Abstract
Objective
Although gastric cancer incidence is declining in China, trends may differ from historical patterns in developed countries. Our aim was to (1) retrospectively estimate the effects of Helicobacter pylori (H. pylori) and smoking on past gastric cancer incidence and (2) project how interventions on these two risk factors can reduce future incidence.
Methods
We used a population-based model of intestinal-type gastric cancer to estimate gastric cancer incidence between 1985 and 2050. Disease and risk factor data in the model were from community-based epidemiological studies and national prevalence surveys.
Results
Between 1985 and 2005, age-standardized gastric cancer incidence among Chinese men declined from 30.8 to 27.2 per 100,000 (12%); trends in H. pylori and smoking prevalences accounted for >30% of overall decline. If past risk factor trends continue, gastric cancer incidence will decline an additional 30% by 2050. Yet, annual cases will increase from 116,000 to 201,000 due to population growth and aging. Assuming that H. pylori prevention/treatment and tobacco control are implemented in 2010, the decline in gastric cancer incidence is projected to increase to 33% with universal H. pylori treatment for 20-year-olds, 42% for a hypothetical childhood H. pylori vaccine, and 34% for aggressive tobacco control.
Conclusions
The decline in gastric cancer incidence has been slower than in developed countries and will be offset by population growth and aging. Public health interventions should be implemented to reduce the total number of cases.
Keywords: Gastric cancer, Helicobacter pylori, Smoking, Cancer prevention, China
Introduction
Gastric cancer is the second leading cause of cancer-related deaths, with an estimated annual 933,000 new cases and 700,000 deaths worldwide (10.4% of all cancer deaths) [1]. In most developed countries, gastric cancer incidence has declined dramatically without targeted interventions. The “unplanned triumph” has been attributed to improvements in sanitation, greater fruit and fresh vegetable consumption, and reduced Helicobacter pylori infection [2]. Responsible for 74–78% of all cases [3], H. pylori is the leading risk factor for gastric cancer. As prevalence has declined, <30% are infected in high-income countries such as the United States and the United Kingdom [4–6]. Other risk factors include low vegetable and fruit intake, high dietary salt, and smoking [7–11].
Helicobacter pylori treatment can potentially prevent gastric cancer by reducing the progress of precancerous lesions, defined as atrophy, intestinal metaplasia, or dysplasia, to invasive cancer. Several clinical trials are under way [12], and results from the first of such studies suggest that treatment for H. pylori has the potential to reduce the risk of gastric cancer development, with the optimal time for treatment being before precancerous lesions are present [13, 14]. Clinical studies on intermediate outcomes also provide indirect support for a benefit on cancer risk via reduced progression of precancerous lesions to cancer [15, 16]. Despite improved understanding of gastric cancer etiology and clinical interventions, the effects of changing risk factor trends and availability of H. pylori treatment on disease rates at the population level have not been quantified.
China accounts for 40% of all gastric cancer cases worldwide [1] and more than 30% of the world’s tobacco consumption [17]. Currently, an estimated 58% of the population is infected with H. pylori [3], and approximately 60% of adult men and less than 5% of adult women smoke [17–19]. To better understand the population-level dynamics of a disease with unique historical pattern of impressive decline in some populations, and large remaining burden in others, we retrospectively (1) estimated the effects of H. pylori and smoking on past gastric cancer trends and (2) projected how interventions on these two factors can reduce future incidence.
Materials and methods
Overview
We conducted a population-based analysis of gastric cancer among men in China to estimate the effects of risk factors on past trends and future projections of gastric cancer incidence. The analysis included all Chinese men born between 1905 and 2034, divided into 5-year birth cohorts. For each cohort, risk factor prevalence was based on community-based epidemiological studies and national prevalence surveys. We leveraged a previously published natural history model of gastric cancer [20], which includes H. pylori infection and smoking as factors that affect disease initiation and progression, to estimate the incidence of gastric cancer for each cohort in the analysis. (See supplementary materials for details.) Gastric cancer rates for the different birth cohorts were then weighted using population figures from the United Nations (UN) Population Division [21] to estimate overall gastric cancer incidence and cases in all ages combined. We conducted the analysis separately for rural and urban regions because there are differences in infection and other epidemiological characteristics [22, 23]. National incidence was based on rural and urban estimates, weighted by the corresponding share of the population.
To separate changes in age-specific incidence from changes in the population age structure, we report age-standardized incidence. We used the Segi world population for standardization [24]. Because population growth and aging are important determinants of the total number of cases, we also analyzed what proportion of the projected increase in gastric cancer cases is due to these demographic changes. To do this, we recalculated the expected number of cases in each year after 2005 using the modeled annual incidence in 2050 multiplied by year-specific population estimates (i.e., fixed incidence and changing population). Conversely, the effect of epidemiological change was estimated using annual incidence in each year and the 2005 population (i.e., changing incidence and fixed population).
Risk factor and mortality data by birth cohort
For each birth cohort, the prevalences of H. pylori seropositivity and smoking were estimated from community-based epidemiological studies and national prevalence surveys [11, 19, 22, 23, 25–28]. We assumed that prevalences were established by age 20 and remained unchanged through the life course of the cohort because (1) individuals become infected with H. pylori in childhood and remain infected unless treated [29] and (2) smoking starts between the ages of 15 and 23, with subsequent lifetime cessation rates of less than 10% in the absence of aggressive tobacco control [19, 30]. We also assumed that prevalences of the two risk factors were not correlated because smoking initiation is unlikely to be related to infection. While individuals may move between rural and urban regions in adulthood, their H. pylori status, which reflects childhood socioeconomic factors [31–33], and smoking prevalence at age 20 were specific to their location at birth and maintained over time.
Epidemiological studies have shown that (1) H. pylori-seropositive individuals have a higher risk of progression to atrophy than those who are H. pylori-negative and (2) smokers face higher risks of progression to intestinal metaplasia and dysplasia than nonsmokers (relative risk = 1.3 and 2.2, respectively) [34, 35]. When these data are used in the gastric cancer natural history model, the relative risks of disease associated with H. pylori and smoking were estimated as 4.9 and 1.5, respectively. These values are within the range of published estimates (3.0–5.9 and 1.4–2.2, respectively [9–11, 36]). Additional model parameter details are provided in the supplementary materials.
Helicobacter pylori prevalence
A study of 46 rural counties estimated that H. pylori prevalence ranged from 55.8% among 35–39-year-olds (corresponding to the 1945–1949 birth cohort) to 67.1% among 50–54-year-olds (1925–1929 birth cohort) [22]. Based on these data, we estimated that H. pylori infection declined by 4.5% between successive 5-year birth cohorts. For urban populations, a study in Shanghai found that H. pylori prevalence varied from 50% among 50–59-year-olds (1935–1939 birth cohort) to 41.4% among 20–29-year-olds (1965–1969 birth cohort), suggesting a 3.1% decline in infection between successive 5-year birth cohorts [23]. We estimated H. pylori prevalence for other birth cohorts by extrapolating the inter-cohort decline rates [4].
Smoking
A retrospective mortality study on smoking-related deaths (1905–1929 birth cohorts) and a nationwide survey of smoking prevalence (1930–1979 birth cohorts) [11, 19] estimated that birth-cohort-specific smoking prevalence in China ranged from 50% (1905–1909 birth cohort) to 75% (1950–1954 birth cohort) and declined again to 50% for the 1975–1979 birth cohort. Data from three rounds of the Ministry of Health National Health Service Survey also showed a decrease in smoking prevalence among 18-year-olds between 1993 and 2003 [25–27]. Based on these data, we estimated that smoking prevalence would decline by 9% between each successive 5-year post-1975 birth cohort. We used the same smoking prevalence for rural and urban areas because of their similar observed prevalence (68 vs. 64%) [19].
Mortality from competing causes and gastric cancer
Background mortality rates (i.e., mortality from causes other than gastric cancer) were estimated using data from 1953–1964, 1964–1982, 1982–1990, 1990–2000, 1999–2000, and 2005–2030 [37–40]. For birth cohorts born prior to 1953, we used 1953–1964 mortality rates because there were limited changes in adult mortality prior to the 1949 founding of the People’s Republic of China [41]. Because gastric cancer treatment has advanced little in the last century, we assumed that case-fatality for incident gastric cancer cases was the same for all birth cohorts.
Retrospective and prospective risk factor scenarios
We considered two types of scenarios to examine trends in gastric cancer and its major risk factors between 1985 and 2050 (Table 1) [11, 13, 15, 16, 19, 22, 23, 25–28, 42, 43]. We report results for years after 1985, the earliest year for which the population consisted only of post-1905 birth cohorts included in our model.
Table 1.
Risk factor scenarios used in the analysis
| Scenario | Notation | Definitiona |
|---|---|---|
| Type 1: Retrospective risk factor exposure, 1985–2005 | ||
| Epidemiologic changes halted | (EPI) |
H. pylori and smoking prevalences remained at 1905–1909 birth cohort levels in subsequent cohorts |
| Observed H. pylori prevalence decline | (HP) | The prevalence of H. pylori declined by 4.5 and 3.1% every 5-year 1905–2034 birth cohort in rural and urban populations, respectively, as observed [22, 23]. Smoking prevalence remained at 1905–1909 birth cohort levels for all cohorts |
| Observed smoking prevalence changes | (SM) | Smoking prevalence changed from 50 to 75% for 1905–1974 birth cohorts, and then declined by 9% for every post-1975 birth cohort as observed in China [11, 19, 25–28]. H. pylori prevalence remained at 1905–1909 birth cohort levels for all cohorts |
| Both observed H. pylori and smoking prevalence changes |
(ALL) | Combination of observed changes in H. pylori prevalence and smoking for each birth cohort |
| Type 2: Prospective risk factor projectionsb, 2005–2050 | ||
| Continued H. pylori and smoking trends | (CONT) | Birth cohort trends for H. pylori and smoking prevalences continue (as described above in ALL) for cohorts born between 1985 and 2034 |
| H. pylori treatment | (HP-T) | Universal H. pylori treatment for 20-year-olds with 100% coverage. Based on clinical studies, we assumed that H. pylori treatment reduced disease progression only among individuals without advanced precancerous lesions (i.e., gastritis or atrophy) [13, 15, 16]. We also assumed that treated individuals remain uninfected as adult reinfection is rare [42, 43]. See supplementary materials for additional details |
| H. pylori vaccine | (HP-V) | Hypothetical childhood H. pylori vaccination with 100% coverage and efficacy, including a catch-up program for those aged 5–19 years. We assumed vaccination reduces the risk of infection to zero, thereby reducing disease risk from 3.7 to 0.7% (96%) |
| Tobacco control | (SM-C) | Tobacco control to reduce initiation among 20-year-olds reduced to 0%c |
All scenarios also incorporated change in background mortality risk from other diseases. Population growth (medium fertility variant) and urbanization were also included, using estimates from the UN population division
All interventions begin in 2010
We also estimated the additional benefit if risk among smokers ages 35 and younger in 2010 was reversible to non-smoking levels
Retrospective risk factor exposure, 1985–2005
To estimate the effects of risk factors on past disease trends, we compared the gastric cancer trend under the observed trends in H. pylori and smoking with those that would have been expected had H. pylori infection and smoking remained at 1905–1909 birth cohort levels (referred to as the “epidemiological halt” scenario). Each risk factor trend was also evaluated separately.
Prospective risk factor projection, 2005–2050
To quantify the potential effects of risk factor interventions on future gastric cancer in addition to risk factor trends, we used several H. pylori prevention/treatment and tobacco control scenarios. We assumed that these were implemented in 2010.
Results
Birth cohort risk factor trends
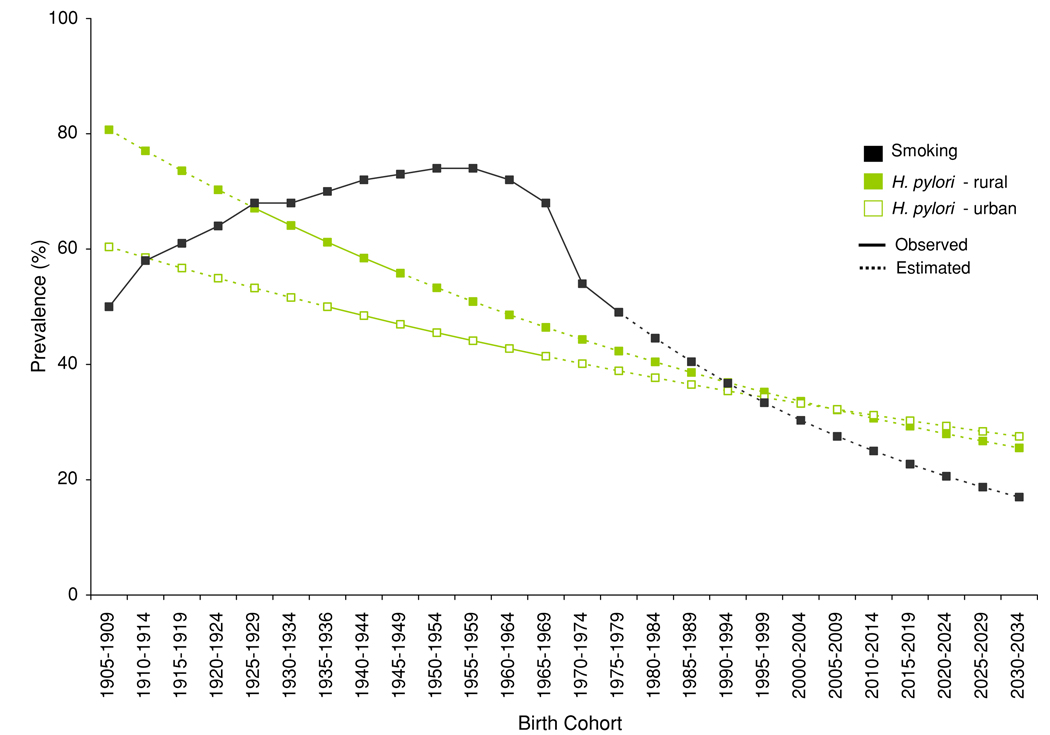
Figure 1 shows H. pylori and smoking prevalences for cohorts of men born between 1905 and 2034 in China. H. pylori prevalence declined from 81% in the 1905–1909 birth cohort to 39% in the 1985–1989 birth cohort in rural regions and from 60 to 37% in urban regions. If past trends continue, H. pylori prevalence for the 2030–2034 birth cohort is expected to decline to 26 and 28%, respectively, which is similar to the H. pylori prevalence among middle-aged Americans in 1990 [44]. If the decline in cohort smoking prevalence continues for post-1975 birth cohorts, less than 20% of individuals born in 2030 in China would become smokers. For comparison, currently 24% of all men in the United States smoke [45].
Fig. 1.
Birth cohort risk factor exposure at age 20. The graph shows the observed exposure for birth cohorts up to the 1975–1979 cohort and projected exposure for birth cohorts born after 1980
Retrospective risk factor exposure and gastric cancer incidence, 1985–2005
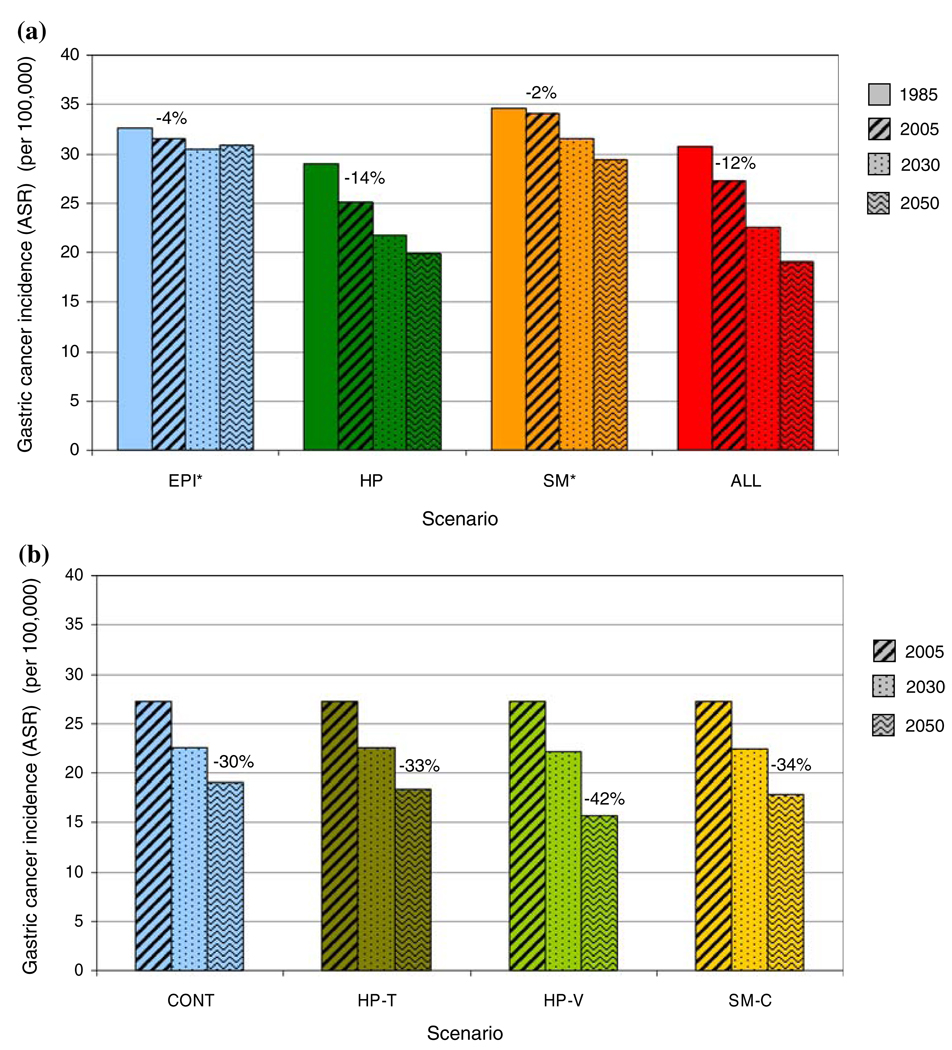
Modeled age-standardized incidence was 30.8 per 100,000 in 1985 and 27.2 per 100,000 in 2005 (a 12% decline; Table 2; Fig. 2a). The 2005 estimate was within 6–18% of reported rates, e.g., from GLOBOCAN [1], Global Burden of Disease study [40] and the national mortality routine reporting system (CHIS) [46]. The modeled 12% decline between 1985 and 2005, based on observed trends in H. pylori and smoking only, was approximately one-third of the observed decline in gastric cancer in China [46]. The effects of this decline reduced the cumulative risk of gastric cancer between 20 and 84 years of age from 4.5 to 4.0%. The decline in gastric cancer incidence was 10% in rural areas and 6% in urban areas. The national decline was larger than both of these because the proportion of the population in urban areas, where prevalence is lower, increased over this period.
Table 2.
Modeled gastric cancer incidence and number of cases for select scenarios
| Age-standardized gastric cancer incidence (per 100,000 men)a |
Crude gastric cancer incidence (per 100,000 men) |
Number of cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1985 | 2005 | 2030 | 2050 | 1985 | 2005 | 2030 | 2050 | 1985 | 2005 | 2030 | 2050 | |
| Type 1: Retrospective | ||||||||||||
| ALL | 30.8 | 27.2 | 22.6 | 19.0 | 23.7 | 25.0 | 35.2 | 37.6 | 74,900 | 116,200 | 194,800 | 200,800 |
| Type 2: Projected | ||||||||||||
| CONT | – | 27.2 | 22.6 | 19.0 | – | 25.0 | 35.2 | 37.6 | – | 116,200 | 194,800 | 200,800 |
| Hp-T | – | 27.2 | 22.5 | 18.3 | – | 25.0 | 35.1 | 36.8 | – | 116,200 | 194,300 | 196,300 |
| Hp-V | – | 27.2 | 22.2 | 15.7 | – | 25.0 | 34.8 | 34.1 | – | 116,200 | 192,700 | 182,100 |
| SM-C | – | 27.2 | 22.4 | 17.9 | – | 25.0 | 35.0 | 36.2 | – | 116,200 | 193,700 | 193,400 |
Age-standardized using the Segi world population
Fig. 2.
Age-standardized gastric cancer incidence for retrospective and prospective scenarios (see Table 1 for scenario definitions). a Age-standardized gastric cancer incidence between 1985 and 2050 for scenarios of past risk factor trends. Numbers show percentage of declines between 1985 and 2005. b Age-standardized gastric cancer incidence between 2005 and 2050 for future risk factor scenarios. Numbers show percentage of declines between 2005 and 2050. * A part of the decline in gastric cancer incidence is due to the increase in the proportion of urban population. For example, if proportion of urban population were held constant at 1985 level, age-standardized gastric cancer incidence between 1985 and 2005 would have increased for EPI (+0.2%) and SM (+2.2%)
Had the prevalence of H. pylori and smoking remained at the 1905–1909 birth cohort level (scenario EPI), age-standardized gastric cancer incidence would have decreased 4% between 1985 and 2005 (32.6 to 31.5 per 100,000). Had smoking prevalence remained unchanged at the 1905–1909 birth cohort level, the reduction in gastric cancer incidence from declining H. pylori prevalence (scenario HP) would have been even higher at 14%, versus the observed 12%.
Projection of risk factor exposure and gastric cancer incidence, 2005–2050
If observed birth cohort risk factor trends continue (scenario CONT), cumulative risk of gastric cancer between the ages of 20 and 84 will decline from 4.0 to 2.9% between 2005 and 2050. Age-standardized gastric cancer incidence will decline an additional 30% from 27.2 to 19.0 per 100,000 (Table 2; Fig. 2b). The annual rate of decline during this period would be approximately one-third higher than in 1985–2005, due largely to declining smoking prevalence among post-1960 birth cohorts.
Helicobacter pylori treatment at age 20 would reduce the lifetime risk of gastric cancer among infected individuals from 3.7 to 3.1% (17%). This effect is relatively small because treatment reduces disease progression only among individuals without advanced precancerous lesions and is not expected to benefit those who have already developed these lesions [13, 15, 16]. In birth cohorts that consist of infected and non-infected individuals, the benefits of treatment will depend on the prevalence of H. pylori infection. For example, universal treatment would reduce the lifetime gastric cancer risk of the 1990 birth cohort, which has an H. pylori prevalence of 35%, from 1.4 to 1.2%. When the effects of universal treatment in all birth cohorts are considered, the projected decline in gastric cancer between 2005 and 2050 would increase from 30 to 33% (18.3 per 100,000; Fig. 2b).
A hypothetical childhood H. pylori vaccine would have a larger benefit, leading to a 42% decline in gastric cancer because of its ability to prevent the development of pre-cancerous lesions present by age 20 in the vaccinated cohorts. The remaining incidence in 2050 will be 15.7 per 100,000 (16.4 per 100,000 if vaccine efficacy was only 80%). If young men do not take up smoking (SM-C), the incidence decline would be 34%, leading to an incidence of 17.9 per 100,000 in 2050. If gastric cancer risk among smokers aged 35 and younger were also reversible, tobacco control would reduce incidence to 16.2 per 100,000.
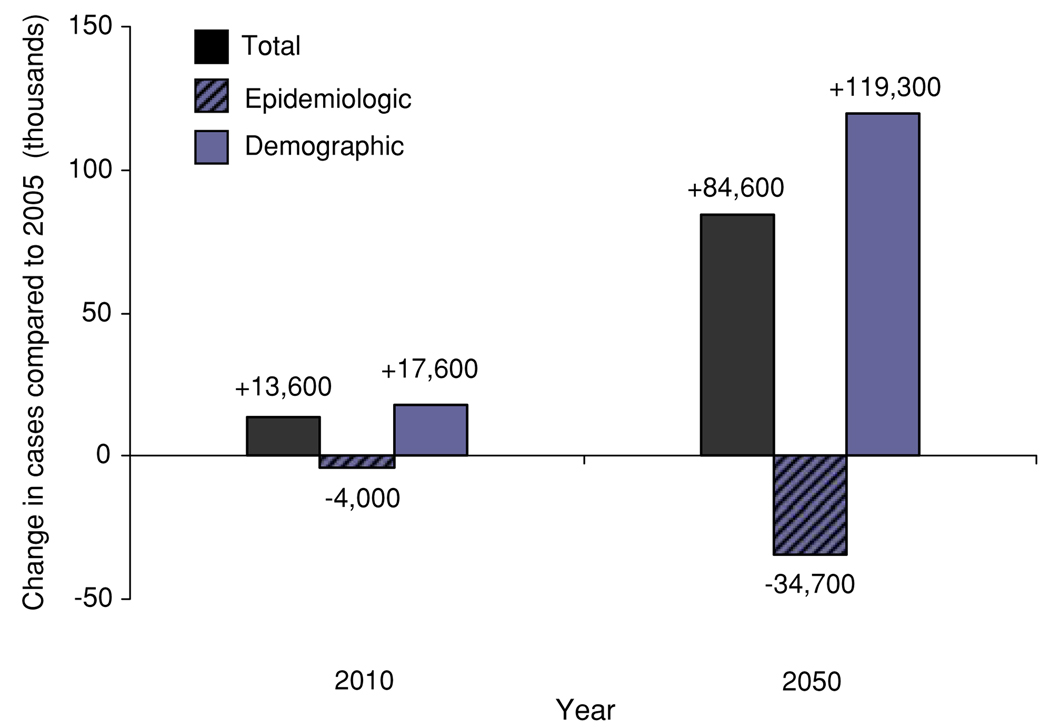
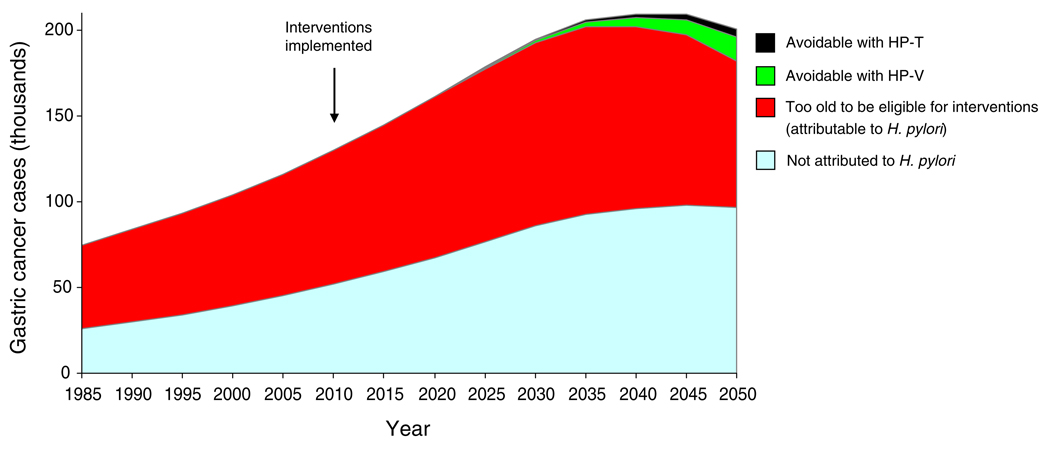
The benefits of lower H. pylori and smoking, seen as a declining age-specific incidence, will be outweighed by the fact that China is projected to have a larger and older population (Fig. 3). As a result, the number of cases among Chinese men is expected to nearly double from 116,000 in 2005 to 201,000 in 2050. Only after 2040 will the total number of cases begin to decline, by approximately 8,000 between 2040 and 2050 (<5%). Approximately 50% of gastric cancers in 2050 will be attributable to H. pylori infection (104,000 cases). Only 5–18% of these cases can be avoided with H. pylori interventions by 2050 as those benefiting will be too young to have realized the full benefits (Fig. 4). However, the benefits of prevention will increase substantially over time, as treated cohorts increase in number and age.
Fig. 3.
Change in the projected number of gastric cancer cases attributed to demographic and epidemiological changes between 2005 and 2010/2050. Number of cases attributed to each component is depicted for each year
Fig. 4.
Gastric cancer cases between 1985 and 2050: proportions attributable to H. pylori and avoidable through interventions
Discussion
Unlike the dramatic decline in developed countries, gastric cancer remains a major public health concern in China. We estimated that the combined changes in H. pylori and smoking trends were associated with a 12% decline in gastric cancer incidence between 1985 and 2005 among Chinese men, although approximately 20% of the total benefits from reductions in H. pylori infection were offset by the rise in smoking. Even if declining H. pylori and smoking trends continue, gastric cancer incidence in China in 2050 will be six times that of current levels in the United States [1], with the vast majority of new cases stemming from H. pylori infections decades earlier. Population growth and aging will overshadow this decline and lead to near doubling of the number of new cases by 2050. Smoking and H. pylori interventions can reduce incidence, but the full benefits will be realized only after several decades. Nonetheless, even in the analysis period, reducing smoking and preventing or treating H. pylori infection can have significant benefits, with the largest benefits from preventing smoking or H. pylori at younger ages.
As with any projections of future disease burden, our estimates are uncertain and affected by our model assumptions and some limitations. First, our estimates of past cohort H. pylori and smoking were based on epidemiologic studies from China but may miss possible within-cohort dynamics that happen later in life. For example, progression of gastritis to atrophy or intestinal metaplasia can lead to loss of H. pylori among a small proportion, or some of the older cohorts in our retrospective analysis may have begun smoking later in life. Future prevalences of H. pylori and smoking are also uncertain, as are those of other factors responsible for the unaccounted portion of variation in incidence, including dietary changes [47]. Second, based on the available epidemiological evidence, we assumed that H. pylori and smoking influence disease progression at specific and distinct stages of gastric cancer natural history. The estimated effects of risk factors would be underestimated if they also affect other disease stages. Third, the estimated model parameters for the effects of H. pylori on gastric cancer were relatively uncertain [20], but the effects on the projected gastric cancer incidence were less than 10%; further, the relative ordering of scenarios was unchanged. Fourth, our model parameters were estimated using epidemiologic data of the high-risk region of Linqu [1, 48]. We adjusted H. pylori and smoking to their national prevalence, but the etiological effects may vary by region. Risk factor interventions may also affect background mortality, especially for smoking because it is a risk factor for all-cause mortality. Lastly, our analysis focused only on men. Although H. pylori prevalence is similar among women, smoking is considerably lower and the projected decline in incidence and burden would likely be lower.
Despite these limitations, our analysis of risk factor trends and gastric cancer burden has several strengths. We used a state-transition model that was developed to reflect current scientific knowledge about the natural history of gastric cancer and which was parameterized using country-specific epidemiological data. The capability of the model to incorporate H. pylori infection and smoking allowed for a more complete representation of heterogeneity in disease progression. Incorporating these risk factors also allowed estimating how past exposure and future interventions affect disease trends and projections. As new data from clinical trials of H. pylori treatment and studies of smoking cessation become available, our results can be updated and refined to provide more accurate estimates of the burden and comparative benefits of gastric cancer prevention policies, providing a basis for discussing public health policy. Further, our estimates can be useful for assessing cost-effectiveness of various interventions, framing questions for epidemiological investigation, and highlighting high priority data that are needed to improve the precision of model estimates.
Projections of future cancer burden at the population level are inherently uncertain, but they are needed to guide the planning of cancer control programmes [49]. As has been observed in many developed countries, gastric cancer incidence is declining and is expected to continue declining in China. However, the relatively low decline rate, coupled with population growth and aging, is expected to nearly double the number of cases over the coming decades. The large absolute burden demonstrates the need for H. pylori treatment and smoking cessation programmes to accelerate the underlying rate of decline. Finding effective strategies for early detection and treatment of gastric cancers should also be a research priority.
Supplementary Material
Acknowledgments
Dr. Yeh was funded by the National Cancer Institute (R25-CA92203).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10552-009-9397-9) contains supplementary material, which is available to authorized users.
Contributor Information
Jennifer M. Yeh, Email: jyeh@hsph.harvard.edu, Center for Health Decision Science, Harvard School of Public Health, 718 Huntington Avenue, 2nd Floor, Boston, MA 02115, USA.
Sue J. Goldie, Center for Health Decision Science, Harvard School of Public Health, 718 Huntington Avenue, 2nd Floor, Boston, MA 02115, USA Department of Health Policy and Management, Harvard School of Public Health, Boston, MA, USA.
Karen M. Kuntz, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA
Majid Ezzati, Department of Global Health and Population, Harvard School of Public Health, Boston, MA, USA; Department of Environmental Health, Harvard School of Public Health, Boston, MA, USA.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM. IARC cancer base no. 5 Version 2.0. Lyon: IARC Press; 2004. GLOBOCAN 2002. Cancer incidence, mortality and prevalence worldwide. [Google Scholar]
- 2.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 4.Haruma K. Trend toward a reduced prevalence of Helicobacter pylori infection, chronic gastritis, and gastric cancer in Japan. Gastroenterol Clin North Am. 2000;29:623–631. doi: 10.1016/s0889-8553(05)70134-5. [DOI] [PubMed] [Google Scholar]
- 5.Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. J Infect Dis. 1993;168:219–221. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- 6.The EUROGAST Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;34:1672–1676. doi: 10.1136/gut.34.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 8.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 9.Chao A, Thun MJ, Henley SJ, Jacobs EJ, McCullough ML, Calle EE. Cigarette smoking, use of other tobacco products and stomach cancer mortality in US adults: the cancer prevention study II. Int J Cancer. 2002;101:380–389. doi: 10.1002/ijc.10614. [DOI] [PubMed] [Google Scholar]
- 10.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116:963–971. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 11.Liu BQ, Peto R, Chen ZM, Boreham J, Wu YP, Li JY, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–1422. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman D. Results of intervention trials in Helicobacter pylori-infected populations. In: Hunt R, Tytgat G, editors. Helicobacter pylori: basic mechanisms to clinical cure 2002. Dordrecht: Kluwer; 2003. pp. 225–230. [Google Scholar]
- 13.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 14.Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 16.You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–983. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 17.Mackay J, Eriksen M, Shafey O. The tobacco atlas. Atlanta: American Cancer Society; 2006. [Google Scholar]
- 18.Lopez A, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tob Control. 1994;3:242–247. [Google Scholar]
- 19.Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, et al. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA. 1999;282:1247–1253. doi: 10.1001/jama.282.13.1247. [DOI] [PubMed] [Google Scholar]
- 20.Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY, Goldie SJ. Development of an empirically calibrated model of gastric cancer in two high-risk countries. Cancer Epidemiol Biomarkers Prev. 2008;17:1179–1187. doi: 10.1158/1055-9965.EPI-07-2539. [DOI] [PubMed] [Google Scholar]
- 21.Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. [cited 22 Jan 2007];World population prospects: the 2004 revision and world urbanization prospects: the 2003 revision. 2004 2004 last update. Available from: http://esa.un.org/unpp.
- 22.Forman D, Sitas F, Newell DG, Stacey AR, Boreham J, Peto R, et al. Geographic association of Helicobacter pylori antibody prevalence and gastric cancer mortality in rural China. Int J Cancer. 1990;46:608–611. doi: 10.1002/ijc.2910460410. [DOI] [PubMed] [Google Scholar]
- 23.Zhou D, Yang H. Epidemiology of Helicobacter pylori infection in the People’s Republic of China. Chin Med J (Engl) 1995;108:304–313. [PubMed] [Google Scholar]
- 24.Segi M. Cancer mortality for selected sites in 24 countries (1950–57) Sendai: Tohoku University of Medicine; 1960. [Google Scholar]
- 25.Ministry of Health (MOH), People’s Republic of China. Reports on the 1993 National Health Services Survey results. Beijing: The Ministry of Health; 1994. [Google Scholar]
- 26.Ministry of Health (MOH), People’s Republic of China. Reports on the 1998 National Health Services Survey results. Beijing: The Ministry of Health; 1999. [Google Scholar]
- 27.Ministry of Health (MOH), People’s Republic of China. Reports on the 2003 National Health Services Survey results. Beijing: The Ministry of Health; 2004. [Google Scholar]
- 28.Yang GH, Ma JM, Liu N, Zhou LN. Smoking and passive smoking in Chinese, 2002. Chin J Epidemiol. 2005;26:77–83. [PubMed] [Google Scholar]
- 29.Xia HH, Talley NJ. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implications. Am J Gastroenterol. 1997;92:1780–1787. [PubMed] [Google Scholar]
- 30.Yang G, Ma J, Chen A, Zhang Y, Samet JM, Taylor CE, et al. Smoking cessation in China: findings from the 1996 national prevalence survey. Tob Control. 2001;10:170–174. doi: 10.1136/tc.10.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D, et al. Childhood living conditions and Helicobacter pylori seropositivity in adult life. Lancet. 1992;339:896–897. doi: 10.1016/0140-6736(92)90931-r. [DOI] [PubMed] [Google Scholar]
- 32.McCallion WA, Murray LJ, Bailie AG, Dalzell AM, O’Reilly DP, Bamford KB. Helicobacter pylori infection in children: relation with current household living conditions. Gut. 1996;39:18–21. doi: 10.1136/gut.39.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moayyedi P, Axon AT, Feltbower R, Duffett S, Crocombe W, Braunholtz D, et al. Relation of adult lifestyle and socio-economic factors to the prevalence of Helicobacter pylori infection. Int J Epidemiol. 2002;31:624–631. doi: 10.1093/ije/31.3.624. [DOI] [PubMed] [Google Scholar]
- 34.Kneller RW, You WC, Chang YS, Liu WD, Zhang L, Zhao L, et al. Cigarette smoking and other risk factors for progression of precancerous stomach lesions. J Natl Cancer Inst. 1992;84:1261–1266. doi: 10.1093/jnci/84.16.1261. [DOI] [PubMed] [Google Scholar]
- 35.Kato I, Vivas J, Plummer M, Lopez G, Peraza S, Castro D, et al. Environmental factors in Helicobacter pylori-related gastric precancerous lesions in Venezuela. Cancer Epidemiol Biomarkers Prev. 2004;13:468–476. [PubMed] [Google Scholar]
- 36.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case-control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banister J, Hill K. Mortality in China 1964–2000. Popul Stud (Camb) 2004;58:55–75. doi: 10.1080/0032472032000183753. [DOI] [PubMed] [Google Scholar]
- 38.Coale AJ. Rapid population change in China, 1952–1982. Washington, DC: National Academy Press; 1984. [Google Scholar]
- 39.Mathers CD, Murray CJL, Lopez A, Salomon JA, Sadana R, Tandon A, et al. Estimates of healthy life expectancy for 191 countries in the year 2000: methods and results. Geneva: World Health Organization; GPE discussion paper no. 38. 2000
- 40.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell HM, Hu P, Chi Y, Chen MH, Li YY, Hazell SL. A low rate of reinfection following effective therapy against Helicobacter pylori in a developing nation (China) Gastroenterology. 1998;114:256–261. doi: 10.1016/s0016-5085(98)70475-5. [DOI] [PubMed] [Google Scholar]
- 43.Parsonnet J. What is the Helicobacter pylori global reinfection rate? Can J Gastroenterol. 2003;17 Suppl B doi: 10.1155/2003/567816. 46B–48B. [DOI] [PubMed] [Google Scholar]
- 44.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359–1363. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control, Prevention. Cigarette smoking among adults—United States, 2003. Morb Mortal Wkly Rep. 2005;54:509–513. [PubMed] [Google Scholar]
- 46.Yang L, Parkin DM, Li LD, Chen YD, Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991–2005. Br J Cancer. 2004;90:2157–2166. doi: 10.1038/sj.bjc.6601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epplein M, Nomura AM, Hankin JH, Blaser MJ, Perez-Perez G, Stemmermann GN, et al. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer Causes Control. 2008;19:869–877. doi: 10.1007/s10552-008-9149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You WC, Blot WJ, Li JY, Chang YS, Jin ML, Kneller R, et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 1993;53:1317–1321. [PubMed] [Google Scholar]
- 49.Bray F, Moller B. Predicting the future burden of cancer. Nat Rev Cancer. 2006;6:63–74. doi: 10.1038/nrc1781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.