in chronic lymphocytic leukemia (CLL) patients treated with fludarabine.33-36 More recently MMF has been associated with cases reports of PML.37 Finally, all patients in this study were treated with cyclosporine. Neurotoxicity, including posterior reversible leukoencephalopathy, may occur in up to 30% of patients receiving cyclosporine.38 Irreversible encephalopathy with the use of cyclosporine in pediatric patients have also been reported.39
We found no influence of F-ara-A pharmacokinetic measures on neutrophil engraftment. However, our engraftment rate was high thereby reducing our ability to detect an effect. Studies in relapsed CLL patients comparing chemotherapy responders vs non-responders suggest that sufficient F-ara-A plasma concentrations are needed to maintain a minimum inhibitory F-ara-ATP concentration in tumor cells.12 It is possible that the higher fludarabine dose, long terminal half-life, combined with the 5 day dosing regimen leads to sufficient intracellular F-ara-ATP needed for engraftment. Interestingly, detectable plasma F-ara-A was still present on day of transplant in all patients. It is possible that patients receiving lower doses of fludarabine (25-30 mg/m2) may not exceed these thresholds. Future studies in HCT should incorporate the evaluation of intracellular F-ara-ATP concentrations and the potential role of genetic polymorphisms involved in fludarabine pathway including cellular transporters, kinases and targets.40-42 Engraftment is a complex process and is influenced by several factors including cell dose, donor source, and MMF exposure and substantially larger sample sizes will be required to evaluate both genetic and non-genetic effects.43
We found a strong association between higher plasma concentrations of fludarabine and increased risk of TRM and reduced overall survival following nonmyeloablative HCT. Whether these relationships hold true for other preparative regimens, especially those containing lower doses of fludarabine have yet to be established. Renal insufficiency led to higher concentrations of fludarabine and dose reductions are warranted to avoid excessive systemic exposure. Future studies are needed to further define the minimal level of F-ara-A exposure required for stem cell engraftment so that toxicity is minimized.
Acknowledgments
The technical assistance of Jim Fisher and Jason Dagit is gratefully acknowledged. We also acknowledge the work and dedication of our study nurse coordinator, Dixie Lewis.
Footnotes
Conflict of Interest/Funding Disclosures: We have no conflicts of interest to disclose. This work was supported by grants from the National Institutes of Health, National Cancer Institute (CA096622) (P.J.), and National Institutes of Health National Center for Research Services (M01-RR00400).
References
- 1.Baron F, Storb R. Allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning as treatment for hematologic malignancies and inherited blood disorders. Mol Ther. 2006;13(1):26–41. doi: 10.1016/j.ymthe.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Brockman RW, Cheng YC, Schabel FM, Jr, Montgomery JA. Metabolism and chemotherapeutic activity of 9-beta-D-arabinofuranosyl-2-fluoroadenine against murine leukemia L1210 and evidence for its phosphorylation by deoxycytidine kinase. Cancer Res. 1980;40(10):3610–5. [PubMed] [Google Scholar]
- 3.Dow LW, Bell DE, Poulakos L, Fridland A. Differences in metabolism and cytotoxicity between 9-beta-D arabinofuranosyladenine and 9-beta-D-arabinofuranosyl-2-fluoroadenine in human leukemic lymphoblasts. Cancer Res. 1980;40(5):1405–10. [PubMed] [Google Scholar]
- 4.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102(6):2021–30. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 5.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderlini P, Saliba R, Acholonu S, Giralt SA, Andersson B, Ueno NT, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin's lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93(2):257–64. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plunkett W, Huang P, Gandhi V. Metabolism and action of fludarabine phosphate. Semin Oncol. 1990;17(5 Suppl 8):3–17. [PubMed] [Google Scholar]
- 8.Danhauser L, Plunkett W, Liliemark J, Gandhi V, Iacoboni S, Keating M. Comparison between the plasma and intracellular pharmacology of 1-beta-D-arabinofuranosylcytosine and 9-beta-D-arabinofuranosyl-2-fluoroadenine 5′-monophosphate in patients with relapsed leukemia. Leukemia. 1987;1(9):638–43. [PubMed] [Google Scholar]
- 9.Danhauser L, Plunkett W, Keating M, Cabanillas F. 9-beta-D-arabinofuranosyl-2-fluoroadenine 5′-monophosphate pharmacokinetics in plasma and tumor cells of patients with relapsed leukemia and lymphoma. Cancer Chemother Pharmacol. 1986;18(2):145–52. doi: 10.1007/BF00262285. [DOI] [PubMed] [Google Scholar]
- 10.Avramis VI, Champagne J, Sato J, Krailo M, Ettinger LJ, Poplack DG, et al. Pharmacology of fludarabine phosphate after a phase I/II trial by a loading bolus and continuous infusion in pediatric patients. Cancer Res. 1990;50(22):7226–31. [PubMed] [Google Scholar]
- 11.Avramis VI, Wiersma S, Krailo MD, Ramilo-Torno LV, Sharpe A, Liu-Mares W, et al. Pharmacokinetic and pharmacodynamic studies of fludarabine and cytosine arabinoside administered as loading boluses followed by continuous infusions after a phase I/II study in pediatric patients with relapsed leukemias. The Children's Cancer Group. Clin Cancer Res. 1998;4(1):45–52. [PubMed] [Google Scholar]
- 12.Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41(2):93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lichtman SM, Etcubanas E, Budman DR, Eisenberg P, Zervos G, D'Amico P, et al. The pharmacokinetics and pharmacodynamics of fludarabine phosphate in patients with renal impairment: a prospective dose adjustment study. Cancer Invest. 2002;20(7-8):904–13. doi: 10.1081/cnv-120005903. [DOI] [PubMed] [Google Scholar]
- 14.Hersh MR, Kuhn JG, Phillips JL, Clark G, Ludden TM, Von Hoff DD. Pharmacokinetic study of fludarabine phosphate (NSC 312887) Cancer Chemother Pharmacol. 1986;17(3):277–80. doi: 10.1007/BF00256699. [DOI] [PubMed] [Google Scholar]
- 15.Hutton JJ, Von Hoff DD, Kuhn J, Phillips J, Hersh M, Clark G. Phase I clinical investigation of 9-beta-D-arabinofuranosyl-2-fluoroadenine 5′-monophosphate (NSC 312887), a new purine antimetabolite. Cancer Res. 1984;44(9):4183–6. [PubMed] [Google Scholar]
- 16.Kuo GM, Boumpas DT, Illei GG, Yarboro C, Pucino F, Burstein AH. Fludarabine pharmacokinetics after subcutaneous and intravenous administration in patients with lupus nephritis. Pharmacotherapy. 2001;21(5):528–33. doi: 10.1592/phco.21.6.528.34549. [DOI] [PubMed] [Google Scholar]
- 17.Knebel W, Davis JC, Jr, Sanders WD, Fessler B, Yarboro C, Pucino F, et al. The pharmacokinetics and pharmacodynamics of fludarabine in rheumatoid arthritis. Pharmacotherapy. 1998;18(6):1224–9. [PubMed] [Google Scholar]
- 18.Malspeis L, Grever MR, Staubus AE, Young D. Pharmacokinetics of 2-F-ara-A (9-beta-D-arabinofuranosyl-2-fluoroadenine) in cancer patients during the phase I clinical investigation of fludarabine phosphate. Semin Oncol. 1990;17(5 Suppl 8):18–32. [PubMed] [Google Scholar]
- 19.Bonin M, Pursche S, Bergeman T, Leopold T, Illmer T, Ehninger G, et al. F-ara-A pharmacokinetics during reduced-intensity conditioning therapy with fludarabine and busulfan. Bone Marrow Transplant. 2007;39(4):201–6. doi: 10.1038/sj.bmt.1705565. [DOI] [PubMed] [Google Scholar]
- 20.Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102(3):820–6. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Weisdorf DJ, Snover DC, Haake R, Miller WJ, McGlave PB, Blazar B, et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76(3):624–9. [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 24.Anonymous. (CDER) CfDEaR, (ed) 1998. Guidance for industry: Pharmacokinetics in patients with impaired renal function-study design, data analysis, and impact on dosing and labeling. [Google Scholar]
- 25.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majhail NS, Brunstein CG, McAvoy S, DeFor TE, Al-Hazzouri A, Setubal D, et al. Does the hematopoietic cell transplantation specific comorbidity index predict transplant outcomes? A validation study in a large cohort of umbilical cord blood and matched related donor transplants. Biol Blood Marrow Transplant. 2008;14(9):985–92. doi: 10.1016/j.bbmt.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Anonymous. Product Information: Fludara (R) (fludarabine phosphate) For Injection. Berlex Laboratories; Richmond, California: 2002. [Google Scholar]
- 28.McCune JS, Batchelder A, Guthrie KA, Witherspoon R, Appelbaum FR, Phillips B, et al. Personalized dosing of cyclophosphamide in the total body irradiation-cyclophosphamide conditioning regimen: a phase II trial in patients with hematologic malignancy. Clin Pharmacol Ther. 2009;85(6):615–22. doi: 10.1038/clpt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCune JS, Salinger DH, Vicini P, Oglesby C, Blough DK, Park JR. Population pharmacokinetics of cyclophosphamide and metabolites in children with neuroblastoma: a report from the Children's Oncology Group. J Clin Pharmacol. 2009;49(1):88–102. doi: 10.1177/0091270008325928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding X, Herzlich AA, Bishop R, Tuo J, Chan CC. Ocular toxicity of fludarabine: a purine analog. Expert Rev Ophthalmol. 2008;3(1):97–109. doi: 10.1586/17469899.3.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheson BD, Vena DA, Foss FM, Sorensen JM. Neurotoxicity of purine analogs: a review. J Clin Oncol. 1994;12(10):2216–28. doi: 10.1200/JCO.1994.12.10.2216. [DOI] [PubMed] [Google Scholar]
- 32.Narimatsu H, Miyamura K, Iida H, Hamaguchi M, Uchida T, Morishita Y. Early central nervous complications after umbilical cord blood transplantation for adults. Biol Blood Marrow Transplant. 2009;15(1):92–100. doi: 10.1016/j.bbmt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez H, Bolgert F, Camporo P, Leblond V. Progressive multifocal leukoencephalitis (PML) in three patients treated with standard-dose fludarabine (FAMP) Hematol Cell Ther. 1999;41(4):183–6. doi: 10.1007/s00282-999-0183-7. [DOI] [PubMed] [Google Scholar]
- 34.Saumoy M, Castells G, Escoda L, Mares R, Richart C, Ugarriza A. Progressive multifocal leukoencephalopathy in chronic lymphocytic leukemia after treatment with fludarabine. Leuk Lymphoma. 2002;43(2):433–6. doi: 10.1080/10428190290006297. [DOI] [PubMed] [Google Scholar]
- 35.Kiewe P, Seyfert S, Korper S, Rieger K, Thiel E, Knauf W. Progressive multifocal leukoencephalopathy with detection of JC virus in a patient with chronic lymphocytic leukemia parallel to onset of fludarabine therapy. Leuk Lymphoma. 2003;44(10):1815–8. doi: 10.1080/1042819031000116625. [DOI] [PubMed] [Google Scholar]
- 36.Vidarsson B, Mosher DF, Salamat MS, Isaksson HJ, Onundarson PT. Progressive multifocal leukoencephalopathy after fludarabine therapy for low-grade lymphoproliferative disease. Am J Hematol. 2002;70(1):51–4. doi: 10.1002/ajh.10085. [DOI] [PubMed] [Google Scholar]
- 37.Neff RT, Hurst FP, Falta EM, Bohen EM, Lentine KL, Dharnidharka VR, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation. 2008;86(10):1474–8. doi: 10.1097/TP.0b013e31818b62c8. [DOI] [PubMed] [Google Scholar]
- 38.Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: a review. J Neurol. 1999;246(5):339–46. doi: 10.1007/s004150050360. [DOI] [PubMed] [Google Scholar]
- 39.Minn AY, Fisher PG, Barnes PD, Dahl GV. A syndrome of irreversible leukoencephalopathy following pediatric allogeneic bone marrow transplantation. Pediatr Blood Cancer. 2007;48(2):213–7. doi: 10.1002/pbc.20731. [DOI] [PubMed] [Google Scholar]
- 40.Mackey JR, Galmarini CM, Graham KA, Joy AA, Delmer A, Dabbagh L, et al. Quantitative analysis of nucleoside transporter and metabolism gene expression in chronic lymphocytic leukemia (CLL): identification of fludarabine-sensitive and -insensitive populations. Blood. 2005;105(2):767–74. doi: 10.1182/blood-2004-03-1046. [DOI] [PubMed] [Google Scholar]
- 41.Lamba JK, Crews K, Pounds S, Schuetz EG, Gresham J, Gandhi V, et al. Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther. 2007;323(3):935–45. doi: 10.1124/jpet.107.128595. [DOI] [PubMed] [Google Scholar]
- 42.Molina-Arcas M, Marce S, Villamor N, Huber-Ruano I, Casado FJ, Bellosillo B, et al. Equilibrative nucleoside transporter-2 (hENT2) protein expression correlates with ex vivo sensitivity to fludarabine in chronic lymphocytic leukemia (CLL) cells. Leukemia. 2005;19(1):64–8. doi: 10.1038/sj.leu.2403582. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson P, Rogosheske J, Barker JN, Green K, Ng J, Weisdorf D, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78(5):486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]