Abstract
Obesity is highly associated with an increased risk of serious health conditions including hypertension, cardiovascular disease, diabetes, and cancer. Changes in redox status with increased oxidative stress have been linked with obesity. Previous studies have shown that administration of the antioxidant Tempol in the food of mice prevents obesity causing significant weight loss without toxicity. To gain a better understanding of the molecular mechanism(s) underlying this effect, the influence of Tempol on the differentiation of mouse 3T3-L1 pre-adipocytes was studied. Tempol inhibited differentiation of 3T3-L1 cells resulting in a reduction in cellular lipid storage, down-regulation of protein levels of key adipogenesis transcription factors (PPAR-γ and PPAR-α), down regulation of prolyl hydroxylase, and up-regulation of HIF-1α. Mice on a Tempol diet demonstrated reduced systemic levels of IGF-1, in qualitative agreement with that observed in vitro in 3T3-L1 cells, which also show lower IGF-1 levels as a result of Tempol treatment. These results show that treatment of 3T3-L1 cells with Tempol inhibits the expression of key adipogenesis factors, adipose differentiation, and lipid storage and may underlie, at least in part, some of the in vivo effects of Tempol on body weight.
Keywords: Adipogenesis, Tempol, oxidative stress, obesity, nitroxide
Introduction
Obesity develops as a result of an imbalance between caloric intake and expenditure, and is associated with increased risk for hypertension, cardiovascular disease, type 2 diabetes, and carcinogenesis [1]. Common suggested treatments for obesity include physical activity, caloric restriction, bariatric surgery, and their combinations. A pharmaceutical approach can also be attempted and includes appetite suppressants and blockers of dietary fat absorption. While in humans, caloric restriction is primarily associated with weight reduction, in animals it has been shown also to lower the incidence of tumorigenesis and to increase lifespan [2, 3]. Obese patients can exhibit both adipocyte hypertrophy and adipocyte hyperplasia [4], the former is thought to be more important in determination of fat mass in adults [5]. It is believed that the total number of fat cells is regulated and rather constant, and that 10% of fat cells are renewed annually at all adult ages and levels of body mass index [6]. To investigate possible drug intervention for the treatment of obesity, diet-induced animal models of obesity and adipocytes in culture, in particular mouse 3T3-L1 cells are commonly used.
3T3-L1 cells have been extensively used for studies of obesity and of adipocyte differentiation. These pre-adipocyte fibroblasts undergo a well-characterized process of differentiation upon induction with insulin, dexamethasone and isobutylmethylxanthine (IBMX). The transition to mature adipocytes involves several stages, which include clonal expansion, growth arrest, and early and terminal differentiation. Differentiated 3T3-L1 cells exhibit many of the characteristics found in adipocytes from fat tissue including the production and storage of fat globules, and secretion of adipokines and other growth factors. 3T3-L1 cells show their distinctive morphology as early as 3 days following initiation of differentiation [7]. The differentiation process of 3T3-L1 cells is highly coordinated by various transcription factors, in particular a member of the peroxisome proliferators-activated receptor (PPAR) family of nuclear hormone receptors, PPARγ [8]. PPARγ, typically regarded as a master regulator of adipocyte differentiation, has been shown to be both necessary and sufficient for adipocyte differentiation [9]. PPARγ specifically functions as a heterodimer with the retinoid X receptor (RXR) through interactions with peroxisome proliferator response elements on target genes [10]. The critical role of PPARγ in the regulation of adipogenesis was demonstrated in knockout mouse models [11, 12], but also in high-fat diet induced obesity in vivo models where antagonism of PPARγ was shown to inhibit obesity [13]. Apart from its role in adipocyte differentiation and adipogenesis, PPARγ also plays an important role in the metabolism of carbohydrates and lipids, as synthetic ligands that activate PPARγ, such as the anti-diabetic drug thiazolidinedione, which has been shown to improve insulin sensitivity, and reduce hyperglyciemia, hyperinsulinemia and hyperlipidemia in animals [14]. PPARγ was also shown to regulate the expression of perilipins, a class of proteins found exclusively at the surface of lipid droplets in adipocytes [15].
Reports have indicated that there is an increased oxidative stress in obesity [16] and that oxidative stress can cause changes in adipocytes and their differentiation. For example, the antioxidant n-acetylcyteine was shown to inhibit 3T3-L1 differentiation [17], while bolus of H2O2 was reported to decrease adipokine expression [18]. Hypoxia (0.01–2.0% O2) was also shown to inhibit adipocytes differentiation by down-regulating PPARγ [19]. It was suggested that HIF-1α is involved in the regulation of adipogenesis, and that hypoxia could decrease insulin signaling pathways [20]. These reports, linking adipogenesis to the redox state are at the base of the current study.
Previously, we have shown that Tempol, a low molecular weight antioxidant, not only lowered tumor incidence in mice, but also decreased the animals’ weight in fashion similar to that of caloric restriction [21]. To better understand the molecular mechanism(s) underlying this effect of Tempol, 3T3-L1 cells were differentiated in the presence and absence of the drug. Adipocyte differentiation was monitored using fluorescence microscopy, and factors relating to both adipogenesis and to hypoxia were assayed. The effect of the Tempol on metabolism and weight gain was also studied in animals.
Material and Methods
Reagents
Tempol (4, hydroxy-TEMPO, 97% pure), Dexamethasone, IBMX, and rabbit polyclonal anti perilipin A were purchased from Sigma-Aldrich (St. Louis, MO, USA). Insulin was purchased from Invitrogen (Carlsbad, CA, USA). Rabbit polyclonal anti-HIF prolyl hydroxylase 2 (PHD), mouse monoclonal anti-HIF-1α , rabbit polyclonal anti-PPARα , and mouse monoclonal anti-PPARγ were purchased from Novus Biologicas (Littleton, CO, USA). Rabbit polyclonal anti fasting-induced adipose factor (FIAF) was purchased from MBL International Corporation, (Woburn, MA, USA). Mouse monoclonal anti actin was purchased from Chemicon International (Temecula, CA, USA). Non-fat dry milk was purchased from Bio Rad, (Hercules, CA, USA). Mouse monoclonal anti p21 and HRP-conjugated secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell Culture
3T3-L1 pre-adipocytes cells [22] were maintained in DMEM containing 4.5 g/L glucose, 10% (v/v) bovine calf serum (Hyclone, South Logan, UT, USA), and supplemented with penicillin/streptomycin. Adipocyte differentiation was initiated 2 days after cells reached confluency (day 0). Differentiation media consisted of DMEM containing 4.5 g/L glucose, 10% fetal calf serum, 110 mg/L sodium pyruvate, penicillin/streptomycin, supplemented with 0.5 mM IBMX, 1 μM dexamethasone, and 5 μg/ml insulin, and was changed daily for 3 days. At the beginning of the 4th day, the differentiation media was replaced with regular media (without the IBMX, dexamethasone and insulin), which was changed daily. To test the effect of Tempol on adipocyte differentiation, Tempol at 500 μM, 1 mM and 2 mM final concentration was added to confluent cells (at day 0) and changed daily for the course of the experiments. Cells were fixed at 1, 2, 3, 4, 5, 6 and 7 days after the beginning of differentiation and stained for lipids. Media were collected to assess cytokine levels, and protein was harvested for immunoblotting.
Quantification of Fat-containing Cells
Cells were harvested and counted 6 days after initiation of differentiation. Duplicate plates with cells were washed (x2) with PBS (pH 7.4), fixed for 30 min at RT with 4% para-formaldehyde, and incubated with 0.1% saponin in PBS for 60 min. Following additional washes, cells were incubated at RT with 5mg/L anti perilipin A for 60 min at RT, washed, and incubated with 10g/L Alexa Fluor® 488 goat anti-rabbit IgG for 30 min. Counterstaining was performed with 5mg/L propidium iodide (PI). Samples were examined using a Carl Zeiss Axiovert 200 fluorescent microscope, and images captured using a CCD camera (Cooke Sensicam, Auburn Hills, MI). Images (a minimum of 4/field, for each sample) were taken using blue light for perilipin and green light for PI staining, respectively, and processed using ImagePro Plus (MediaCybernetics, Silver Spring, MD). The percentage of fat cells was determined by taking the total area of the image filled by the perilipin staining divided by the total area stained by the PI.
Measurement of IGF-1
One and six days after initiation of differentiation of 3T3-L1 cells media was collected and assayed for IGF-1 using a Quantikine Immunoassay ELISA kit for IGF-1 (R & D Systems, Minneapolis, MN). Mouse plasma IGF-1 levels were also measured following the release of IGF-1 from its binding protein by acid/ethanol extraction [23]. Briefly, plasma was extracted in 10 volumes of 12.5% v/v 2N HCl in ethanol for 30 min at RT. After centrifugation for 3 min at 10,000 rpm, the supernatant was neutralized with an equal volume of 0.43 M Tris.
Immunoblotting
Cells were washed twice with ice-cold PBS, harvested, and lysed in lysis buffer (Tris-buffered saline (TBS), 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide, 100 mg/L PMSF, 10 ml/L protease inhibitor cocktail, 1 mM sodium orthovanadate) to isolate whole cell extract. Proteins from extracts were resolved by 4-20% gradient SDS-PAGE and transferred to a nitrocellulose membrane (Invitrogen, Carlsbad, CA, USA). Following the transfer, membrane was blocked overnight at 4°C with 3% non-fat dry milk in TBS containing 0.1% Tween (TBST), and immunoblotted with either 2 mg/L anti-PHD, 5 mg/L anti-HIF-1a, 2 mg/L anti-PPARa, 2 mg/L anti-PPARg, 1 mg/L anti FIAF, or 0.2 mg/L anti actin at RT for 60 min. The membrane was then washed with TBST three times for 15 min, reacted with secondary antibody (1:2000–1:10,000), washed and incubated with Western LightingTM Chemiluminescent Reagent Plus (PerkinElmer Las Inc., Boston, MA, USA) according to the manufacturer’s protocol. Proteins were visualized using FluorChem SP imager (Alpha Innotech, San Leandro, CA). Densitometric analysis was accomplished with an image analyzer software package (Alpha Innotech, San Leandro, CA). Density values for each protein were normalized to actin.
Animals
Female C3H/Hen mice were supplied through the Frederick Cancer Research Center-Animal Production, Frederick, MD. The animals were housed five per cage in climate controlled, circadian rhythm-adjusted rooms and allowed food and water ad libitum. At 9 weeks of age the animal’s diet was changed to bacon-flavored chow (Bio-Serv, Frenchtown, NJ) with Tempol at a concentration of 10 mg/g (Tempol thoroughly mixed into chow and “cold-pressed” into pellets). Control animals received bacon-flavored chow minus the Tempol. All animal studies and research was conducted according to the principles outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council and was approved by the NCI Animal Care and Use Committee. Average weights of mice were determined once a week by weighing specific groups of animals and dividing their total weight by the number of animals. At specific times, mice were euthanized and serum was collected and stored at −80°C until processed.
Statistics
Data were analyzed with SigmaStat software (version 2.03; Systat Systems, Point Richmond, CA). Mean ± SEM was calculated and significance was analyzed using a two-tailed Student’s t test. Differences with p<0.05 were considered to be statistically significant.
Results
The Effect of Tempol on 3T3-L1 Differentiation
Un-differentiated 3T3-L1 pre-adipocytes resemble fibroblasts with a few cells having the appearance of differentiated adipose cells. Upon differentiation (induction with insulin, dexamethasone, and IBMX) the cells gradually change to their distinctive morphology of mature adipocytes, which store lipid globules. 3T3-L1 pre-adipocytes were differentiated in the presence and absence of Tempol. Differentiated cells (positive control) showed lipid accumulation as early as two days after initiation of differentiation (data not shown). Undifferentiated, differentiated, and differentiated-TEMPOL-treated cells were fixed, stained and counterstained with perilipin A and PI, respectively 6 days after initiating differentiation. Perilipin A is an intracellular neutral lipid storage droplet surface protein commonly used for quantification of adipocytes. The data presented in Figure 1, demonstrate the number of positively stained mature adipocytes (perilipin A: green) with an over lay of the total number of cells (PI: red) in the field. Undifferentiated cells (negative control) retained their fibroblastic appearance throughout the duration of the experiments and showed few positively stained Perilipin A containing fat cells (Figure 1A). In contrast, differentiated 3T3-L1 (positive control) showed substantial lipid accumulation 6 days post-differentiation (Figure 1B). The ratio of positive stained adipocytes to the total number of cells was ~70% (Figure 1E). Treatment of differentiated cells with Tempol reduced the number of positive stained adipocytes in a concentration dependent manner. Tempol (1 mM) reduced the ratio of positive cells to the total number of cells to 40% (Figure 1C, E), while 2 mM Tempol decreased the ratio to almost zero (Figure 1D, E). The ratio of cell density (the number of PI stained cells/the total area of the field) was not markedly different between the un-differentiated, differentiated, and differentiated-Tempol-treated cells (data not shown). Addition of Tempol (1 or 2 mM) to already differentiated cells had no effect on the number of positively stained adipocytes indicating that Tempol probably inhibited growth of the cells rather than exerted cytotoxicity (data not shown). Likewise, addition of Tempol-H (the reduced form of Tempol) had no effect on the number of positively stained adipocyte cells (data not shown). To study the effect of Tempol on the proliferation of the cells, duplicate plates were also harvested for counting. While addition of insulin, dexamethasone and IBMX (differentiated cells) resulted in a ~two-fold increase in the number of cells, co-administration with 1 mM Tempol partially inhibited proliferation (Figure 1F). The addition of 2 mM Tempol completely inhibited clonal expansion (Figure 1F). The fact that 2 mM Tempol treatment did not decrease the total number of cells (compared to control) and the cells appeared morphologically normal (Figure 1D) suggests that Tempol did not exert cytotoxicity.
Figure 1. Tempol reduces the number of mature 3T3-L1 adipocytes and inhibits proliferation.
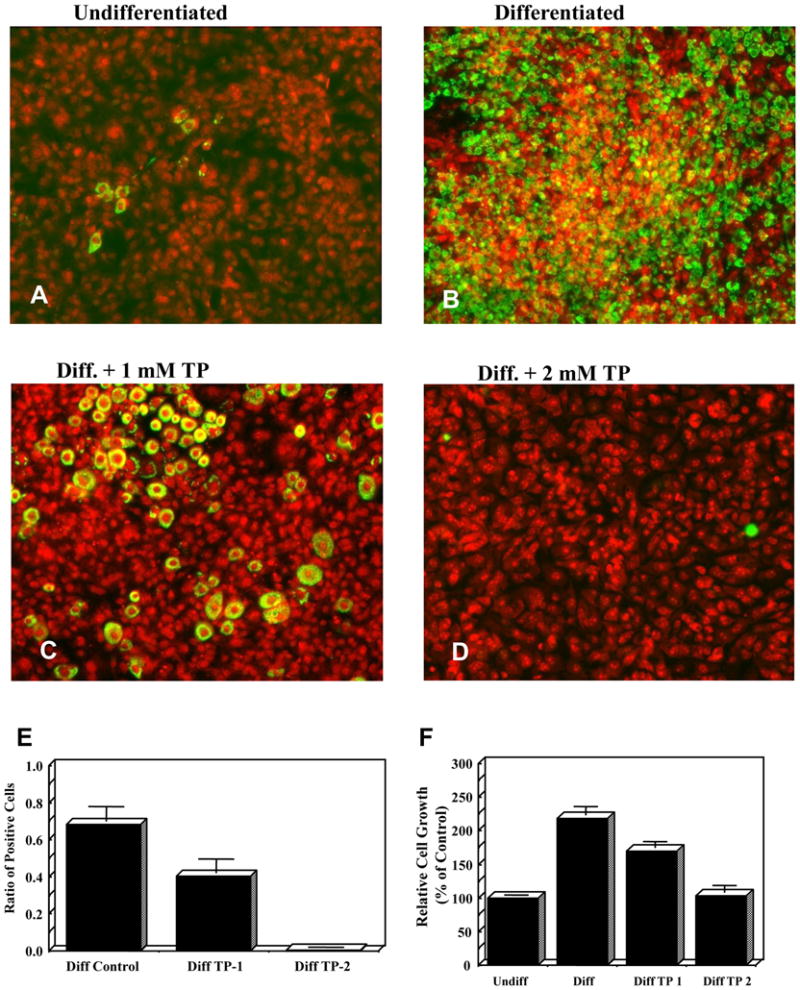
3T3-L1 cells were differentiated in the presence and absence of 1 or 2 mM Tempol. Six days after initiation of differentiation cells were fixed, stained with FITC-conjugated perilipin (green), counterstained with PI (red) and counted: photos of undifferentiated (1A), differentiated (1B), 1 mM Tempol-treated differentiated 3T3-L1 cells (1C) and 2 mM Tempol-treated-differentiated 3T3-L1 cells (1D). (1E) The ratio of FITC-conjugated perilipin positive stained cells to total number of cells decreased upon treatment with Tempol. (1F) Duplicate plates were trypsinized and counted and relative cell growth is shown. 1 mM Tempol partially inhibited 3T3-L1 proliferation, while 2 mM Tempol completely inhibited proliferation. Data presented as mean ± SEM of 4–7 independent experiments (1F).
The Effect of Tempol on Adipocyte Differentiation Proteins
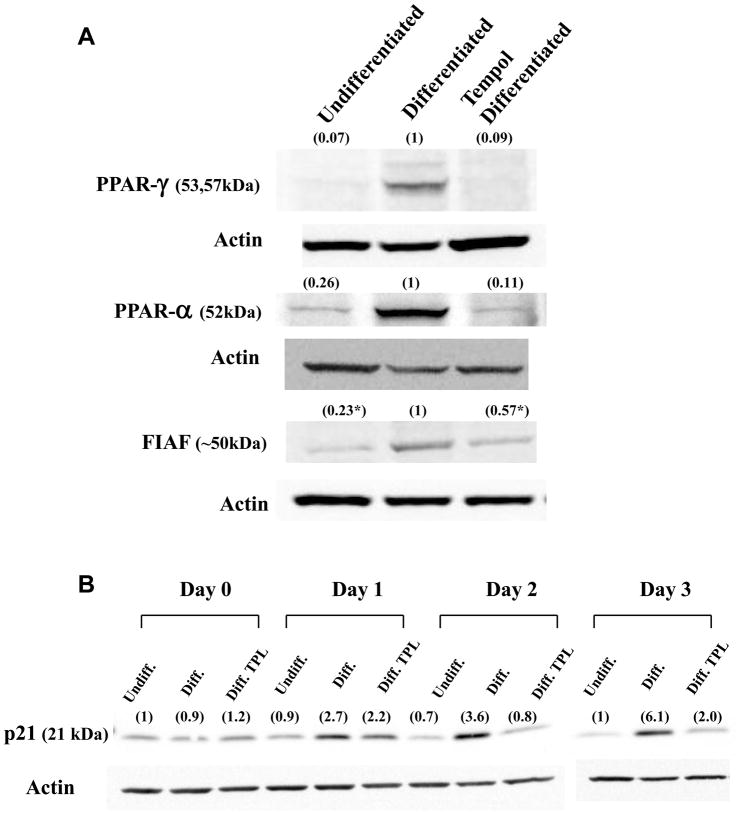
To better understand the molecular mechanism underlying the effect of Tempol, untreated, differentiated, and 2 mM Tempol-treated-differentiated 3T3-L1 cells were also assayed for content of various proteins associated with adipocyte differentiation. Tempol was used at a 2 mM concentration since it conferred a larger inhibitory effect on differentiation of adipocytes. The levels of transcription factors PPARγ, PPARα , and of the secreted protein FIAF are shown in Figure 2A. While the expression levels of both PPARγ and PPARα increased upon differentiation of the cells, in accord with previous published results [19], addition of 2 mM Tempol inhibited this elevation. The expression level of FIAF, a secreted protein, which is positively regulated by PPARγ and PPARα, also increased upon differentiation, and similarly decreased with concomitant addition of Tempol (p < 0.05). The effect of Tempol on cell cycle was also evaluated by measuring the levels of p21 (Figure 2B), a cyclin dependent kinase inhibitor, which is known to increase in pre-differentiated post mitotic arrested cells. While differentiated cells showed a gradual increase in the protein levels of p21 in the first 3 days following initiation of differentiation, co-administration of 2 mM Tempol inhibited this rise. The protein levels of p21 in Tempol treated cells were similar to those of un-differentiated 3T3-L1 cells.
Figure 2. Tempol decreases the protein levels of PPAR-γ, PPAR-α , and FIAF proteins in differentiated 3T3-L1 cells.
A) Proteins from undifferentiated, differentiated, and Tempol-treated (2 mM)-differentiated whole cell extracts were resolved by SDS-PAGE, blotted, and probed with anti-PPAR-γ, -PPAR-α , and FIAF, and HRP-conjugated secondary antibodies. B) Levels of p21 increased upon 3T3-L1 differentiation and decreased with co-administration of 2 mM Tempol. Above each lane is the optical density value (in parenthesis) for each protein band corrected for the actin loading control using the differentiated protein level as the relative control. For FIAF, *p < 0.05, n = 3). For the p21 time course study the first lane (undifferentiated) was used as the relative control (Day 0–2 for one gel and Day 3 for the other gel). *p < 0.05.
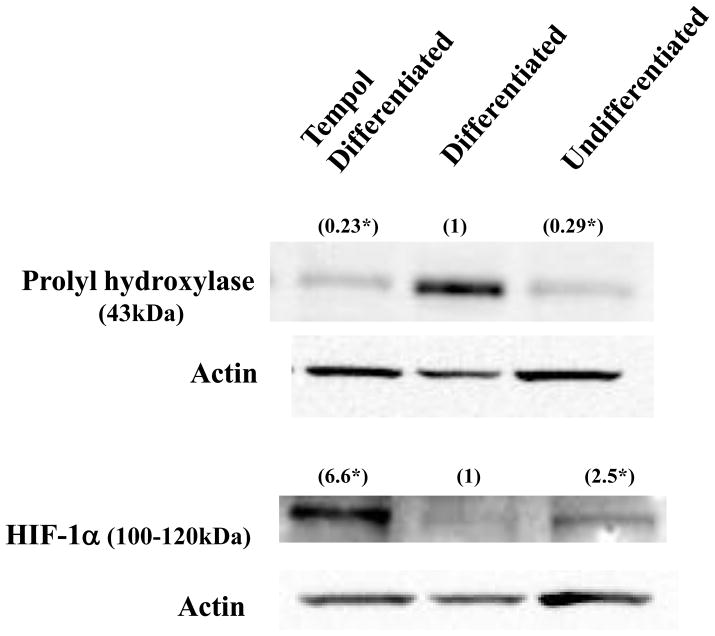
The levels of hypoxia-related gene products known to affect adipogenesis are shown in Figure 3. Under normoxia HIF-1α undergoes hydroxylation by prolyl-hydroxylase (PHD). This targets HIF-1α for proteasomal degradation via the von Hippel-Lindau ubiquitylation complex. Differentiation of 3T3-L1 cells up-regulated the protein levels of PHD and down-regulated that of HIF-1α , in agreement with previously published results [24]. Addition of 2 mM Tempol inhibited this increase in the levels of PHD (p < 0.05), and also up-regulated the levels of HIF-1α (p < 0.05) in a similar manner to the effect of hypoxia on these proteins. Co-administration of ascorbate alone (1 mM) or in combination with Tempol (to reduce Tempol to Tempol-H) did not cause these changes, indicating that the oxidized form of Tempol is required for this effect and that a reducing agent such as ascorbate is incapable of up-regulating HIF-1a or preventing the up-regulation of PHD (data not shown).
Figure 3. Tempol treatment mimics hypoxia in 3T3-L1 cells.
Proteins from undifferentiated, differentiated, and Tempol-treated-differentiated whole cell extracts were resolved by SDS-PAGE, blotted, and probed with anti-PHD, anti-HIF-1α , and conjugated secondary antibodies. The levels of PHD and HIF-1α proteins increased and decreased, respectively, upon differentiation. Conversely, treatment with 2 mM Tempol lowered the levels of PHD protein but increased HIF-1α , thus essentially mimicking hypoxia. Above each lane is the optical density value (in parenthesis) of each protein band corrected for the actin loading control using the differentiated protein level as the relative control. (*p < 0.05, n = 3)
The Effect of Tempol on Weight and IGF-1 Levels
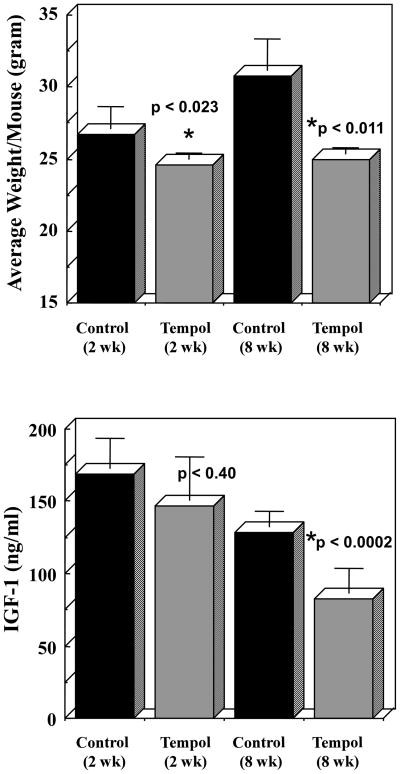
While 3T3-L1 cells are widely used as a model for studying adipocyte differentiation and adipogenesis, they do not reflect the complex relationship of adipose tissue with liver and muscle, nor do they allow the study of hormonal changes. To answer whether Tempol affects metabolic parameters C3H mice were kept on bacon-flavored chow diet with or without Tempol. Animals were weighed weekly and the results are shown in Figure 4A. The average weight of control animals was significantly higher than that of Tempol-fed at all time points measured. Furthermore, while the average weight of control animals gradually increased with time, the mean weight of Tempol-fed animals remained constant. Since caloric restricted animals are known to have to changes in their insulin metabolism, serum from the animals was assayed for levels of IGF-1. The serum from control animals exhibited a decrease in IGF-1 levels with time (Figure 4B). This decrease was even more pronounced in Tempol-fed animals, and was significantly different at the 8 weeks time point. Thus, Tempol-fed animals not only weighed less but also showed decreased levels of IGF-1.
Figure 4. Tempol mimics caloric restriction and reduces serum level of IGF-1 in mice.
9 week old control and Tempol-fed animals were monitored for changes in weight and IGF-1 serum levels. (A) The average weight of control animals was significantly higher than that of Tempol-fed at all time points measured. (B) Serum IGF-1 levels from Tempol-fed animals was significantly lower than those of control. Data are presented as mean ± SEM.
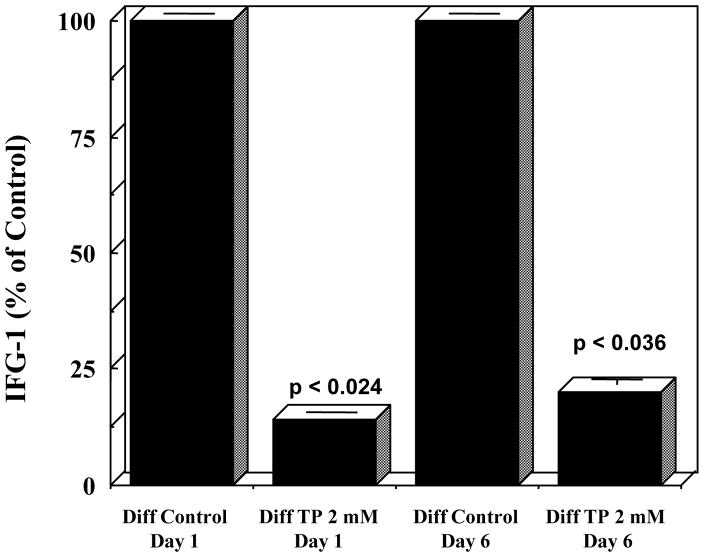
The levels of IGF-1, which mimic insulin in glucose and lipid metabolism [25], were also assayed in the medium from in vitro 3T3-L1 cultures and are shown in Figure 5. IGF-1 levels from Tempol-treated-differentiated cells were lower than those of differentiated cells on days 1 and 6 following initiation of differentiation. Thus, administration of Tempol resulted in reduction of levels of IGF-1 both in 3T3-L1 cells and in animals.
Figure 5. Tempol treatment decreases secreted IGF-1 levels in 3T3-L1 cells.
Media from differentiated, and Tempol-treated-differentiated cells were collected on Day 1 and Day 6 after initiation of differentiation, and assayed for IGF-1 levels. Treatment with 2 mM Tempol decreased the levels of IGF-1. Data are presented as % of control ± SEM.
Discussion
We have previously reported that administration of Tempol in food mimics caloric restriction in mice and caused a significant decrease in body weight [21]. To better understand the molecular mechanism underlying this effect we studied the differentiation process of 3T3-L1 pre-adipocytes in the presence of Tempol. The results from the present study demonstrate that Tempol inhibited differentiation of 3T3-L1 pre-adipocytes, but had no cytotoxic effects. The nitroxide also lowered the protein levels of transcription factors PPAR-α , PPAR-γ, and increased the protein levels of HIF-1α in a manner similar to that seen in hypoxia. Hypoxia is known to negatively regulate cellular differentiation in 3T3-L1 cells [19]. Tempol has been previously shown to increase HIF-1α levels in MCF7 cells [26]. The findings of the present study show that Tempol-mediated inhibition of 3T3-L1 differentiation is correlated to the elevation of HIF-1α levels.
Previously, under hypoxic conditions, 3T3-L1 pre-adipocytes were shown to be maintained in their precursor phenotype with their adipogenic genes PPAR-γ and C/EBPα repressed [19]. This transcriptional repression of PPAR-γ was only seen in pre-adipocytes but not in mature adipocytes [24]. Adipocyte differentiation was also shown to be blocked upon expression of DEC/Stra13, a transcription repressor for PPAR-γ and a target of HIF-1α [19]. It has also been shown that hypoxia inhibited adipocyte differentiation in a histone deacetylase-independent manner by inhibition of PPARγ, and by activation of AMP-activated protein kinase (AMPK) which impairs the clonal expansion phase of pre-adipocytes [27]. Such activation of AMPK was recently suggested as a means to down-regulate PPAR-γ, and to inhibit differentiation both in 3T3-L1 cells in vitro and in vivo using a mouse diet induced obesity model [28, 29]. Our results indicate that Tempol inhibits the mitotic clonal expansion, which occurs when differentiating 3T3-L1 cells are treated with insulin, dexamethasone, and IBMX cocktail. The inhibition was concentration dependent with 1 mM Tempol inhibiting the clonal expansion by ~ 23% (218% to 169%). The inhibition of lipid storage with 1 mM Tempol was also reduced by ~ 30% (Fig 1B). Hence, there appears to be a correlation between the reduction in lipid storage and clonal expansion. The role of clonal expansion in 3T3-L1 differentiation is controversial [30–32]. An inhibitor of PKA (protein kinase A) was found to completely inhibit clonal expansion while still differentiating the 3T3-L1 cells into adipose cells [32]. Thus it seems possible that while Tempol does inhibit clonal expansion that it may also have other effects on the differentiation process. The close relationship between growth arrest and adipocytes differentiation has also been reported [30]. A transient elevation in p21 levels during the pre-differentiated state of post-mitotic growth arrest was shown to be inversely correlated with the level of PPARγ [33]. Inoue et. al., showed that p21 is induced during 3T3-L1 differentiation and is very important in the latter stages of differentiation [34]. Although not absolutely required, it is a contributor to the differentiation process [34]. Our findings, in accord with these observations, showed that differentiated 3T3-L1 cells demonstrated a gradual increase in p21 levels in the first 3 days of differentiation, followed by a decline. Treatment with 2 mM Tempol attenuated the p21 increase, essentially mirroring the expression profile of PPARγ.
The mechanisms by which Tempol mimics hypoxia and inhibits differentiation are unclear. Studies have shown that reactive oxygen species can mediate the stabilization of HIF-1α [26]. In particular, it was reported that down regulation of MnSOD caused an increase in the protein levels of HIF-1α . Under hypoxia, treatment with Tempol (mM range) also caused an increase in HIF-1α , indicating the possible regulatory role of superoxide radicals [26]. Tempol acts as an SOD-mimic and thus can affect the cellular redox state [35, 36]. Modulation of the redox state of 3T3-L1 pre-adipocytes by addition of N-acetylcysteine was previously shown to inhibit differentiation [17]. In our study, similar attempts to modulate the redox state by addition of ascorbic acid failed to inhibit differentiation. Although Tempol facilitates H2O2 generation upon dismutation of superoxide radicals, the reported concentrations of H2O2 in the presence of Tempol were too low to constitute an additional oxidizing stress [26]. Still, H2O2 treatment was shown to reduce insulin responsiveness, to impair insulin-induced GLUT3 translocation, and to stimulate insulin receptor phosphorylation in 3T3-L1 and isolated adipocytes [37–39].
In our study, Tempol not only caused up-regulation of HIF-1α , but more importantly, down-regulated the levels of prolyl hydroxylase (PHD) protein, the upstream enzyme that targets HIF-1α to degradation by the ubiquitin-proteasome system. While the importance of PHD has been recognized, its relationship with HIF-1α is complex. Pharmacologic inhibition of PHD was shown to inhibit adipogenesis in 3T3-L1 cells, but did not consistently correlate with an increase in the levels of HIF-1α protein, suggesting additional hydroxylation-independent mechanisms [40]. Tempol could also affect the activity of PHD in a more direct fashion. PHD utilizes oxygen as co-substrate providing the molecular basis of the oxygen-sensing function of the enzyme, but it also requires 2-oxoglutarate and iron as cofactors. Since Tempol is known to interact with redox active metals such as iron [41], the nitroxide could inhibit the activity of the enzyme, causing an increase in the levels of HIF-1α .
Insulin-like growth factor (IGF-1), which is normally secreted from liver, muscle, and adipose tissues, is known to mimic insulin in glucose and lipid metabolism [42]. The hormone is also found in higher levels in serum of obese patients and mice when compared to normal individuals [3, 43]. In our study, Tempol treated animals displayed a significant decrease in body weight both after 2 and 8 weeks, but also a reduction in the levels of IGF-1 in serum. This is in accord with other reports showing that caloric restriction resulted in lower IGF-1 level [44, 45]. When we assayed the medium for secreted IGF-1 from 3T3-L1 cells in cultures we found that Tempol substantially reduced the amount of secreted IGF-1 when compared to the differentiated 3T3-L1 cells. Although we have used different concentrations of Tempol in the cell work (mM) and in animals (μM range, unpublished results), both systems follow a similar trend of Tempol treatment decreasing the levels of IGF-1. Interestingly, while the treatment of differentiated cells with Tempol changed the protein levels of PPARγ, FIAF, PHD and HIF-1α to those of undifferentiated cells, the levels of IGF-1 from differentiated Tempol-treated cells was significantly lower and different from those of undifferentiated cells. This suggests that the effect of Tempol on IGF-1 is not only dependent on the differentiation status of the cells but is directly affected by the nitroxide. The reduction in the levels of IGF-1 in 3T3-L1 cells upon treatment with Tempol might also have an indirect effect on the differentiation of the cells via the IGF-binding proteins. The latter was recently shown to affect the differentiation of 3T3-L1 by inhibiting PPAR-γ heterodimerization [46]. IGF-1 receptor tyrosine kinase signaling is also required for induction of 3T3-L1 pre-adipocyte differentiation and lead to their clonal expansion [47].
It is important to note that 3T3-L1 cells, which represent a distinct population of white adipose tissue, are extensively used as a model for adipocyte differentiation and adipogenesis. Mammals also have brown adipose tissue, which is important for energy expenditure, via the expression of uncoupling proteins [4]. Previously, we have shown that Tempol-fed animals, which exhibited weight loss also showed increased skeletal muscle production of UCP-2 [21]. The possibility that Tempol exerts additional effects on the levels of UCP in brown adipose tissue, or that it affects lipid metabolism (lipogenesis/lipolysis) in liver warrants further investigation.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yanovski SZ, Yanovski JA. Obesity. N Engl J Med. 2002;346:591–602. doi: 10.1056/NEJMra012586. [DOI] [PubMed] [Google Scholar]
- 2.Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? BioEssays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- 3.Wheatley KE, Williams EA, Smith NC, Dillard A, Park EY, Nunez NP, Hursting SD, Lane MA. Low-carbohydrate diet versus caloric restriction: effects on weight loss, hormones, and colon tumor growth in obese mice. Nutr Cancer. 2008;60:61–68. doi: 10.1080/01635580701510150. [DOI] [PubMed] [Google Scholar]
- 4.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 6.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 7.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 8.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 10.Cowherd RM, Lyle RE, McGehee REJ. Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 11.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 12.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 13.Nakano R, Kurosaki E, Yoshida S, Yokono M, Shimaya A, Maruyama T, Shibasaki M. Antagonism of peroxisome proliferator-activated receptor gamma prevents high-fat diet-induced obesity in vivo. Biochem Pharmacol. 2006;72:42–52. doi: 10.1016/j.bcp.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Gerhold DL, Liu F, Jiang G, Li Z, Xu J, Lu M, Sachs JR, Bagchi A, Fridman A, Holder DJ, Doebber TW, Berger J, Elbrecht A, Moller DE, Zhang BB. Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-gamma agonists. Endocrinology. 2002;143:2106–2118. doi: 10.1210/endo.143.6.8842. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- 16.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JR, Ryu HH, Chung HJ, Lee JH, Kim SW, Kwun WH, Baek SH, Kim JH. Association of anti-obesity activity of N-acetylcysteine with metallothionein-II down-regulation. Exp Mol Med. 2006;38:162–172. doi: 10.1038/emm.2006.20. [DOI] [PubMed] [Google Scholar]
- 18.Kamigaki M, Sakaue S, Tsujino I, Ohira H, Ikeda D, Itoh N, Ishimaru S, Ohtsuka Y, Nishimura M. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;339:624–632. doi: 10.1016/j.bbrc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 20.Regazzetti C, Peraldi P, Gremeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 2009;58:95–103. doi: 10.2337/db08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 22.Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974;1:113–116. [Google Scholar]
- 23.Daughaday WH, Mariz IK, Blethen SL. Inhibition of access of bound somatomedin to membrane receptor and immunobinding sites: a comparison of radioreceptor and radioimmunoassay of somatomedin in native and acid-ethanol-extracted serum. J Clin Endocrinol Metab. 1980;51:781–788. doi: 10.1210/jcem-51-4-781. [DOI] [PubMed] [Google Scholar]
- 24.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 26.Kaewpila S, Venkataraman S, Buettner GR, Oberley LW. Manganese superoxide dismutase modulates hypoxia-inducible factor-1 alpha induction via superoxide. Cancer Res. 2008;68:2781–2788. doi: 10.1158/0008-5472.CAN-07-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KH, Song MJ, Chung J, Park H, Kim JB. Hypoxia inhibits adipocyte differentiation in a HDAC-independent manner. Biochem Biophys Res Commun. 2005;333:1178–1184. doi: 10.1016/j.bbrc.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 28.Giri S, Rattan R, Haq E, Khan M, Yasmin R, Won JS, Key L, Singh AK, Singh I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr Metab (Lond) 2006;3:31. doi: 10.1186/1743-7075-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YK, Lee WS, Hwang JT, Kwon DY, Surh YJ, Park OJ. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J Agric Food Chem. 2009;57:305–310. doi: 10.1021/jf802737z. [DOI] [PubMed] [Google Scholar]
- 30.Yeh WC, Bierer BE, McKnight SL. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. Proc Natl Acad Sci U S A. 1995;92:11086–11090. doi: 10.1073/pnas.92.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu Z, Wei Y, Chen N, Jiang M, Wu J, Liao K. DNA synthesis and mitotic clonal expansion is not a required step for 3T3-L1 preadipocyte differentiation into adipocytes. J Biol Chem. 2001;276:11988–11995. doi: 10.1074/jbc.M011729200. [DOI] [PubMed] [Google Scholar]
- 32.Martini CN, Plaza MV, Vila Mdel C. PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts differentiation. Mol Cell Endocrinol. 2009;298:42–47. doi: 10.1016/j.mce.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Morrison RF, Farmer SR. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J Biol Chem. 1999;274:17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 34.Inoue N, Yahagi N, Yamamoto T, Ishikawa M, Watanabe K, Matsuzaka T, Nakagawa Y, Takeuchi Y, Kobayashi K, Takahashi A, Suzuki H, Hasty AH, Toyoshima H, Yamada N, Shimano H. Cyclin-dependent kinase inhibitor, p21WAF1/CIP1, is involved in adipocyte differentiation and hypertrophy, linking to obesity, and insulin resistance. J Biol Chem. 2008;283:21220–21229. doi: 10.1074/jbc.M801824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuni A, Krishna CM, Riesz P, Finkelstein E, Russo A. A novel metal-free low molecular weight superoxide dismutase mimic. J Biol Chem. 1988;263:17921–17924. [PubMed] [Google Scholar]
- 36.Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, Russo A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990;29:2802–2807. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- 37.Hayes GR, Lockwood DH. Role of insulin receptor phosphorylation in the insulinomimetic effects of hydrogen peroxide. Proc Natl Acad Sci U S A. 1987;84:8115–8119. doi: 10.1073/pnas.84.22.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudich A, Kozlovsky N, Potashnik R, Bashan N. Oxidant stress reduces insulin responsiveness in 3T3-L1 adipocytes. Am J Physiol. 1997;272:E935–40. doi: 10.1152/ajpendo.1997.272.5.E935. [DOI] [PubMed] [Google Scholar]
- 39.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes. 1998;47:1562–1569. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 40.Floyd ZE, Kilroy G, Wu X, Gimble JM. Effects of prolyl hydroxylase inhibitors on adipogenesis and hypoxia inducible factor 1 alpha levels under normoxic conditions. J Cell Biochem. 2007;101:1545–1557. doi: 10.1002/jcb.21266. [DOI] [PubMed] [Google Scholar]
- 41.Krishna MC, Russo A, Mitchell JB, Goldstein S, Dafni H, Samuni A. Do nitroxides antioxidants act as scavengers of superoxide or as SOD mimics? J Biol Chem. 1996;271:26026–26031. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- 42.Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990;70:591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- 43.Wabitsch M, Blum WF, Muche R, Heinze E, Haug C, Mayer H, Teller W. Insulin-like growth factors and their binding proteins before and after weight loss and their associations with hormonal and metabolic parameters in obese adolescent girls. Int J Obes Relat Metab Disord. 1996;20:1073–1080. [PubMed] [Google Scholar]
- 44.Takenaka A, Oki N, Takahashi SI, Noguchi T. Dietary restriction of single essential amino acids reduces plasma insulin-like growth factor-I (IGF-I) but does not affect plasma IGF-binding protein-1 in rats. J Nutr. 2000;130:2910–2914. doi: 10.1093/jn/130.12.2910. [DOI] [PubMed] [Google Scholar]
- 45.Williams EA, Perkins SN, Smith NC, Hursting SD, Lane MA. Carbohydrate versus energy restriction: effects on weight loss, body composition and metabolism. Ann Nutr Metab. 2007;51:232–243. doi: 10.1159/000104143. [DOI] [PubMed] [Google Scholar]
- 46.Chan SS, Schedlich LJ, Twigg SM, Baxter RC. Inhibition of adipocyte differentiation by insulin-like growth factor-binding protein-3. Am J Physiol Endocrinol Metab. 2009;296:E654–63. doi: 10.1152/ajpendo.90846.2008. [DOI] [PubMed] [Google Scholar]
- 47.Jin S, Zhai B, Qiu Z, Wu J, Lane MD, Liao K. c-Crk, a substrate of the insulin-like growth factor-1 receptor tyrosine kinase, functions as an early signal mediator in the adipocyte differentiation process. J Biol Chem. 2000;275:34344–34352. doi: 10.1074/jbc.M004927200. [DOI] [PubMed] [Google Scholar]