The molecular changes that account for the cognitive and behavioral features of acute and extended abstinence states associated with ethanol dependence are not well understood. One potential target that has received growing attention in this regard is the nicotinic acetylcholine receptor (nAChR). Specifically, the β2*-subunit containing nAChR (β2*-nAChR) is involved in a number of ethanol behaviors including ethanol-induced locomotion (Blomqvist et al., 1992), ethanol-induced ataxia (Taslim et al., 2008), alterations of the startle response (Owens et al., 2003), in genetic polymorphisms that mediate the effects of ethanol (Butt et al., 2003) and in ethanol withdrawal (Butt et al., 2004).
There is conflicting evidence on the effects of ethanol on nAChR binding levels during exposure to ethanol and during abstinence (Gorbounova et al., 1998; Robles and Sabria, 2008; Yoshida et al., 1982). Many rodent studies indicate that moderate lengths of ethanol exposure, e.g., 15–21 days, do not change numbers of β2*-nAChRs during abstinence (de Fiebre and Collins, 1993; Ribeiro-Carvalho et al., 2009). In human brain, no difference was observed in high-affinity nicotine binding sites postmortem between alcoholics and controls (Hellstrom-Lindahl et al., 1993), and a more recent human neuroreceptor imaging suggests no difference in β2*-nAChR availability between heavy drinkers and controls (Esterlis et al., 2009). However, few studies have examined the neurochemical changes occurring during abstinence from chronic ethanol exposure on β2*-nAChR expression in living animals. We used the radiotracer [123I]5-IA-85380 ([123I]5-IA) and SPECT brain imaging to examine β2*-nAChR availability during acute and extended abstinence from chronic ethanol administration in nonhuman primates.
Five male rhesus monkeys (Macaca mulatta) served as subjects. Of the five, 2 were adolescent (M-M, M-R), and 3 were young adult (M-B, M-D, M-J), weighing 5.5–12.9 kg. Two animals were alcohol naïve (M-M, M-R) and the other 3 had previous exposures to ethanol. Each previous exposure was approximately 6 months; M-B and M-D had 1 previous exposure and M-J had 2 previous exposures. The housing facility is fully accredited by the American Association for the Accreditation of Laboratory Animals (AAALAC) and all experiments were conducted in accordance with the Institutional Care and Use Committee guidelines.
Fasted animals were scanned under anesthesia as previously described (Staley et al., 2006). [123I]5-IA was synthesized as previously described (Zoghbi et al., 2001) and administered as a bolus to constant infusion at a ratio of 6.0 for 6 hours. This paradigm has demonstrated high test-retest reliability in rhesus monkeys (Staley et al., 2006). Animals were injected with a bolus (63.96±10.05, mean±SD, MBq) and constant infusion (10.62±1.68, mean±SD, MBq/h). Equilibrium (<5% change/hr) was established by 2.5–4.5 hours for all regions except thalamus, which was between 5–6 hours. Up to 12 consecutive SPECT images (approximately 30 min each) were acquired in the NeuroFocus™ Model 200 camera (NeuroPhysics Corp, MA) for up to 6 h. Animals were scanned at “baseline”, e.g., prior to ethanol consumption, during acute abstinence at 24 h after their last drink and during extended abstinence at 5–13 weeks after their last drink. A magnetic resonance image (MRI) of each animal was previously obtained to perform coregistration and guide the placement of regions of interest (ROIs). The ROIs were: cingulate cortex, right and left parietal, frontal, temporoinsular and occipital cortices, the right and left midbrain, thalamus and right and left cerebellum. The regional brain activities are average value of the right and left hemispheres. The primary outcome measure is the binding potential (BPND,) which is the ratio of specific to nonspecific binding with the cerebellum as the reference region.
A dose escalation procedure was used to obtain self-administration of ethanol, which was sweetened with saccharin (0.3% wt/vol) and diluted with tap water. Animals had free daily access (24 h/dy) to ethanol via a drinking spout protruding into the cage attached to a secured bottle. The concentration of ethanol was increased over a 4-week period (2%, 4%, to 6% vol/vol). Amount of alcohol (g/kg) consumed was recorded daily. Animals consumed ethanol daily for 18 ± 1 week and had access to water for a minimum of 2 hr/day. Behavior was monitored with a checklist (e.g., to record pacing behavior, yawns, scratches) twice daily for a 15-min period over the first week of alcohol abstinence.
Linear mixed models with time (baseline, 24 h, and 5–13 wk) as a within-subjects factor were used to assess β2*-nAChR availability measured across phase of abstinence. Cortical analysis included region (frontal, parietal, cingulate, occipital, temporal) as within-subjects factors, and random subject effects. The Bonferroni correction was used for comparisons within the five cortical regions. Associations between percent changes from baseline in BPND and alcohol consumption history were explored using Spearman correlations. All tests were two-sided and considered significant at P<0.05. All analyses were conducted using SAS, version 9.1 (Cary, NC).
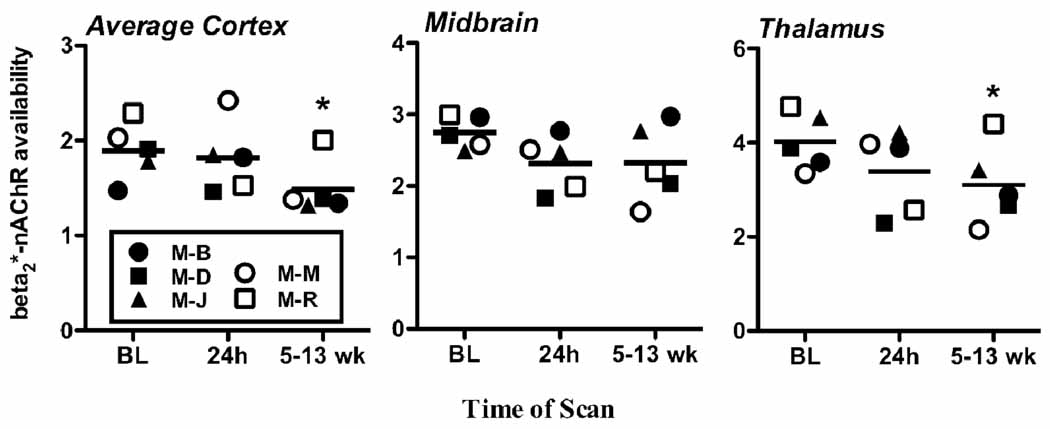
The animals consumed an average of 2.57 ± 1.09 g/kg/day over 18 ± 1 weeks, with 0.25 g/kg considered 1 standard drink for a human. No overt behavioral signs of ethanol withdrawal were observed during the first week of abstinence and no significant change from baseline behavior was recorded. Analysis of β2*-nAChR availability in the cortex revealed an overall time effect (F(2,56)=28.2, unadjusted p<0.0001, adjusted p<0.0005) such that availability decreased relative to baseline after extended (5–13 wk) abstinence (F(1,56)=11.5, unadjusted p=0.0013, adjusted p=0.0065) but not acute (24 h) abstinence (F(1,56)=0.08, unadjusted p=0.78, adjusted p=3.9) (Figure 1). The interaction between time and region was not significant (F(8,56)=0.77, unadjusted p=0.63, adjusted p=3.2), and post-hoc tests confirmed the decrease at 5–13 weeks was present in each of the five cortical regions (all unadjusted p<0.02, adjusted p<0.10). Similar abstinence effects were observed in the thalamus (F(2,4)=24.1, p=0.006) explained by significant decreased β2*-nAChR availability following extended (t(5)=5.6, p=0.005) but not acute (t(4)=1.2, p=.30) abstinence. Overall β2*-nAChR availability in the midbrain did not significantly change across the different stages of abstinence (F(2,8)=2.4, p=0.15). There were no significant correlations observed between the percent change in β2*-nAChR availability from baseline to 24 h abstinence and history of alcohol consumption. Negative correlations were observed between decreases in β2*-nAChR availability, expressed as percent change from baseline to 5–13 wks abstinence, in the parietal cortex with lifetime grams of ethanol consumed (Spearman rho=−0.9, p=0.037) and in the midbrain with average daily g/kg ethanol consumed (Spearman rho=−0.9, p=0.037).
Figure 1.
β2*-nAChR availability (BPND) in 5 animals at baseline, e.g., prior to this ethanol exposure, at 24 h and 5–13 wks abstinence in the average cortex (average of frontal, parietal, cingulate, temporal and occipital), midbrain, and thalamus. Each symbol represents an individual data point and the lines represent the mean. * indicates significantly different from baseline at p<0.05.
This study provides the first in vivo evidence in nonhuman primates that extended, but not acute, abstinence after chronic ethanol consumption is associated with decreased β2*-nAChR availability. Significant decreases in β2*-nAChR availability during extended abstinence compared to baseline were found throughout the cortex (22%) and in the thalamus (24%), but not the midbrain (15%). There was variability in the decrease from baseline to prolonged withdrawal. Specifically, while each individual animal demonstrated decreased β2*-nAChR availability, this decrease was less pronounced in M-R. Additionally, the animals that had consumed greater grams of ethanol over their life and more average daily ethanol (g/kg) had greater decreases in the parietal cortex and midbrain, respectively.
While there was no significant difference in β2*-nAChR availability between baseline and 24h abstinence, there was individual variability. The potential sources of these inter-individual differences are not yet known. One potential hypothesis is that during acute abstinence there are individual differences in endogenous ACh levels as a result of nAChR neuroadaptations to ethanol exposure. ACh is capable of interfering with [123I]5-IA binding (Fujita et al., 2003) and alcohol consumption and withdrawal modulates endogenous ACh and cholinergic function (Arendt et al., 1989; Kohila et al., 2004; Nestby et al., 1997). Thus, changes in changes in β2*-nAChR availability during acute alcohol withdrawal may reflect alterations in receptor number combined with changes in the levels of occupancy of these receptors by ACh.
In vitro, acute ethanol has been shown to increase excitatory cellular response to ACh (Mancillas et al., 1986) by synergistically diminishing the M-current in combination with ACh (Moore et al., 1990). This may lead to receptor downregulation after more chronic ethanol exposure. Chronic ethanol exposure (28 weeks) in rats produced a long-lasting reduction, e.g., 5 months post-ethanol treatment, in muscarinic AChR function (Rothberg and Hunter, 1991). Chronic ethanol (28 wks) also resulted in robust and persistent reductions in ACh content, in acetylcholinesterase (AChE) activity, in vitro release and synthesis of ACh, and choline uptake (Arendt et al., 1989; Arendt et al., 1988) and a reduction in the number of AChE-positive neurons in rat brain in the basal nucleus of Meynert Complex (Arendt et al., 1988). The evidence of reduced ACh levels associated with chronic exposure to ethanol reduces the likelihood that the current data are substantially contaminated by increases in the occupancy of nAChR’s by ACh. The current data add to an emerging body of evidence that chronic ethanol exposure leads to a reduction in cholinergic activity and function and in β2*-nAChR availability that remains during extended abstinence.
There are several limitations to this preliminary study including the small sample size and that 3 of the 5 animals were not alcohol-naïve at their “baseline” scan. Despite these limitations, all 5 animals demonstrated decreased cortical and thalamic β2*-nAChR availability following prolonged withdrawal. Such long-lasting changes in receptor availability have treatment implications. Specifically, due to the nature of alcohol dependence, e.g., a chronic disorder characterized by frequent relapse, treatments may need to be geared at preventing relapse for many months after an individual has stopped drinking. The β2*-nAChR offers a receptor target that may be critically involved in the recovery from alcohol dependence.
Acknowledgments
The authors would like to thank Louis Amici for technical assistance and Drs. Marina Picciotto and Stephanie O’Malley for helpful discussion on the manuscript. We would like to dedicate this work to Dr. Julie Staley who passed away July 25, 2009.
Funding: This research was supported in part by National Institute of Health grants KO2 DA21863 (Staley), KO1 DA20651 (Cosgrove), P50 DA13334 and P50 AA15632 (O’Malley), the Peter McManus Charitable Trust (Cosgrove), and the VA Alcohol Research Center (Krystal). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, or the National Institutes of Health.
References
- Arendt T, Allen Y, Marchbanks RM, Schugens MM, Sinden J, Lantos PL, Gray JA. Cholinergic system and memory in the rat: effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience. 1989;33(3):435–462. doi: 10.1016/0306-4522(89)90397-7. [DOI] [PubMed] [Google Scholar]
- Arendt T, Henning D, Gray JA, Marchbanks R. Loss of neurons in the rat basal forebrain cholinergic projection system after prolonged intake of ethanol. Brain Res Bull. 1988;21(4):563–569. doi: 10.1016/0361-9230(88)90193-1. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel J. Ethanol-induced locomotor activity: Involvement of central nicotinic acetycholine receptors? Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hutton SR, Stitzel JA, Balogh SA, Owens JC, Collins AC. A polymorphism in the alpha4 nicotinic receptor gene (Chrna4) modulates enhancement of nicotinic receptor function by ethanol. Alcohol Clin Exp Res. 2003;27(5):733–742. doi: 10.1097/01.ALC.0000067973.41153.BC. [DOI] [PubMed] [Google Scholar]
- Butt CM, King NM, Stitzel JA, Collins AC. Interaction of the nicotinic cholinergic system with ethanol withdrawal. J Pharmacol Exp Ther. 2004;308(2):591–599. doi: 10.1124/jpet.103.059758. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, Collins AC. A comparison of the development of tolerance to ethanol and cross-tolerance to nicotine after chronic ethanol treatment in long- and short-sleep mice. J Pharmacol Exp Ther. 1993;266(3):1398–1406. [PubMed] [Google Scholar]
- Esterlis I, Cosgrove K, Petrakis I, McKee S, Bois F, Krantzler E, Stiklus S, Perry E, Tamagnan G, Seibyl J, Krystal J, Staley J. SPECT imaging of nicotinic acetylcholine receptors in nonsmoking heavy alcohol drinking individuals. 2009 doi: 10.1016/j.drugalcdep.2009.12.006. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Al-Tikriti M, Tamagnan G, Zoghbi S, Bozkurt A, Baldwin R, Innis R. Influence of acetylcholine levels on the binding of a SPECT nicotinic acetylcholine receptor ligand [123I]5-I-A-85380. Synapse. 2003;48:116–122. doi: 10.1002/syn.10194. [DOI] [PubMed] [Google Scholar]
- Gorbounova O, Svensson A, Jonsson P, Mousavi M, Miao H, Hellstrom-Lindahl E, Nordberg A. Chronic ethanol treatment decreases [3H]epibatidine and [3H]nicotine binding and differentially regulates mRNA levels of nicotinic acetylcholine receptor subunits expressed in M10 and SH-SY5Y neuroblastoma cells. J Neurochem. 1998;70:1134–1142. doi: 10.1046/j.1471-4159.1998.70031134.x. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Winblad B, Nordberg A. Muscarinic and nicotinic receptor changes in the cortex and thalamus of brains of chronic alcoholics. Brain Res. 1993;620:42–48. doi: 10.1016/0006-8993(93)90268-r. [DOI] [PubMed] [Google Scholar]
- Kohila T, Parkkonen E, Tahti H. Evaluation of the effects of aluminium, ethanol and their combination on rat brain synaptosomal integral proteins in vitro and after 90-day oral exposure. Arch Toxicol. 2004;78(5):276–282. doi: 10.1007/s00204-003-0530-3. [DOI] [PubMed] [Google Scholar]
- Mancillas JR, Siggins GR, Bloom FE. Systemic ethanol: selective enhancement of responses to acetylcholine and somatostatin in hippocampus. Science. 1986;231(4734):161–163. doi: 10.1126/science.2867600. [DOI] [PubMed] [Google Scholar]
- Moore SD, Madamba SG, Siggins GR. Ethanol diminishes a voltage-dependent K+ current, the M-current, in CA1 hippocampal pyramidal neurons in vitro. Brain Res. 1990;516(2):222–228. doi: 10.1016/0006-8993(90)90922-x. [DOI] [PubMed] [Google Scholar]
- Nestby P, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH, Schoffelmeer AN. Ethanol, like psychostimulants and morphine, causes long-lasting hyperreactivity of dopamine and acetylcholine neurons of rat nucleus accumbens: possible role in behavioural sensitization. Psychopharmacology (Berl) 1997;133(1):69–76. doi: 10.1007/s002130050373. [DOI] [PubMed] [Google Scholar]
- Owens JC, Balogh SA, McClure-Begley TD, Butt CM, Labarca C, Lester HA, Picciotto MR, Wehner JM, Collins AC. Alpha 4 beta 2* nicotinic acetylcholine receptors modulate the effects of ethanol and nicotine on the acoustic startle response. Alcohol Clin Exp Res. 2003;27(12):1867–1875. doi: 10.1097/01.ALC.0000102700.72447.0F. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A, Lima CS, Medeiros AH, Siqueira NR, Filgueiras CC, Manhaes AC, Abreu-Villaca Y. Combined exposure to nicotine and ethanol in adolescent mice: effects on the central cholinergic systems during short and long term withdrawal. Neuroscience. 2009;162(4):1174–1186. doi: 10.1016/j.neuroscience.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Robles N, Sabria J. Effects of moderate chronic ethanol consumption on hippocampal nicotinic receptors and associative learning. Neurobiol Learn Mem. 2008;89(4):497–503. doi: 10.1016/j.nlm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Rothberg BS, Hunter BE. Chronic ethanol treatment differentially affects muscarinic receptor responses in rat hippocampus. Neurosci Lett. 1991;132(2):243–246. doi: 10.1016/0304-3940(91)90311-g. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O'Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26(34):8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslim N, Al-Rejaie S, Saeed Dar M. Attenuation of ethanol-induced ataxia by alpha(4)beta(2) nicotinic acetylcholine receptor subtype in mouse cerebellum: a functional interaction. Neuroscience. 2008;157(1):204–213. doi: 10.1016/j.neuroscience.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Engel G, Liljequist S. The effect of chronic ethanol administration on high affinity 3H-nicotinic binding in rat brain. Naunyn-Schmiedeberg's Arch Pharmacol. 1982;321:74–76. doi: 10.1007/BF00586353. [DOI] [PubMed] [Google Scholar]
- Zoghbi S, Tamagnan G, Fujita M, Baldwin R, Al-Tikriti M, Amici L, Seibyl J, Innis R. Measurement of plasma metabolites of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy) pyridine (5-IA-85380), a nicotinic acetylcholine receptor imaging agent, in non-human primates. Nucl Med Biol. 2001;28:91–96. doi: 10.1016/S0969-8051(00)00188-8. [DOI] [PubMed] [Google Scholar]