Abstract
Deficiency of adiponectin (APN), an adipocyte-derived vascular protective molecule, contributes to diabetic vascular injury. The current study determined whether obesity/hyperlipidemia may alter the vascular response to APN, and investigated the involved mechanisms and pathologic significance. Adult male Sprague-Dawley rats were fed a regular or high-fat diet (HF) for 4–16 weeks. Circulating APN levels, aortic pAMPK/AMPK, peNOS/eNOS, and APN receptor expression levels were determined. Compared to time-matched animals fed control diet, plasma APN levels in HF-diet animals were significantly increased at 8 weeks, and rapidly declined thereafter. Despite unchanged or elevated circulating APN levels, phosphorylated AMPK and eNOS in vascular tissue were significantly reduced at all observed time points. Recombinant full length APN (rAPN) induced AMPK/eNOS phosphorylation and vasodilatation were significantly reduced in 16-week obese/hyperlipidemic aortic segments. Vascular APN receptor 1 (AdipoR1) and receptor 2 (AdipoR2) expression were significantly reduced 16 weeks after HF-diet. Pre-incubation of rAPN with obese/hyperlipidemic plasma, but not with normal plasma, significantly reduced its AMPK and eNOS activation effect, and blunted its protective effect against TNFα-induced HUVEC apoptosis. This study demonstrated for the first time that obesity/hyperlipidemia reduces vascular responsiveness to APN. Modification/inactivation of APN by unidentified factors present in obese/hyperlipidemic plasma, decreased vascular AdipoR1/R2 expression, and reduced circulating APN levels contribute to reduced vascular responsiveness to APN at different stages of the obese condition. Reduced APN bioactivity allows unmitigated TNFα pro-apoptotic and pro-inflammatory actions, contributing to vascular injury in obesity/hyperlipidemia.
Keywords: Vascular injury, Cytokines, Obesity, Dyslipidemia, Adiponectin
1. Introduction
Cardiovascular disease is the leading cause of death in developed countries. Despite extensive research efforts in the past decade, effective therapeutic interventions reducing cardiovascular mortality remain limited. It is increasingly recognized that early intervention, including the elimination of cardiac disease risk factors, is critical in the prevention of cardiovascular morbidity and mortality[1].
The metabolic syndrome is a cluster of physiologic dysregulations encompassing obesity, insulin resistance, dyslipidemia, and hypertension[2]. Numerous clinical epidemiology studies have demonstrated that the metabolic syndrome is a major risk factor for cardiovascular disease[3]. Multiple pathologic alterations in these patients collectively facilitate development of arteriosclerosis, contributive to coronary heart disease and stroke[4]. However, the specific molecular link connecting the metabolic syndrome to cardiovascular injury remains largely unknown.
The hormone adiponectin (APN) circulates in plasma as multimeric complexes at relatively high concentrations (2–10 μg/ml)[5]. In recent years, APN has garnered much attention due to its pleiotropic beneficial effects upon obesity-related complications. Atop its well-defined insulin sensitization and metabolic regulatory effects, APN has been confirmed by recent experimental and clinical studies to mediate various vascular processes[6]. It stimulates endothelial nitric oxide production, induces vascular relaxation, inhibits inflammatory response, and reduces oxidative/nitrative stress[7]. Numerous clinical studies have also reported altered plasma APN levels in obesity, type-2 diabetes, and the metabolic syndrome. As such, APN has been proposed as a molecular link between the metabolic syndrome and its detrimental cardiovascular consequences[8]. However, although many clinical studies have reported that APN levels are significantly decreased in obesity, hypertension, and type-2 diabetes[9, 10], others have identified unchanged or even increased APN levels in rats with the metabolic syndrome[11–13]. Such conflicting clinical results suggest the existence of mechanisms contributive to vascular APN malfunction and loss of vascular regulatory function in the metabolic syndrome beyond mere APN reduction.
In the metabolic syndrome, insulin resistance is a well-recognized pathologic alteration. Emerging evidence suggests that the metabolic syndrome also causes APN resistance in insulin sensitive tissues. Specifically, APN response has been found to be significantly reduced in skeletal muscle (from patients[14, 15] or experimental animals[13, 16] with the metabolic syndrome) and liver tissue (from hypertensive[13] or insulin receptor transgenic/knockout[17] animals). Moreover, a recent study has demonstrated that APN resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats[18], suggesting that APN resistance may contribute to insulin resistance development. However, whether the pathologic response to APN may also develop in vascular tissue, a system critically determinant of the metabolic syndrome’s clinical effects, has never been previously investigated.
Therefore, the aims of the current study were 1) to examine whether diet-induced obesity/hyperlipidemia may cause vascular APN resistance; 2) to identify the mechanisms that are responsible for obesity/hyperlipidemia-induced vascular APN resistance, and 3) to determine the pathologic significance of vascular APN resistance.
2. Materials and Methods
2.1 Animals and diets
Adult male Sprague-Dawley rats were randomized to receive a regular chow (12% kcal from fat, control) or a high-fat (60% kcal from lard, Research Diets, New Brunswick, NJ) diet. Food and water were provided ad libitum. Animals were maintained in a temperature-controlled barrier facility, with a 12-hour alternating light/dark cycle. Prior to dietary intervention and weekly thereafter, body weight was recorded, and 0.5 ml blood samples were collected from the tail artery under isoflurane anesthesia. All procedures were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals, and were approved by the Thomas Jefferson University Committee on Animal Care.
2.2 Terminal blood and tissue collection
4 to 16 weeks after high-fat feeding, the animals were fasted overnight, and anesthetized with 2% isoflurane. Thoracotomy was performed, and terminal blood collection in heparinized tubes was achieved via cardiac puncture. Plasma was removed for both biochemical assays and for in vitro incubation of recombinant full length APN (rAPN). The aortic segment from heart to iliac bifurcation was excised, cleaned of adherent tissues, and either homogenized for the immunoblotting assay, or cut into 3–4 mm vascular segment for ex vivo experiments as described below.
2.3 Plasma lipid and APN determinations
Plasma cholesterol and triglyceride levels were determined by a biochemistry analyzer (COBAS INTEGRA 400 Plus, Roche Inc., Switzerland). Plasma APN levels were quantified with a rat APN ELISA assay kit (Alpco, Salem, NH) per manufacturer’s instructions.
2.4 Organ chamber experiments
APN-induced vascular relaxation was performed as previously described[19, 20]. Briefly, aortic rings were mounted onto hooks, suspended in organ chambers filled with Krebs buffer and aerated with 95% O2 and 5% CO2 at 37°C, and connected to force transducers (WPI, Sarasota, FL) to record changes via a Maclab data acquisition system. After equilibration for 60 min at preload of 1g, the rings were pre-contracted with norepinephrine (NE, 0.1 nM). Once a stable contraction was achieved, the rings were exposed to cumulative concentrations of full length recombinant rat APN (rAPN, BioVendor, Candler, NC, Cat# RD272023100, 0.1–10 μg/ml). After the cumulative response stabilized, the rings were washed and allowed to equilibrate to baseline. The procedure was then repeated with an endothelium-independent vasodilator (SNAP, 0.03–3 μg/ml) to determine smooth muscle function and sensitivity to NO.
2.5 Determination of NO production from aortic segments
To determine rAPN stimulated nitric oxide production by endothelium in situ, isolated aortic segments were placed in culture medium containing vehicle or rAPN (3 μg/ml), and incubated in a cell culture incubator (5% CO2 37°C). 1400W (10 μM), a highly selective iNOS inhibitor[21], was added to all samples to block potential iNOS mediated NO production. After 1 hour incubation, NO and its oxidative metabolic products (NO2 and NO3), collectively known as NOx, were determined as described in our previous study.[20] The amount of NO released was expressed in nmol/mg protein.
2.6 Immunoblotting
The vascular tissues were homogenized in lysis buffer, and Western blotting was performed as previously reported[22].
2.7 In vitro incubation of full length recombinant APN with normal plasma or high-lipid plasma
To each well of a 24-well cell culture plate, 20 μg of rAPN and 1 ml of plasma from either normal-diet fed rat (normal plasma, NP, 8 weeks) or a high-fat-diet fed rat (high-lipid plasma, HLP, 8 weeks) was added. The plate was placed in a cell culture incubator and incubated at 37°C for 2 to 8 hours.
2.8 APN activity assay using cultured endothelial cells
Human umbilical vein endothelial cells (HUVEC, Lonza Inc, Allendale, NJ) were cultured as previously described[23]. Experiments were carried out at 3 or 4 cell-passage state. After 3 hours of serum-starvation (serum-free growth medium incubation), the HUVECs were randomized to receive one of the following treatments: normal plasma (NP, 50% culture medium, 50% normal plasma from healthy rats), high-lipid plasma (HLP, 50% culture medium, 50% plasma from high-fat-diet rats), NP pre-incubated rAPN (50% culture medium, 50% rAPN incubated with normal diet fed rat plasma as described above, final rAPN concentration: 10 μg/ml), or HLP pre-incubated rAPN (50% culture medium, 50% rAPN incubated with high-lipid diet fed rat plasma as described above). After 1 hour of treatment, endothelial cells were collected, sonicated, and AMPK/eNOS phosphorylation was determined by Western blotting.
2.9 Apoptosis induction and APN treatment
After 3 hours serum-starvation, the HUVEC were randomized to receive one of the following treatments: normal plasma (NP, 50% culture medium, 50% normal plasma from healthy rats), high-lipid plasma (HLP, 50% culture medium, 50% plasma from high-fat-diet rats), TNFα (25 ng/ml, Sigma-Aldrich, St. Louis, MO), TNFα+NP pre-incubated rAPN (50% culture medium, 50% rAPN incubated with normal diet fed rat plasma as described above), or TNFα+HLP pre-incubated rAPN (50% culture medium, 50% rAPN incubated with high-lipid diet fed rat plasma as described above). Twenty hours after treatment, apoptosis was determined using a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) kit (Roche Applied Science, Indianapolis, IN) per manufacturer’s instructions. Nuclei were counter-stained with 4,6-diamidino-2-phenylindole (DAPI), samples were visualized on an Olympus BX51 Fluorescence Microscope, and multiple digital images scanning the entire culture dish were acquired with IP Lab Imagine Analysis Software (BioVison, Exton, PA). Apoptotic index (number of TUNEL stain positive nuclei/total nuclei x 100%) was automatically calculated for further analysis.
2.10 Statistical Analysis
All values in the text and figures are presented as means ± SEM of n independent experiments. Data (except Western blot density) were subjected to t test (two groups) or ANOVA (Three or more groups) followed by Bonferoni correction for post hoc t-test. Western blot densities were analyzed with the Kruskal-Wallis test followed by Dunn’s post test. Probabilities of 0.05 or less were considered to be statistically significant.
3. RESULTS
3.1 Body mass and lipid profile
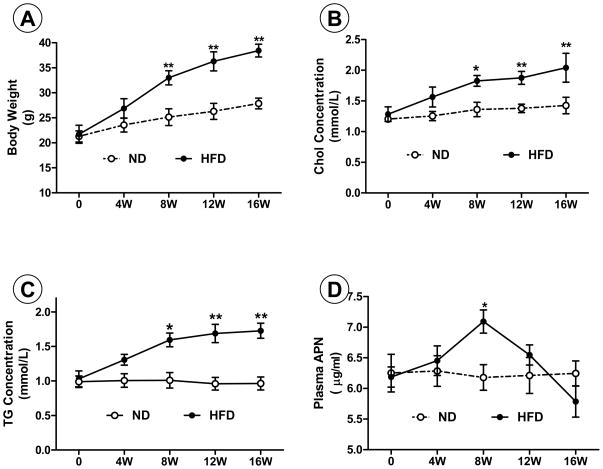
No significant difference in any parameter was found between groups before HF-diet feeding. Rats fed a normal diet exhibited a slight increase in body weight, and maintained stable plasma cholesterol, triglyceride, and APN concentrations over the 16-week observation period (Figure 1). In contrast, body weight, plasma cholesterol, and triglyceride levels continuously increased in rats fed HF-diet, reaching statistical significance compared to control after 8 weeks, remaining elevated thereafter (P<0.01, Figure 1). Plasma APN levels were unchanged 4 weeks after HF-diet, significantly increased 8 weeks after HF-diet (P<0.05), and rapidly declined thereafter (Figure 1).
Figure 1.
Body weight (A), plasma cholesterol (B), triglyceride (C), and APN (D) concentrations in rats fed normal diet (ND) or high-fat diet (HFD). Chol=cholesterol; TG=triglyceride; APN=adiponectin. *P<0.05 and **P<0.01 vs. time-matched control rats fed ND. N=8–10 animals/group.
3.2. Evidence of vascular APN resistance in HF-diet animals
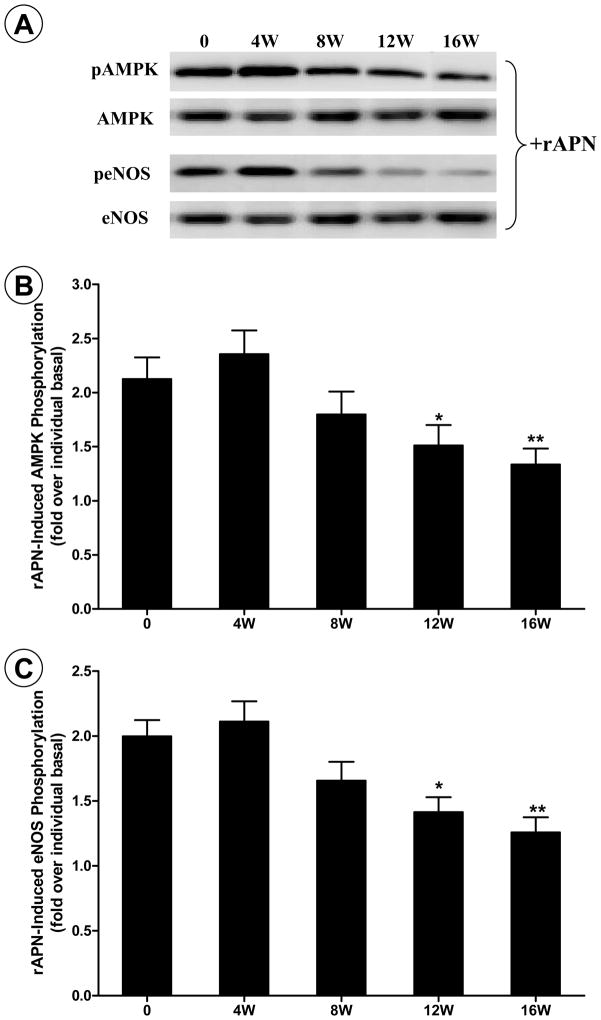
APN is an upstream molecule of the AMPK-eNOS signaling axis, and AMPK-eNOS phosphorylation has been well accepted as a readout of APN intracellular signaling. Results obtained from our studies indicate that, despite unchanged or elevated plasma APN levels (presented here in Figure 1), vascular AMPK and eNOS phosphorylation levels were significantly reduced in HF-diet animals (data not shown here). The disassociation between plasma APN levels and vascular AMPK/eNOS phosphorylation in obese/hyperlipidemic rats suggests reduced vascular response to APN in HF-fed animals. However, since AMPK and eNOS can be activated by many signaling molecules, reduction in AMPK and eNOS phosphorylation in HF-fed animals could reflect a wide variety of alterations induced by high fat feeding. To obtain more direct evidence supporting that APN system malfunction might be one of the mechanisms contributory to diminished AMPK and eNOS phosphorylation, freshly isolated aortic segments from HF-diet animals at different time points were stimulated with rAPN. Although basal vascular AMPK and eNOS phosphorylation were significantly reduced at 4 and 8 weeks after HF-diet (data not shown), rAPN-induced AMPK and eNOS phosphorylation was not reduced at this time point (Figure 2). These results indicate that vascular response to rAPN is unimpaired at this time point. However, in vascular segments isolated from 12- and 16-week HF-fed rats, both basal and rAPN-stimulated AMPK/eNOS phosphorylation was significantly reduced (P<0.05 and <0.01, respectively). These results provided direct evidence that vascular responsiveness to APN was impaired in HF-diet induced obesity animals.
Figure 2.
Recombinant APN-induced AMPK and eNOS phosphorylation in aortic segments isolated from control rats, or rats fed high-fat diet (HFD) for 4 to 16 weeks. A: representative Western blot photos from aortic tissues stimulated with APN. B: ratio of pAMPK and AMPK with (solid bars) and without (open bars) APN stimulation; C: ratio of peNOS and eNOS with (solid bars) and without (open bars) APN stimulation. *P<0.05 and **P<0.01 vs. control rats. N=8–10 animals/group.
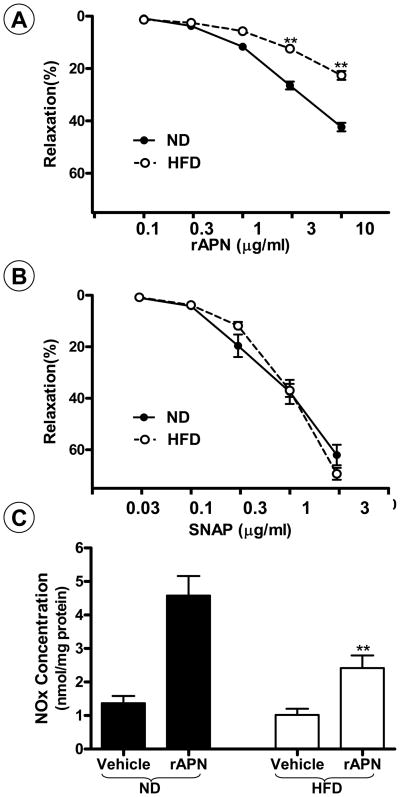
To obtain more evidence in supporting this conclusion, two additional experiments were performed. In the first experiment, rAPN-induced vasodilatation was assessed in aortic vascular segments isolated from animals fed with either a normal or HF-diet for 16 weeks. As summarized in Figure 3A and B, rAPN and SNAP both induced concentration-dependent vasodilatation in vascular segments obtained from animals with normal diet. Although vasodilatory response to SNAP is normal in vascular segments isolated from animals with HF-diet, rAPN-induced vasodilatation was significantly reduced in these vascular segments (P<0.01). In the second experiment, rAPN-stimulated NO production by in situ vascular endothelial cells was determined. As summarized in Figure 3C, treatment of vascular segments from age-matched animals on normal diet with 3 μM rAPN caused a 3.3-fold increase in NO production. This rAPN-stimulated NO production was significantly blunted in vascular segments from animals on HF-diet for 16 weeks (P<0.01).
Figure 3.
rAPN and SNAP-induced vasorelaxation (A, B) and NO production (C) in aortic segments isolated from rats fed with a normal diet (ND), or a high-fat diet (HFD) for 16 weeks. **P<0.01 vs. ND rats. N=12 to 14 vascular segments/group from at least 5 rats/group.
3.3 Vascular AdipoR1/R2 expression in obese/hyperlipidemic rats
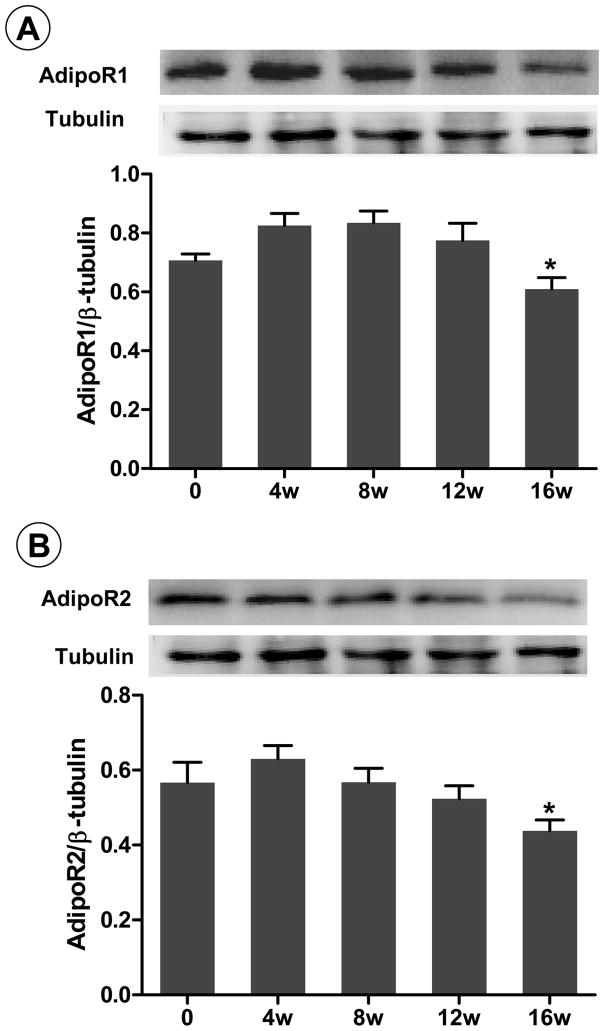
Figures 2 and 3 provides direct evidence that vascular response to rAPN was significantly reduced in rats fed HF-diet 12 weeks or longer. To define the mechanisms responsible for HF-diet-induced APN resistance, vascular AdipoR1 and AdipoR2 expression were determined. As illustrated in Figure 4, vascular AdipoR1 and AdipoR2 expression remained unchanged from 4 to 12 weeks of HF-diet, and significantly declined after 16 weeks HF-diet (P<0.05). AdipoR1 and AdipoR2 expression remains constant over the 16-week observation period in normal-diet-fed rats (data not shown), indicating that reduction of AdipoR expression in HF-fed rats is not a generalized effect due to aging/development.
Figure 4.
AdipoR1 (A) and AdipoR2 (B) expression in aortic tissues from control rats or rats fed HFD for 4 to 16 weeks. Inserts are representative Western blot photos; bar graphs are data summary from 8–10 animals. *P<0.05 vs. control rats.
3.4 Reduction of APN activity by high-lipid plasma (HLP)
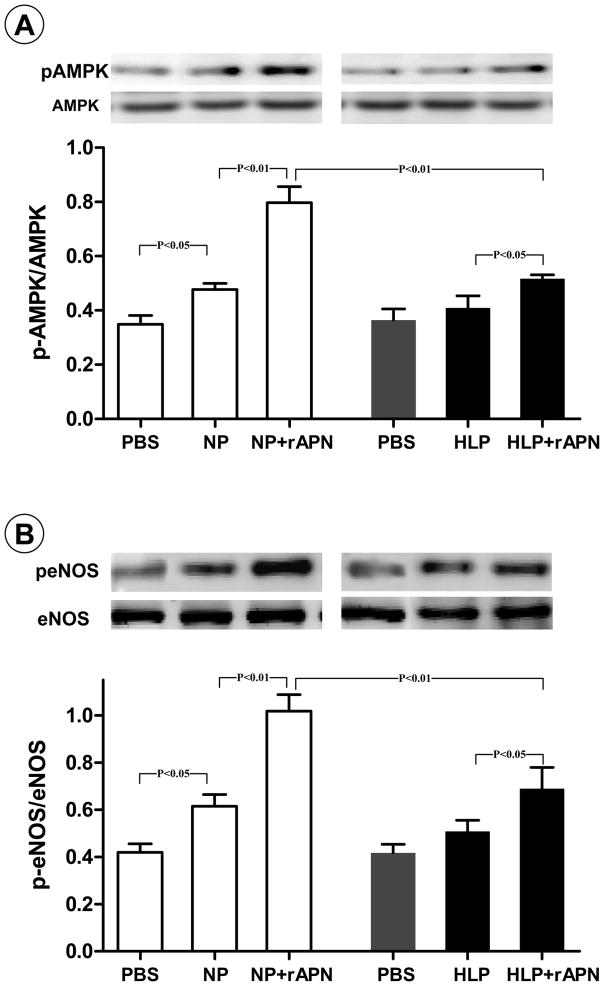
The aforementioned experimental results suggest that reduced AdipoR1 and AdipoR2 expression was likely responsible for the reduced response to rAPN in aortic segments isolated from rats fed with HF-diet for 16 weeks. However, our in vivo experimental results demonstrated that vascular AMPK and eNOS phosphorylation was significantly reduced as early as 4 weeks after HF-diet (data not shown). These results suggest that besides reduced vascular APN receptor expression and resultant reduced vascular APN sensitivity, other mechanisms exist that contribute to vascular malfunction in hyperlipidemic animals. Although it is likely that the causes of reduced AMPK and eNOS phosphorylation in hyperlipidemic vessels are multifactorial, previous studies have demonstrated that pathogenic molecules present in diabetic serum can modify and alter functions of lipids and proteins [24, 25]. Such data stimulated us to test a novel hypothesis that circulating APN might be modified and inhibited by yet-unidentified molecules present in HF-diet-fed animal plasma. Though this hypothesis would be best tested by isolating APN from HF-diet-fed animals and determining its activity directly, a technique isolating rat plasma APN in sufficient quantity to perform in vitro experiments is currently unavailable. As an alternative approach, plasma from animals fed control diet or HF-diet for 8 weeks was freshly isolated, and incubated with rAPN in vitro. The rAPN-induced AMPK and eNOS phosphorylation in cultured HUVEC was utilized as a reporter system for APN activity. Our pilot experimental results demonstrated that in vitro incubation of rAPN with normal plasma for 4 hours only slightly reduced its AMPK activation activity (11.4±3.2% reduction), but longer incubation time (>6 hours) caused significant reduction of rAPN activity. Therefore, the duration 4 hours of in vitro incubation was selected for the remainder of our study. As summarized in Figure 5, normal plasma (NP), but not high-lipid plasma (HLP), caused a statistically significant amount of APMK (A) and eNOS (B) phosphorylation. More importantly, treatment of HUVEC with NP-incubated rAPN caused a 1.7- and 1.6-fold increase in AMPK (P<0.01) and eNOS phosphorylation (P<0.01) respectively. However, AMPK and eNOS activation effects were markedly blunted when rAPN was pre-incubated with plasma isolated from animals fed 8 weeks of HF-diet (P<0.01 vs. normal plasma pre-incubated rAPN).
Figure 5.
Effects of pre-incubation of rAPN with normal plasma (NP) or high-lipid plasma (HLP, 8 weeks after HF-diet) on its biological activity as determined by AMPK (A) and eNOS (B) phosphorylation in cultured endothelial cells. N=14–16 wells/group from 5–7 independent experiments.
3.5 High-lipid plasma incubation significantly inhibited rAPN’s anti-TNFα and anti-apoptotic effects
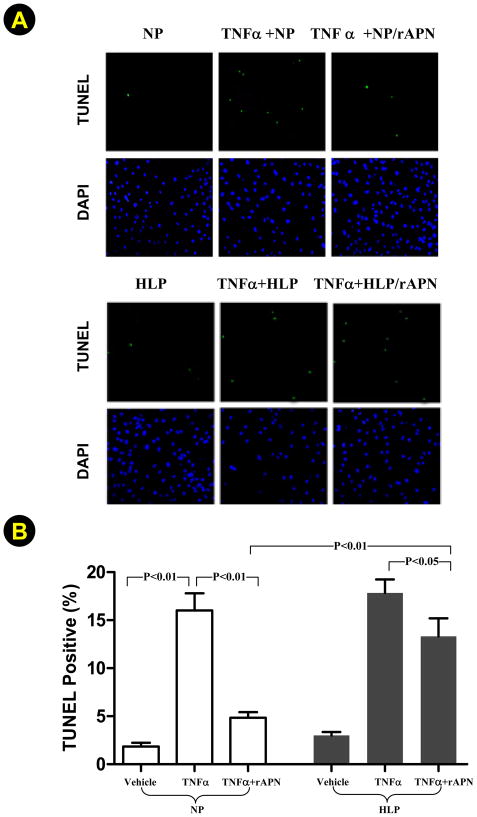
Having demonstrated that high-lipid plasma incubation significantly reduced rAPN activity determined by AMPK and eNOS phosphorylation, we further investigated whether high-lipid plasma-induced rAPN modification/inhibition is pathologically significant. As illustrated in Figure 6A and summarized in Figure 6B, exposure of HUVEC to TNFα for 20 hours caused an 8.7-fold increase in TUNEL-positive staining cells, which was significantly inhibited by rAPN pre-incubated with normal plasma (69.8% reduction, P<0.01). However, the anti-TNFα and anti-apoptotic effect of rAPN was significantly blunted when pre-incubated with high-lipid plasma (P<0.01 vs. normal plasma pre-incubated rAPN).
Figure 6.
Effects of pre-incubation of rAPN with normal plasma (NP) or high-lipid plasma (HLP) on its anti-TNFα and anti-apoptotic effect in cultured endothelial cells. A: representative microscopic photos; B: data summary of 14–16 wells/group from 5–7 independent experiments.
4. Discussion
Numerous epidemiological studies have identified APN deficiency as an independent risk factor for endothelial dysfunction, hypertension, coronary heart disease, myocardial infarction, and other cardiovascular complications[26]. Conversely, elevation of circulating APN concentrations by either genetic or pharmacological approaches has been shown to improve various vascular dysfunctions in animal models[27, 28]. Although the reduction of APN production is undoubtedly a major contributor to vascular injury observed in the metabolic syndrome, repeated reports of unchanged or even elevated plasma APN levels existent in the metabolic syndrome belie the existence of additional mechanisms contributive to vascular injury by APN malfunction.
Analogous to insulin resistance, APN resistance has recently been observed in insulin-sensitive tissues of obese/diabetic individuals. Specifically, APN-induced AMPK activation is reduced in liver tissue from hypertensive[13] or insulin receptor transgenic/knockout animals[17], and the ability of APN to stimulate AMPK/ACC and FA oxidation is impaired in cultured myotubes[15] and skeletal muscle strips[14] isolated from obese/diabetic individuals. In addition, Mullen a nd colleagues demonstrated that high-fat diet can induce the loss of acute ACC phosphorylation and stimulation of FA oxidation in rodent muscle by APN[16], and that APN resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats[18]. Considerable evidence supports, in conjunction with lower circulating APN, an impaired skeletal/liver response to adiponectin in the metabolic syndrome contributes to APN system malfunction.
In insulin-sensitive tissues, such as liver and muscle cells, APN achieves its metabolic regulative actions primarily through phosphorylative activation of AMPK[29]. In vascular tissue, APN exerts its vascular specific actions principally through phosphorylative activation of endothelial nitric oxide synthase (eNOS)[30]. Utilizing AMPK and eNOS phosphorylation as molecular markers, and rAPN-induced vasodilatation and NO production as function markers, we have provided the first direct evidence that significant APN resistance develops in the vascular tissue of high-fat-diet induced obese/hyperlipidemic animals. APN is a potent vasculoprotective molecule, and vascular injury is the defining end point of the metabolic syndrome. Therefore, impaired vascular response to APN may be the critical mechanism responsible for accelerated vascular injury observed in obese/hyperlipidemic patients.
Although APN resistance has been previously reported in skeletal and liver tissues, its underlying mechanisms remain largely unknown. Our study demonstrated that different mechanisms are involved in vascular APN resistance in HF-fed rats at different stages of obesity/dyslipidemia. Specifically, APN modification/inactivation by yet-unidentified factors present in obese/hyperlipidemic plasma is likely responsible for early phase APN resistance. This conclusion is supported by following experimental results: 1) plasma APN levels were not reduced after 4 weeks HF-diet, and significantly increased after 8 weeks HF-diet; 2) vascular AdipoR1 and AdipoR2 expression were not changed 4 to 12 weeks after HF-diet; 3) tissue levels of phosphorylated AMPK and eNOS were significantly reduced at all observed time points; 4) vascular segments isolated from rats fed HF-diets for 4 and 8 weeks responded normally to rAPN; and most importantly, 5) pre-incubation of rAPN with obese/hyperlipidemic plasma significantly reduced its AMPK and eNOS activation effect in HUVEC.
During the later stages of obesity/hyperlipidemia, multiple pathological alterations including APN modification/inactivation, APN receptor downregulation, and circulating APN reduction collectively occur, deranging normal APN function. Our in vivo and ex vivo experimental results clearly demonstrated that the most significant reduction of pAMPK and peNOS was observed after 16 weeks HF-diet. Moreover, we also have demonstrated that AdipoR1 and AdipoR2 expression was significantly reduced in vascular tissue from rats fed HF-diet for 16 weeks. This latter result is consistent with previously observed data, in which reduced AdipoR1 expression in muscular tissue from diabetic animals is believed to be responsible for reduced APN response[31]. Reduced plasma APN levels are a common finding in late stage diabetic and obese individuals. In the current experiments, APN reduction did not reach statistical significance during our observation period. However, plasma APN levels rapidly reduced after 8 weeks, and a clear trend of decline was observed in the late stage of hyperlipidemia (Figure 1D). It is possible that a longer feeding period is required to significantly inhibit APN production from adipose tissue in this particular obesity/hyperlipidemic model.
APN resistance in skeletal and liver tissues causes systemic pathologic alterations, such as impaired glucose/fatty acid utilization. Development of systemic hyperglycemia and hyperlipidemia consequently causes vascular injury and cardiovascular complications. Although vascular APN resistance may have limited impact on systemic metabolic abnormalities, vascular APN malfunction tilts homeostatic processes in favor of cytotoxic factors such as TNFα and accelerates vascular injury, the common end point of the metabolic syndrome. Arteriosclerosis, the most significant vascular complication in the metabolic syndrome, is inhibited by APN at multiple levels[6]. APN reduces oxidative/nitrative stress, protects the endothelium from apoptosis, and inhibits endothelial adhesion molecule expression, the leukocyte-endothelium interaction, macrophage activation, foam cell formation, and smooth muscle proliferation[7]. It is well-recognized that cytokine production (particularly TNFα) is significantly elevated in obese/diabetic individuals, and plays a critical pathogenic role in cardiovascular complications[32]. Moreover, a reciprocal inhibitory action between TNFα and APN exists[33]. TNFα not only inhibits adipocyte-APN production, but also inhibits peripheral, including cardiovascular, tissue APN receptor expression[34]. Conversely, APN has been found to counteract many of TNFα’s pathologic effects, including sustained endothelial oxidative stress, apoptosis, and angiogenesis[7, 35]. Consistent with previously reported results, we have observed that TNFα-induced HUVEC apoptosis was markedly inhibited by rAPN. However, we have demonstrated for the first time that this anti-TNFα and anti-apoptotic effect of rAPN was significantly inhibited when pre-incubated with hyperlipidemic plasma. Although the specific factor or factors present in hyperlipidemic plasma responsible for rAPN inhibitory modification remain unknown, these results provide direct evidence that APN system malfunction is pathologically significant. The loss of APN physiologic function allows unmitigated TNFα pro-apoptotic and pro-inflammatory actions, contributing to vascular injury in obesity/hyperlipidemia.
In summary, we have demonstrated that diet-induced obesity/hyperlipidemia caused significant vascular APN resistance. Similar to reports regarding insulin resistance, our current results suggest that APN resistance may develop at pre-receptor, receptor, and post-receptor levels. A detailed characterization of the vascular APN signaling cascade will potentially provide insight into novel therapeutic approaches ameliorating the elevated cardiovascular risks associated with obesity. Future studies are required to fully understand how hyperlipidemia causes vascular APN resistance, and determine whether restoration of APN sensitivity will yield functional and clinical improvement.
5. Limitations
Our current study demonstrated for the first time that APN modification/inactivation and APN receptor downregulation is likely responsible for reduced APN signaling in the early and late phase of hyperlipidemia, respectively. However, the identity of the factor or factors present in high-lipid plasma responsible for deleterious APN modification/inhibition remains unidentified. Our most recent in vitro experimental results demonstrated that advanced glycation end products (AGE) are capable of inhibiting APN activity. However, to what extent AGEs are responsible for hyperlipidemic serum modification of APN, and how APN is modified and inhibited by AGE (e.g., alteration of oligomeric state, molecular weight, and higher order structure of APN, or impairment of APN binding ability with its receptors) require further investigation. Moreover, questions such as the signaling mechanisms responsible for APN receptor downregulation and whether reversal of APN resistance, such as overexpressing of AdipoR1 and AdipoR2, may restore APN vascular protection against obese/hyperlipidemic injury remains to be answered. These issues warrant direct investigation and their resolution will advance understanding of diabetic cardiovascular injury. However, given the complexity of hyperlipidemic pathology and APN signaling, these important questions will have to be investigated in our future studies.
Acknowledgments
Funding Sources
This research was supported by the following grants: NIH 2R01HL-63828, American Diabetes Association Research Award 7-08-RA-98, American Heart Association Grant-in-Aid 0855554D (to X.L.M.), NSFC 30700308 (to RL).
Footnotes
Disclosures: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, et al. Reduction in Acute Myocardial Infarction Mortality in the United States: Risk-Standardized Mortality Rates From 1995–2006. JAMA. 2009;302:767–73. doi: 10.1001/jama.2009.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C for the Conference Participants. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Cooper-Dehoff RM, Pepine CJ. Metabolic Syndrome and Cardiovascular Disease: Challenges and Opportunities. Clin Cardiol. 2007;30:593–7. doi: 10.1002/clc.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heusch G. Obesity--a risk factor or a RISK factor for myocardial infarction? Br J Pharmacol. 2006;149:1–3. doi: 10.1038/sj.bjp.0706833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–6. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 2008;114:361–74. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabin KR, Kamari Y, Avni I, Grossman E, Sharabi Y. Adiponectin: linking the metabolic syndrome to its cardiovascular consequences. Expert Rev Cardiovasc Ther. 2005;3:465–71. doi: 10.1586/14779072.3.3.465. [DOI] [PubMed] [Google Scholar]
- 9.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 10.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 11.Semple RK, Halberg NH, Burling K, Soos MA, Schraw T, Luan J, et al. Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes. 2007;56:1712–7. doi: 10.2337/db06-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanety H, Hemi R, Ginsberg S, Pariente C, Yissachar E, Barhurdar E, et al. Total and high molecular weight adiponectin are elevated in patients with Laron syndrome despite marked obesity. Eur J Endocrinol. 2009;161(6):837–44. doi: 10.1530/EJE-09-0419. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez A, Catalan V, Becerril S, Gil MJ, Mugueta C, Gomez-Ambrosi J, et al. Impaired adiponectin-AMPK signalling in insulin-sensitive tissues of hypertensive rats. Life Sci. 2008;83:540–9. doi: 10.1016/j.lfs.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Bruce CR, Mertz VA, Heigenhauser GJ, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154–60. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- 15.Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O’Brien PE, Dixon JB, et al. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90:3665–72. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- 16.Mullen KL, Smith AC, Junkin KA, Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat-fed rats. Am J Physiol Endocrinol Metab. 2007;293:E83–E90. doi: 10.1152/ajpendo.00545.2006. [DOI] [PubMed] [Google Scholar]
- 17.Lin HV, Kim JY, Pocai A, Rossetti L, Shapiro L, Scherer PE, et al. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes. 2007;56:1969–76. doi: 10.2337/db07-0127. [DOI] [PubMed] [Google Scholar]
- 18.Mullen KL, Pritchard J, Ritchie I, Snook LA, Chabowski A, Bonen A, et al. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R243–R251. doi: 10.1152/ajpregu.90774.2008. [DOI] [PubMed] [Google Scholar]
- 19.Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun. 2005;332:200–5. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, et al. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413–9. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–63. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, et al. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835–44. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Mahadev K, Fuchsel L, Ouedraogo R, Xu SQ, Goldstein BJ. Adiponectin suppresses IkappaB kinase activation induced by tumor necrosis factor-alpha or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab. 2007;293:E1836–E1844. doi: 10.1152/ajpendo.00115.2007. [DOI] [PubMed] [Google Scholar]
- 24.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9441–5. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munzel D, Lehle K, Haubner F, Schmid C, Birnbaum DE, Preuner JG. Impact of diabetic serum on endothelial cells: an in-vitro-analysis of endothelial dysfunction in diabetes mellitus type 2. Biochem Biophys Res Commun. 2007;362:238–44. doi: 10.1016/j.bbrc.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 26.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–16. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009;284:22426–35. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 31.Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008;34:52–61. doi: 10.1016/j.diabet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, et al. Effects of TNF-alpha neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2007;293:E102–E109. doi: 10.1152/ajpendo.00089.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N. Adiponectin Suppresses Pathological Microvessel Formation in Retina Through Modulation of Tumor Necrosis Factor-{alpha} Expression. Circ Res. 2009;104:1058–65. doi: 10.1161/CIRCRESAHA.109.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito Y, Fujioka D, Kawabata KI, Kobayashi T, Yano T, Nakamura T, et al. Statin reverses reduction of adiponectin receptor expression in infarcted heart and in TNF{alpha}-treated cardiomyocytes in association with improved glucose uptake. Am J Physiol Heart Circ Physiol. 2007;293:H3490–7. doi: 10.1152/ajpheart.00310.2007. [DOI] [PubMed] [Google Scholar]
- 35.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–16. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]