Abstract
The continuous incorporation of new neurons in the dentate gyrus of the adult hippocampus raises exciting questions about memory and learning, and has inspired new computational models to understand the function of adult neurogenesis. These theoretical approaches suggest distinct roles for new neurons as they slowly integrate into the existing dentate gyrus network: immature adult-born neurons appear to function as pattern integrators of temporally adjacent events, thereby enhancing pattern separation for events separated in time; whereas maturing adult-born neurons possibly contribute to pattern separation by being more amenable to learning new information, leading to dedicated groups of granule cells that respond to experienced environments. We review these hypothesized functions and supporting empirical research and point to new directions for future theoretical efforts.
The Challenge of New Neurons
Although the nervous systems of other vertebrates exhibit varying degrees of widespread neurogenesis[1, 2], in mammals neurogenesis almost completely ceases after development, with only two regions retaining an ongoing incorporation of new neurons throughout life[3]. One of these regions, the olfactory bulb, is populated by neurons that were originally born in the sub-ventricular zone (SVZ). Immature neurons migrate from the SVZ and give rise to several local interneuron populations in the olfactory bulb[4, 5]. In contrast, in the dentate gyrus (DG) region of the hippocampus, new neurons arise from a local population of neuronal progenitor cells (NPCs) and eventually become excitatory granule cells (GCs), the principal projection neurons of the DG[6].
From a theoretical point of view, the incorporation of new neurons into the adult brain represents a unique challenge: since the neural network responsible for memory is continuously changing with the addition of new neurons, the DG and hippocampus cannot be considered to be architecturally stable. The dynamic nature of the neural network underlying memory is emphasized by the tight regulation of neurogenesis rates and their correlation with hippocampus-related behaviors. For instance, learning and many factors known to be beneficial for memory (e.g., running, enrichment) also increase the numbers of new neurons[7–10]; likewise, factors that impair memory, such as aging, stress, and several diseases, are associated with lower neurogenesis levels[11, 12].
Evidence increasingly suggests that neurogenesis is important for hippocampal function, but the exact function of new neurons is unclear. Much of the work has used behavioral paradigms designed to show changes after full hippocampal lesions[13, 14]. Despite the development of effective animal models for neurogenesis knockdown, the results from these more global behavioral tests have been (perhaps predictably, in retrospect) inconclusive[6]. Instead, theoretical approaches using computational modeling suggest that there may be multiple functions for new neurons that are more subtle than previously thought and can best be described as part of the hypothesized functions of the DG as opposed to an isolated process in hippocampal function. At different stages in its maturation, each new neuron has distinct properties and, at any given time, the DG population consists of GCs of many different ages. This heterogeneity has led to several proposed functions of new neurons.
This review summarizes the literature supporting the view that the function of new neurons is dynamic. After a brief overview of the classic view of the DG’s role in hippocampal function, we discuss the unique biological properties of immature neurons and the hypothesized computational functions of immature GCs. These young GCs, which are thought to be more excitable in the network, could complement the pattern separation function of mature GCs by adding a degree of similarity between events experienced close in time. Next, the biological events that affect long-term maturation are described along with how they relate to the proposed, long-term computational consequences of the addition of new neurons. Several models suggest that directing plasticity towards maturing neurons can preserve the DG’s representations of old memories while maintaining its capacity to learn new information. In both cases, behavioral studies that support and challenge these proposed functions are discussed.
The Function of the DG in Hippocampal Processing
Despite its large number of neurons and key position in the hippocampal formation (Figure 1), the DG has not been investigated as extensively as the other principal hippocampal areas, the CA3 and CA1. Nevertheless, several functions have been proposed for the DG[15, 16], most prominently that it is responsible for the pattern separation of cortical inputs to the hippocampus. The separation, or decorrelation, of encoding events is believed to be important to avoid interference between memories formed in the attractor-like networks in the CA3 and other regions[17, 18].
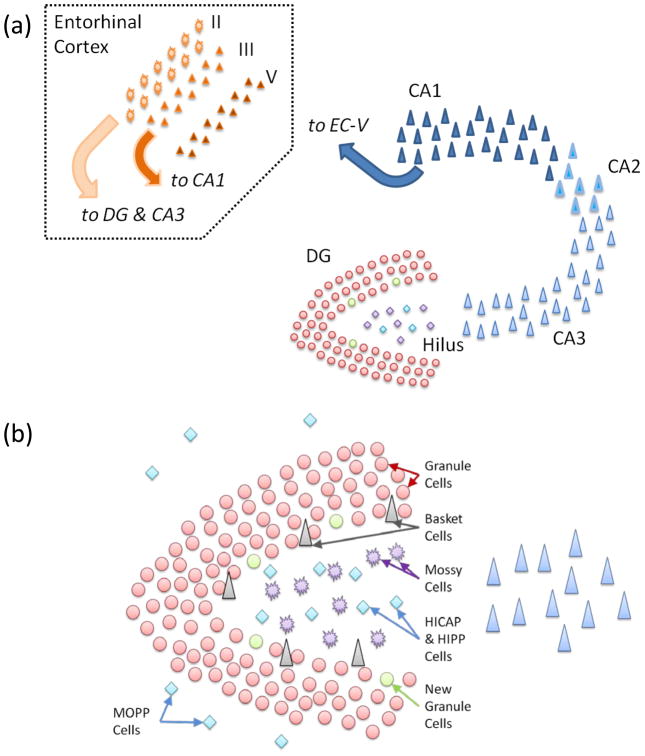
Figure 1. Hippocampal and DG anatomy.
(a) Cartoon schematic of the hippocampus and its principal input, the entorhinal cortex. (b) Schematic of the dentate gyrus and the hilus. The major neuron classes of the DG are labeled.
The general hypothesis concerning the DG’s pattern separation function relating to CA3 is that, as events are experienced, information is communicated to the hippocampus from the entorhinal cortex (EC) via the perforant path, which projects to both DG and CA3[19]. While each GC receives input from thousands of EC neurons, the basal level of inhibition is high, allowing only those neurons most specifically activated by their inputs to fire robustly[20]. Any neurons weakly activated by the stimulus will quickly be suppressed due to feedback inhibition. This tight tuning is believed to enable any shift in EC inputs to result in a substantially different active DG population. Notably, hippocampal (and DG) separation is generally thought to be between events rather than discrimination between features within events. The effect of this sparse activation is amplified by the mossy fiber projections of GCs to CA3; those GCs most highly activated will burst, allowing them to single-handedly induce action potentials in target CA3 pyramidal neurons[21]. Meanwhile, single spikes from weakly activated GCs do not “detonate” downstream principal cells but rather have a relatively stronger effect on the CA3 interneuron population, which in turn raises the global inhibition of the CA3 network[22]. Such a system would ensure that only those CA3 cells receiving inputs from bursting cells would be activated while all other CA3 neurons are suppressed. This co-activation of select CA3 neurons will preferentially potentiate their recurrent connections and the synapses from their direct EC input, thus creating a unique, DG-independent “memory” in the CA3[23–25]. While this process has not been demonstrated at a population level in vivo, behavioral studies in rodents with DG impairments are consistent with a role in pattern separation during memory encoding[26–28]. Similarly, recent fMRI studies suggest that activity in the DG/CA3 regions is correlated with pattern separation[29].
Despite this evidence, the role of the DG in pattern separation is far from clear[15, 30]. First, the results from in vivo DG recordings have been difficult to interpret: while pattern separation can be measured in the DG’s response, it appears to rely more on rate remapping (same neurons responding at different firing rates to different contexts) than population remapping (different populations of neurons responding to different contexts)[31]. Second, while rats with a lesioned DG have impairments in pattern separation and memory encoding[16], the mechanism of this dysfunction is unclear. These results could be explained directly by the loss of the DG’s contribution or by a second-order effect due to malfunction of the perturbed remaining hippocampal circuit. A recent study reported that mice whose Schaffer collaterals (the excitatory projection from CA3 to CA1) were silenced with tetanus toxin, thus essentially lacking CA3 and DG, remained capable of learning many hippocampal tasks that are thought to require the full hippocampus[32], suggesting that the interactions of DG, CA3, and CA1 may be more complex than simply assigning roles to each independently.
Finally, how a pattern separation function involves the considerable network plasticity observed in the DG is unclear. There is substantial synaptic plasticity in the DG: the perforant path inputs onto DG were where long-term potentiation (LTP) was first discovered[33]. An additional form of plasticity in the DG is the focus of this review: the fact that the DG continues to incorporate new GCs throughout life. While there have been several theories about how LTP may improve pattern separation - competitive learning can produce better separation between GCs[24] - the potential relationship of neurogenesis to pattern separation is less clear.
Direct Functions of Immature, Adult-Born Neurons
New neurons do not arise from existing neurons but rather grow into the adult circuitry de novo, arising from a population of NPCs that reside in the SGZ of the DG[34]. Newly born neurons are entirely distinct from the mature DG neurons and must undergo a considerable growth process before becoming recognizable as GCs. While the maturation process is vital to the growing neurons’ survival and shapes their ultimate characteristics, the properties of neurons at different developmental stages may have implications for DG function as well. Functionally, it is convenient to describe the maturation of new neurons in several stages. In reality, these stages are not discrete but represent a gradual, continuous shift from an immature, newborn state to a mature GC state (Figure 2).
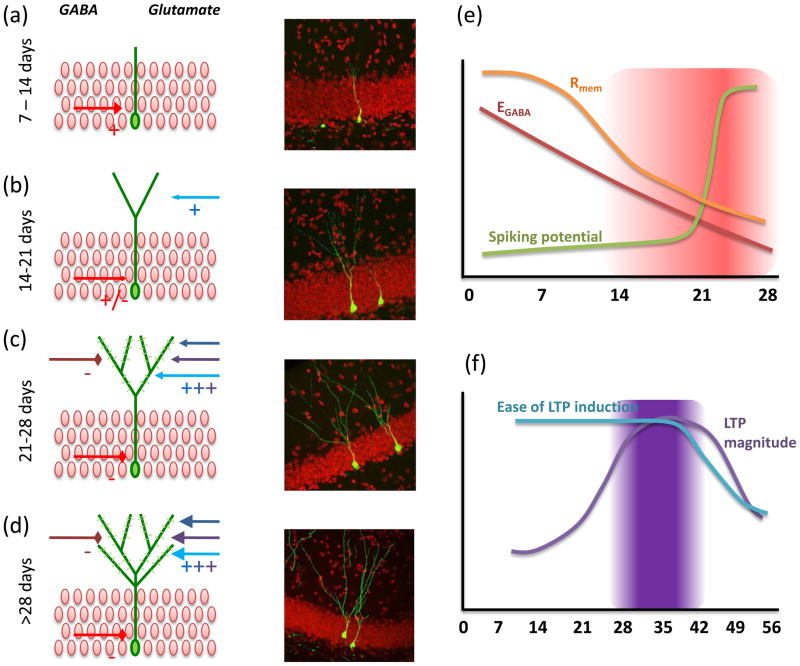
Figure 2. Maturation of adult-born neurons.
(a) Seven- to 14-day-old neurons have limited dendritic arborization and receive only GABA inputs (red arrow), which are excitatory. Right: GFP labeled 10-day-old neuron. (b) Fourteen- to 21-day-old neurons begin to develop spines and receive glutamatergic inputs (blue arrows). GABA transitions from depolarizing to hyperpolarizing. Right: GFP-labeled 14-day-old neuron. (c) The 21- to 28-day-old neurons have significant spine formation and show increased plasticity of perforant path inputs; Right: GFP-labeled 21-day-old neuron. (d) By 56 days, neurons are comparable to embryonic-born GCs; Right: GFP-labeled 56-day-old neuron. (e) Cartoon schematic of maturation of basic neuronal electrophysiology properties. Period of potentially increased excitability is highlighted. F) Cartoon schematic of maturation of synaptic connectivity and plasticity. Period of potentially increased learning is highlighted. Image courtesy of Zhao et al., Journal of Neuroscience 2006[39].
Early in maturation, new GCs are likely functionally “silent,” in that they do not appear to have connections to other neurons in the network or fire typical action potentials (Figure 2a)[35–38]. Physiologically they are quite distinct from mature cells. Lacking much of the neuronal machinery, their resistance is extremely high, whereas their capacitance is very low due to their size. Interestingly, the new neurons do receive GABAergic inputs (possibly initially dendritic), which are depolarizing[35]. It is unclear whether these very young neurons spike in vivo, although under slice conditions broad action potentials can be induced as early as five days after the last cell division[35]. This early development is critical for the ultimate survival of the new neurons (Box 1), but whether these unique properties can affect local network activity is unclear.
Box 1. Biochemical/molecular Basis for New Neuron Maturation.
Subsequent to neuronal fate commitment, the maturation of newborn neurons is regulated by local network activity. Animals’ experiences accompanied by the stimulation of the hippocampus, such as hippocampus-dependent learning, voluntary running, and environmental enrichment, enhance the survival of newborn neurons[70, 95–97].
Neurotransmitters, as mediators of neural activities, are key players in the regulation of the maturation of newborn neurons. GABA is important for the survival, migration and neurite outgrowth of newborn neurons younger than two weeks of age[44, 98]. At this stage, GABA is excitatory for the newborn neurons, due to their high intracellular [Cl-] concentration [44]. Another neurotransmitter, NMDA, is essential for the survival of newborn neurons between two and three weeks of age[99]. This time window corresponds with the appearance of dendritic spines and the formation of excitatory synapses[35, 39, 40, 44]. The newborn neurons seem to compete with their mature counterparts through a NMDA-mediated signaling pathway to survive and integrate into the network[99].
The survival of newborn neurons can also be affected by the emotional status of animals. Anti-depressants, such as fluoxetine and rolipram, enhance newborn neuron survival[100]. The anti-depressants increase levels of monoamines (e.g., serotonin) whose signaling regulates the efficacy of anti-depressants on both neurogenesis and emotional behavior[12]. The BDNF-TrkB signaling pathway, which has been implicated in mood regulation, is also involved in the survival, dendritic morphogenesis, and LTP induction of newborn neurons[101, 102]. In contrast, stress may lead to decreased newborn neuron survival under certain circumstances[103]. The effects of stress on neurogenesis are likely mediated by the alteration of HPA axis in response to stress, such as elevation in the levels of glucocorticoids[104].
The next stage of maturation begins around 16 days with the onset of dendritic spine formation (Figure 2b)[39, 40]. In addition to rapid spine growth, the mossy fiber axons, which grow towards the CA3 within their first week[41], begin to make synapses on downstream CA3 neurons[42]. Therefore, it is important to begin to consider neurons after this age as functionally relevant to the network, even though the full nature of the synapses formed by these young neurons is unknown. These neurons develop rapidly physiologically at this stage, and several features contribute to what appears to be a high excitability. Between 19 and 28 days, the new neurons exhibit dramatic changes in their spiking potential (e.g., ability to burst) (Figure 2c)[43]. Basic physiological properties - such as activation thresholds, membrane resistance and capacitance - are still transitioning to mature levels[35]. Importantly, GABA is still transitioning from being depolarizing to being the potent hyperpolarizing transmitter seen in mature GCs, suggesting that these neurons are not as inhibited as their mature counterparts[44, 45].
After about four weeks, the new neurons appear to settle into a more steady maturation phase in which the density and composition of afferent spines continue to develop[39]; by about eight weeks, the number of synapses on new neurons begins to plateau (Figure 2d). Similarly, the physiology of neurons gradually begins to approach that seen in mature neurons. While these neurons can be considered hyperexcitable around one month of age, by eight weeks they are essentially indistinguishable from mature cells[46, 47]. Finally, as will be discussed in the next section, this maturation phase is characterized by very robust synaptic plasticity[48, 49].
In summary, adult-born GCs appear to pass through several maturation stages, during which their physiology and connectivity suggest that their functional contribution may be distinct from that of mature GCs in the DG network. Specifically, this evidence suggests that immature neurons may be “hyperexcitable” when compared to mature GCs (Figure 2e).
Insights from computational models investigating a mixed DG population
Most neural network studies of the hippocampus that directly attribute a pattern separation role to the DG implicitly assume fairly simple statistics for the DG: only a small number of GCs are randomly activated for any given event. This sparse, uncorrelated activation provides a very strong separation effect since the small fraction of GCs activated in one event would not be expected to be activated in a second event by chance. For instance, Treves and Rolls’ model of CA3, which was an important demonstration of how sparse, orthogonal signals from the DG are necessary for memory formation, treated the DG as a random activation of a sparse set of neurons[25]. Similarly, while O’Reilly and McClelland’s model related pattern separation to sparsity, all GCs were uniform, with a winner-takes-all algorithm leading to separation[50]. Myers and Scharfman’s recent model of the DG[51], which demonstrated one mechanism by which feedback interneuron populations can provide this sparse coding, also utilized a homogeneous population of GCs.
While these studies have demonstrated how sparse DG coding relates to pattern separation and memory formation, it is worth considering whether these results are maintained with GCs of many different ages (Figure 3). The computational model of the DG network developed by Aimone and colleagues[52] demonstrated that the increased excitability of immature neurons can result in the new GCs being more broadly tuned than mature neurons. By being less discriminating than mature GCs, the immature neurons are more likely to be incorporated into the DG’s representations of events, adding similarity between the DG’s outputs. Because new neurons only comprise a small fraction of the neurons in the DG, the overall pattern separation effect is maintained, but the new neurons’ contribution, which is referred to as pattern integration, communicates a relationship between events encoded by the same new neurons. One hypothesis is that this integration permits the hippocampus to retain some link between events in the representations encoded by the downstream network (Figure 3c).
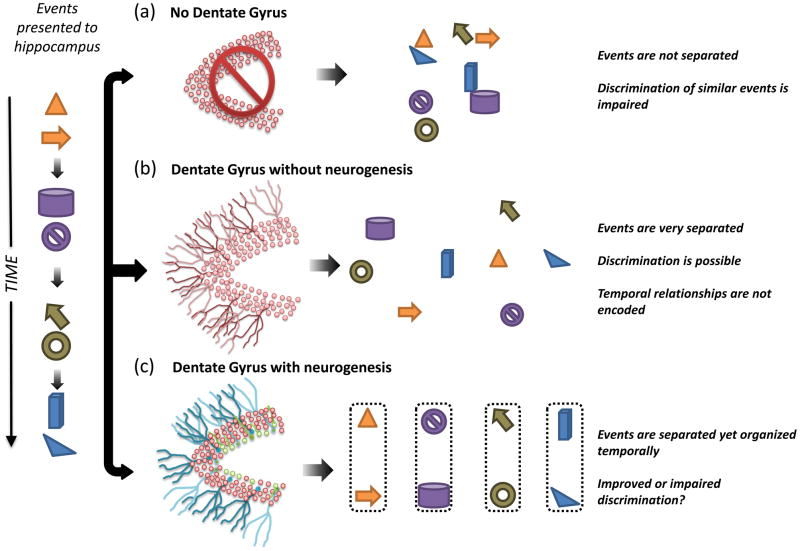
Figure 3. How DG and new neurons may affect pattern separation.
Each panel represents how a series of temporally discrete events (left) would be encoded by the hippocampus. (a) Events encoded by the hippocampus without the DG would not be adequately separated, leading to possible clustering of memories based on content. (b) Events encoded by the DG without neurogenesis would be highly separated. (c) Events encoded by the DG with neurogenesis would also be highly separated but would potentially retain their temporal structure. This temporal integration would be provided by the heterogeneous mix of immature neurons.
A similar effect on pattern separation was observed in the full hippocampal model recently described by Weisz and Argibay[53]. In contrast to the Aimone model, Weisz and Argibay treated neurogenesis as a one-time increase of DG size, with the new neurons more likely to be activated and to have increased learning rates compared to older neurons. While plasticity has several effects on learning and retrieval in the hippocampus (which are described below), the higher activation decreases pattern separation between new memories, consistent with a pattern integration effect.
If immature neurons are indeed encoding a relationship between events through this pattern integration effect, what might this relationship represent? In the Aimone model[52], while events that occurred on the same day activated the same immature neuron populations, events occurring many days apart activated a distinct set of immature neurons. Instead of pattern integration, this activation of different new neuron populations increased pattern separation. This dependence on time suggests that the relationship communicated by pattern integration is actually a “temporal context”[52, 54]. Likewise, Weisz and Argibay noted that their results would result in less overlap between recent and remote memories but increased overlap between new memories[53].
Several other mechanisms have been proposed for how new neurons encode temporal information. Becker and Wojtowicz suggest that waves of neurons arising from common progenitors might mature to encode separate features of common experiences. Because the young cells mature out of their more plastic states, these different waves of new neurons would temporally segregate memories into relevant episodes[55]. A separate hypothesis is that new neurons can provide information about the age of a memory, which appears to be a robust, yet unexplained characteristic of human memories[56].
Behavioral impact of immature neurons
Pattern separation is usually assayed using pattern discrimination tasks in behavioral studies. How does pattern integration affect the behavior of animals on discrimination tasks? One possibility is that pattern integration acts to simply reduce pattern separation; in effect, it impedes the animal’s event discrimination ability. Alternatively, pattern integration may play a fundamentally different (or complementary) role than pattern separation. For example, the artificial links between events due to integration could help to retrieve all related events for comparison when a discrimination decision needs to be made. Another possibility is that pattern integration functions to put similar events into distinct contexts by linking them to different but temporally related events. In both situations, pattern integration may enhance pattern separation and hippocampal learning.
How pattern integration is reflected in animal behavior and its relationship with DG pattern separation in one of the above-mentioned mechanisms have not been tested directly experimentally, but an integration function may be useful to explain the results observed in several behavioral tasks on animals without neurogenesis. If immature neurons, as pattern integrators, create artificial links among events that occur simultaneously or close in time, one would predict that the events would be encoded more separately in the absence of these immature neurons. The findings reported by Saxe and colleagues support this hypothesis[57]. They subjected mice, whose neurogenesis was ablated specifically in the hippocampus, to a working memory paradigm in an eight-arm radial maze, where the animals needed to learn the location of a sample arm in the sample phase. In the test phase, the sample arm and another arm (i.e., test arm) were made accessible and the mice were required to learn to retrieve food rewards only from the test arm. The neurogenesis-ablated mice performed better than sham controls if the sample arms and test arms were employed repeatedly and interchangeably in different trials. One possible explanation for such performance improvement is that the lack of newborn neurons allows each sample-test trial to be encoded more independently, so that memories of multiple trials would not interfere with each other.
However, other behavioral findings suggest that a simple “new neurons reduce pattern separation” function is insufficient. Recently, Clelland and colleagues developed two new behavioral paradigms to examine the role of neurogenesis in spatial discrimination[58]. In the first task, mice with neurogenesis ablation were defective in a working memory task in an eight-arm radial maze when the sample arm and the test arm were close to each other in space but not when the two arms were spatially distant. In the second task, using touch screen apparatus, neurogenesis-deficient mice performed similarly to control mice when the two lighted boxes were distant in space but were impaired when the two lighted boxes were proximate to each other. At first glance, these results appear contradictory to those from the Saxe study. However, there are important differences between these studies. The Saxe study specifically looked at the effect of interference on working memory: animals were either trained on two different sample arms and then tested for working memory for each arm, always bating the corresponding adjacent arms, or they were trained on trials with sample arms and target arms were used inter-changeably. Notably, interference could arise both spatially (between sample arms) and temporally (between trials). Such “between events” interference can be manifested by the pattern integration described above. In contrast, the Clelland study did not use interference trials or require the animals to maintain multiple memory traces simultaneously; the animals simply had to remember the sample location and to choose either matched (touch screen task) or non-matched (RAM task) locations. Another difference is how the animals were trained in each task. In the Saxe tasks, animals were trained only on one level of separation (adjacent arms). In both Clelland tasks, animals were simultaneously trained on both the easy (high separation) and hard (low separation) conditions. One possibility is that pattern separation may underscore the generalization of a common rule between two different conditions. By this perspective, the inability to integrate a strategy across conditions in the absence of neurogenesis may reveal itself in impaired performance on the harder spatial discrimination condition. Notably, in a recent study using the Clelland touch-screen task, running (high neurogenesis) and non-running (normal neurogenesis) mice were trained on one separation level, showing no difference in time to reach criterion, but when the mice were then tested on a more difficult, lower separation condition, the running mice performed significantly better[59].
Pattern integration may also facilitate learning and memory by associating multiple elements from complex environments and from experiences of animals. Such a function may be engaged in locating a hidden platform in the Morris water maze (MWM) or in associating a distinctive context with an aversive stimulus in contextual conditioning. Consistent with this view, mice or rats whose neurogenesis is ablated by several different approaches (see Box 2) display compromised spatial navigation learning and/or memory in MWM[60–64] and contextual learning in contextual fear conditioning[14, 62, 65, 66], despite the fact that such defects were not revealed by other studies, possibly due to differences in experimental subjects and conditions (see ref [6] for detailed discussion). As discussed above, computational studies suggest that the hyper-excitability of immature newborn neurons may be the mechanism for pattern integration. Recently a transgenic mouse model was developed that enabled the number of newborn neurons at a particular maturation stage in adult animals to be reduced[67]. Using this model, it was demonstrated that the reduction of the newborn neuron population at the immature stage - but not at the mature stage - leads to impairments in learning and memory in MWM.
Box 2. Knockdown Methods for Neurogenesis.
The reductionist approach to study the function of neurogenesis is to investigate the effects of neurogenesis ablation on animals using various behavioral assays. A number of approaches have been developed in the past decade to abolish neurogenesis, with most methods targeting the proliferating NPCs. Early approaches took advantage of the vulnerability of proliferating cells to chemical and physical insults. Treating animals with X-ray or gamma-ray irradiation or anti-mitotic drugs such as MAM and TMZ induces apoptosis of NPCs[64, 68, 105, 106]. But such approaches may cause undesired side effects, which in turn complicate interpretation of the experimental data[107, 108].
Recently, several transgenic mouse models for neurogenesis ablation were generated that allow more precise control of neurogenesis ablation. To manipulate neurogenesis, most of these models use NPC-specific promoters such as Nestin and GFAP to express proteins, such as CreER, rtTA and TK, whose activity is inducible by drugs[14, 62, 63, 67]. Cell type-specific and adult-inducible ablation of neurogenesis can be achieved through the combination of these genetic tools with drug treatment and/or other reagents that result in cell death. For example, treating Nestin-TK or GFAP-tk mice with ganciclovir, a nucleotide analog, results in the ablation of dividing Nestin- or GFAP-expressing NPCs, respectively[14, 67]. Combining Nestin-rtTA with the TRE-Bax transgene can induce apoptosis in Nestin-expressing NPCs upon treatment with doxycycline[63]. In Nestin-CreER; NSE-floxed(stop)-DTA mice, treatment with tamoxifen results in the expression of diphtheria toxin A in the neuronal progeny of the Nestin-expressing NPCs and the subsequent ablation of the newborn neurons arising from these progenitors[62]. In summary, these models allow the manipulation of adult neurogenesis with cellular specificity and temporal precision, which minimizes the side effects.
In addition to spatial pattern association, hyper-excitability of immature newborn neurons may contribute to temporal pattern integration by the association of input patterns across short time intervals. Consistent with this hypothesis, rats with neurogenesis ablation are impaired in learning to associate a conditional signal with an unconditional stimulus across a short time interval in either a trace conditioning tasks or delayed non-matching-to-sample task[13, 66, 68]. Interestingly, the studies demonstrating these impairments were performed on animals lacking a relatively immature population of newborn neurons.
Long-term Functions of Adult-born Neurons
While the evidence regarding new neuron maturation is consistent with the theoretical effects of immature neurons on pattern separation described above, these functions alone are not sufficient to explain the lifelong persistence of adult-born neurons. While a fraction of the immature neurons that contribute to the aforementioned functions likely die before fully integrating into the network, the majority of these neurons appear to survive and eventually attain anatomical and physiological characteristics that make them essentially indistinguishable from embryonic and postnatal GCs[39, 46]. This long-term survival of new neurons leads to new questions: what are the effects of the long-term addition of new neurons on DG function? Do these maturing adult-born neurons learn to represent any specific information? While the answers to these questions will likely depend on new basic information about the timing and extent of new neuronal integration in the DG, the plasticity and survival of adult-born GCs are likely to have a significant impact.
As described in the previous section, as new neurons enter the final phases of their maturation, they appear physiologically very similar to existing GCs. However, these “adolescent” GCs differ from the older GCs in one important aspect: they appear to be considerably more plastic than fully mature GCs (Figure 2f). Several groups have observed that LTP is easier to induce in young neurons; immature neurons roughly two to six weeks of age have considerably lower thresholds for LTP[38, 48, 49]. In addition, the magnitude of LTP is higher in GCs about four to six weeks after birth[48]. These observations are consistent with several studies showing that LTP is difficult to elicit in rodents after irradiation[14, 69].
A long-term function for neurogenesis implies a tightly controlled regulation of which new neurons survive in the network. While there is considerable death of young neurons, enriched environment and activity can increase their survival[70]. Recently, a critical time at which experience affects new neurons’ survival was identified[71]. By subjecting mice to environmental enrichment at different time points after labeling new neurons with BrdU (a nucleotide analogue that only incorporates into dividing cells), Tashiro and colleagues revealed that the first three weeks after the birth of newborn neurons are critical for the enrichment-enhanced survival of newborn neurons. (See Box 1)
Insights from computational models of long-term neurogenesis function
Not surprisingly, the first computational studies to investigate adult neurogenesis were interested in how the incorporation of “naïve” new neurons (with accompanying “death” of existing neurons) affected the function of mature networks[72–74]. Because these early studies were mostly performed prior to the biological characterization of new neuron maturation, their results must be re-interpreted, incorporating what is now known about the system. Nevertheless, the basic conclusion of these studies remains relevant: adult neurogenesis can facilitate the encoding of new memories by providing neurons that are not already fully tuned to an existing “memory,” and therefore are more amenable to learning new information. While this role was accompanied by the forgetting of old memories in abstract feed-forward networks that performed both encoding and retrieval, Becker’s model, which limited the DG’s role to encoding, demonstrated that neurogenesis could facilitate the encoding of new, separated memories while not significantly disrupting older memories[75].
Recently, computational models of neurogenesis have investigated whether the distinct properties of immature neurons improve memory acquisition long-term. Specifically, that immature neurons have higher plasticity compared to mature neurons may have a considerable impact on function. Wiskott and colleagues investigated the possibility that new neurons are useful for the avoidance of catastrophic interference in the hippocampus[76, 77]. Their models have shown that directing learning to new neurons allows the network to store new memories while preserving old information. Without neurogenesis, the learning of new memories eventually would disrupt all previously stored memories. Weisz and Argibay’s study showed a similar result[53]. Beyond a critical memory load, their full hippocampal model showed diminished retrieval for all memories. However, if the network received a one-time boost in GC number (with the new neurons having higher plasticity), the capacity of the network was considerably increased. Importantly, they did not observe this benefit by simply using a larger DG population from the onset.
One corollary of the prediction that new neurons facilitate encoding by learning new information (thereby preserving old information) is that the new neurons should subsequently be preferentially activated by that information. Seeking to address this directly, in the model developed by Aimone and colleagues[52] all the GC neurons matured through a neurogenesis process while the “environment” that was presented to the model changed periodically. Later, when re-exposed to each of the previously experienced environments, the GCs were activated preferentially by the environments that they matured within. This “imprinting” or “specialization” of young neurons to the information experienced during their development may be important for improved future encoding of information by the DG. Since the majority of DG development is postnatal[78, 79], most DG neurons may essentially be representations of past events. As a result, a new memory encoded by the DG utilizes a unique combination of past memories. This could be a possible mechanism for a memory-coding scheme, such as the constructive memory hypothesis espoused by Schacter and others[80, 81]. Such a function is entirely consistent with the long-term theories described above, but importantly, it makes a specific biological prediction: re-exposing an animal to previously experienced events should reactivate those GCs that had matured within that environment.
In conclusion, these computational studies suggest two general functions for new neurons over their lifetimes beyond the functions due to their immature properties (Figure 4). First, the addition of new neurons to the network continuously provides new capacity of sparse code generation by the DG. Since these sparse codes are the basis for memory formation in the CA3, the essentially unbounded supply of unique codes due to neurogenesis greatly reduces the probability that a new memory will interfere with existing memories. Secondly, the new neurons, and thus the sparse codes that they provide, are themselves functions of past experiences. How this specialization function would affect subsequent learning, however, is still unclear.
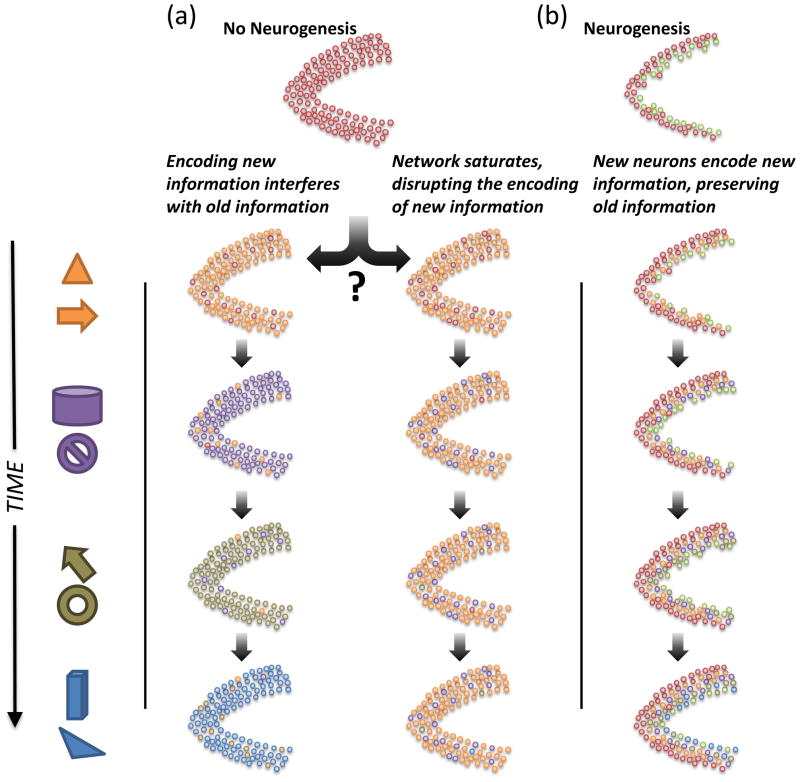
Figure 4. Alternative theories of neurogenesis depletion on the DG long-term.
Each column represents how the DG would acquire information about events presented over extended time scales. (a) Without neurogenesis, the DG (and the rest of hippocampus) might continue to incorporate new information that, in effect, overwrites old information. Such a mechanism could lead to “catastrophic interference” in the network. (b) An alternative possibility is that, without neurogenesis, the DG network could quickly use its finite set of sparse codes for early events. All subsequent new memories would be encoded by utilizing already existing, and thus less optimal, sparse codes. (c) Most models of neurogenesis suggest that immature neurons are the most plastic in the network, allowing new information to be encoded by new neurons and leaving old information intact.
Biological evidence for a long-term function
As with the theoretical short-term functions of neurogenesis, these proposed long-term functions for new neurons have not been tested explicitly by experiments. The hypothesis that new neurons are important for memory capacity predicts that, in the absence of neurogenesis, the hippocampus would be “overloaded” with memories, causing difficulty in acquiring new information or maintaining old memories once the system is saturated. In practice, it is difficult to examine information overload in laboratory rodent models (see Box 2). However, the impairment of long-term memory but not short-term memory in MWM is consistent with the capacity theory[60, 62, 64, 67].
The theory suggesting an “imprinting” or “specialization” of new neurons to previous experiences is particularly amenable to examination with immediate early genes (IEGs), such as c-fos, arc, zif, etc., whose expression has been associated with neuronal activity[82] Because of the changes of physiological characteristics during maturation, the responsiveness of newborn neurons may be a function of their age, and studies of IEG expression suggest that the newborn neurons have to become mature enough to express IEGs. In mouse, the expression of IEGs is not detected when newborn neurons are about three to four weeks of age[83]. In rat, newborn neurons are able to express IEGs as early as two weeks of age, and the proportion of IEG-expressing newborn neurons peaks when they are about three weeks of age[84]. This discrepancy is likely due to the fact that newborn neurons mature faster in rats[84]. As predicted by computational studies, newborn neurons are more likely to be activated by inputs in the hippocampus than their mature counterparts. For example, when rats were allowed to explore a novel activity chamber, a higher proportion of newborn neurons (five months old) expressed IEG than did mature neurons[85].
The specialization function was explicitly investigated in several studies that examined how the experience of animals during a particular stage of newborn neuron maturation would influence their activities after these neurons became fully matured. In one study, Kee and colleagues birth-dated newborn neurons with BrdU and trained mice in a spatial version of MWM at several different time points after new neurons were labeled[86]. Ten weeks after the initial BrdU labeling, the animals were subjected to retrieval trials on the MWM and immediately tested for IEG expression. The study found that a higher proportion of BrdU-labeled neurons expressed IEGs compared to unlabeled GCs only if the training took place when the young neurons were older than 4 or 6 weeks of age. Although this study did not identify a functional role for these neurons, it did suggest that immature neurons were preferentially recruited into the memory circuit, consistent with the theory that inputs on the relatively immature newborn neurons could specialize their responsiveness when they became older.
A similar study was performed by Tashiro and colleagues, who suggested that even younger GCs could acquire information about the animal’s environment[71]. They allowed mice to explore an enriched environment when the BrdU-labeled newborn neurons were about two weeks old. Four weeks later, they either trained the mice on MWM or re-exposed them to the previously encountered enriched environment; then they examined IEG expression in BrdU-positive newborn neurons. They found that a higher proportion of BrdU-labeled neurons was activated in the mice that were re-exposed to enriched environments than in those trained in MWM. Furthermore, a lower proportion of newborn neurons expressed IEG in the MWM if the mice were previously exposed to an enriched environment. Hence, events encoded when the newborn neurons were immature (hyper-excitable and/or hyper-plastic) may suppress their responsiveness to other inputs after maturation, thus “specializing” the newborn neurons.
Newborn neurons may also play a role in strengthening memories in the specialized environment or context. Trouche and colleagues mass-trained mice in MWM when the labeled newborn neurons were nine days old and subjected them to various behavioral procedures in the same MWM before examining IEG expression[87]. They found that a lower proportion of labeled newborn neurons was activated by probe trials than by training trials. In addition, re-training animals thirty days after initial training activated even more labeled newborn neurons, suggesting that newborn neurons that have specialized to a context may be involved in strengthening the previously learned memories. Whether these newborn neurons are involved in learning new information in a familiar environment awaits examination.
The IEG studies described above support the hypothesis that input specialization of the newborn neurons during the immature stage can influence their function in the long run. However, it is currently unclear what the advantages of such specialization are and how the behavior of animals would be affected if lacking this imprinting. Despite the assumption in the IEG studies that the newborn neurons activated during memory encoding were reactivated during memory retrieval, it is not clear currently whether neurogenesis is involved in encoding, consolidation, retrieval or storage of memory (see Box 3).
Box 3. New Neurons in Encoding, Consolidation, and Retrieval?
Recent theories of neurogenesis have focused on the role of new neurons in the encoding of new memories[52, 53, 75]. This perspective originated from computational theories of DG function that suggest that the DG is principally involved in encoding and is possibly bypassed during retrieval[25, 109]. Indeed, one key difference between the pattern integration proposals for neurogenesis and pattern completion is that integration and separation are typically thought of in terms of the encoding process, whereas completion is considered a retrieval function.
A recent study suggests that new neurons may have a role in a different stage of memory formation: consolidation[110]. Memory consolidation, typically described as the transfer of memories from the hippocampus to the cortex, has been attracting increased attention, but the process itself remains a mystery. Consolidation is classically tested by training animals on hippocampus-dependent tasks, such as context fear conditioning, and the hippocampus is lesioned after a delay of several days or weeks. At first, the memory is lost when the hippocampus is removed, but with enough of a delay the memory can be retrieved and is considered hippocampus-independent. In Kitamura et al., the authors observed that a neurogenesis lesion results in memories remaining hippocampus-dependent after delays that should have allowed for transfer to cortex, whereas increased neurogenesis due to running accelerates this consolidation process.
Understanding the role for neurogenesis in consolidation is difficult since the DG’s role in consolidation is itself unclear. Recent evidence has shown a clear dependence of the CA3[111] and CA1[112] on this transfer of memories from hippocampus to cortex, and in vivo recordings have shown “replay” of memory ensembles in both regions[113, 114]. Future work will hopefully reveal what role the DG plays in this process. Furthermore, the separation of encoding deficits from consolidation deficits is not trivial behaviorally. Perhaps, in Kitamura, consolidation is actually normal, but without new neurons, memories are encoded in a qualitatively different manner that changes how they are later transferred to the cortex.
Encoding, retrieval, and consolidation are likely not as distinct from one another as once thought. These results suggest not only that the role of neurogenesis (and DG) in consolidation should be further studied but also that it is worth challenging the idea that the DG and neurogenesis are not involved during retrieval. The development and use of more temporally precise and reversible neurogenesis knockdowns should shed light on some of these mysteries.
Conclusions and Future Directions
This review seeks to link the current theoretical views on neurogenesis function with relevant experimental findings. These different approaches increasingly suggest that the role of neurogenesis is highly complex and affects multiple aspects of learning, as opposed to being a clearly definable function. This subtlety is potentially responsible for the large variance observed between neurogenesis behavioral studies (see [6] for more extensive discussion), and it will continue to present a challenge in the design of behavioral tasks and knockout techniques to examine neurogenesis.
It is important to consider at least two functional periods for adult-born neurons. The distinct physiological properties of immature neurons make them well suited to affect the canonical pattern separation function of the DG in unique ways. By modulating pattern separation, young cells that are just beginning to integrate into the network could have a marked impact on learning. The second function is less temporally defined and pertains to the long-term survival and integration of the neurons into the network. The addition of new GCs to the network can have considerable effects on memory capacity in artificial networks, and it is possible that they affect hippocampal memory capacity similarly. The suggestion that these neurons are specializing to what the animal experiences during their maturation is an exciting possibility, although the behavioral implications are less clear.
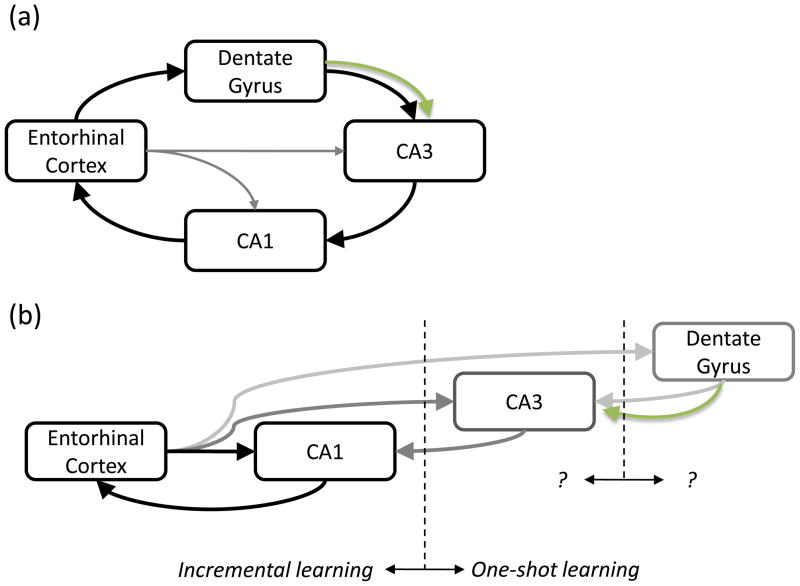
Importantly, the function of neurogenesis will be constrained by the DG’s influence on the rest of hippocampal function (Figure 5). It is increasingly accepted that the hippocampus is more complex than just the classic “tri-synaptic loop” (the EC-DG-CA3-CA1-EC loop shown in Figure 5A)[88], but it is unclear whether the direct cortical inputs are the principal drives to each hippocampal sub-region[32, 89–91]. If the latter is true, the DG’s role may be to bias CA3 and CA1 encoding, making neurogenesis a “modulator of a modulator.” Hippocampus-dependent behaviors that involve extended training (such as MWM) require only the EC-CA1-EC loop to be functional[32], which is interesting because several neurogenesis knockdowns have shown deficits on MWM[60–64, 67]. One possibility is that, without neurogenesis, the remaining DG/CA3 circuitry is dysfunctional. In this sense, neurogenesis, through pattern integration or another contribution, may be essential to keep the DG/CA3 circuit operating within proper parameters. Regardless, it is increasingly clear that further work is required to relate neurogenesis and the DG to the rest of the hippocampus.
Figure 5. Role of DG and neurogenesis in hippocampal function.
(a) According to theories based on the classic trisynaptic loop[23, 25, 88], the DG is the principal input to the hippocampus, positioning neurogenesis for a significant impact on memory formation. (b) An alternative possibility envisions that direct connections from EC to CA1 are sufficient for some hippocampal processing and loops through the CA3 and the DG are important under specific circumstances, such as one-shot learning[32, 89]. This positioning would suggest that DG modulates CA3 function and neurogenesis modulates this modulation.
The development of functional imaging approaches that can measure neurogenesis in humans and live animals (Box 4) represents an important and exciting advance in the field. Some of the proposed functions of neurogenesis, such as forming temporal associations in episodic memory, may be more readily examined in human cognitive studies across populations with different neurogenesis levels (due to aging, disease, or environment) as opposed to rodent behavior. Indeed, an important future direction in this field is to design approaches that examine these proposed neurogenesis functions in humans. Neurogenesis appears to be implicated in several clinical conditions that are associated with deficits in cognition and memory, including depression (Box 5) and several neurological diseases. Because neurogenesis is regulated by many intrinsic and exogenous factors, it may represent an important target for therapeutic interventions to treat these diseases.
Box 4. Adult Neurogenesis in Humans.
One of the more controversial topics regarding adult neurogenesis is the extent to which new neurons integrate into the human brain. The initial study to show direct evidence for human neurogenesis by Eriksson and colleagues measured BrdU, which had been used for diagnostic purposes[115]. Another serendipitous approach took advantage of cells radiolabeled by above-ground nuclear testing in the 1950’s to trace proliferative populations[116]. Outside of these unique circumstances, quantifying and tracking new neurons in the human brain remain significant challenges. BrdU and other tracing molecules used in animal studies are not applicable in human studies, and histological identification of dividing and maturing cells in postmortem tissue is of limited value to functional studies.
A promising development is a technique utilizing functional MRI to measure a signal from the DG region in humans that has been shown to correlate well with neurogenesis in animals[117, 118]. One such approach utilizes cortical blood volume (as opposed to flow) to identify a correlate of neurogenesis[118]; another approach quantifies the magnitude of a signal from a specific lipid believed to localize to a proliferative population[117]. Such ex vivo quantification of neurogenesis should prove a valuable tool for use in conjunction with psychological tests of neurogenesis and DG function[29].
In addition to its use in functional experiments, improved measurement of neurogenesis rates in humans will be a key step in understanding its role in different neurological disorders. Neurogenesis has been shown to be affected in mouse models of Alzheimer’s and Parkinson’s disease[119–122], but the data regarding human patients are limited[123, 124]. Similarly, improved imaging techniques will be valuable in examining potential roles for neurogenesis in epilepsy, stroke, and other neurological conditions[3].
Box 5. Adult Neurogenesis and Depression.
One of the most promising, but also most controversial, aspects of adult neurogenesis is its relationship with depression. Anti-depressants increase neurogenesis considerably[125], and new neurons are required for anti-depressants’ efficacy in several assays for depression[106]. The findings that neurogenesis is suppressed by stress[126] and that hippocampal volume is also significantly reduced in humans suffering from severe depression[127, 128] point to a promising potential interaction between adult neurogenesis and depression.
However, the relationship between neurogenesis and depression has only become less clear in recent years. Knocking out neurogenesis in animals did not produce a “depressed” phenotype on those tasks where neurogenesis is required for anti-depressant function[106]. Furthermore, not all behavioral tasks for depression appear to require neurogenesis for anti-depressant efficacy[129]. While neurogenesis is required for SSRI efficacy in the novelty suppressed feeding task, irradiation does not affect SSRI-based improvement in the open field or forced swim tasks. One possibility is that anti-depressants act through neurogenesis-dependent and neurogenesis-independent mechanisms[129, 130].
Several questions remain in translating this relationship to our understanding of human depression. First, the relationship of human depression to the “depressed” phenotype observed in rodents, which requires artificial inducement, remains unclear. Second, the causal relationship between learning and depression needs to be better explored. If losing neurogenesis impairs learning, an eventual consequence may be something akin to learned helplessness, which is used to induce a depressed state that affects neurogenesis in rodents[131]. While knockout animals may not show a depressed phenotype initially, it may be interesting to investigate whether they develop one after significant cognitive challenges.
The potential relationship between neurogenesis and depression-related cognitive deficits has been investigated theoretically. Becker and Wojtowicz discussed how a lack of neurogenesis due to depression could exacerbate the condition by impairing the encoding of positive contextual features[55]. As a follow-up, they recently tested depressed individuals on several cognitive tasks, correlating their impaired performance on the delayed match to sample task to their computational results[132]. Similarly, in our computational study, we simulated the effects of acute drops in neurogenesis due to stress or depression, observing that several functions, including encoding temporal information, would be compromised[52]. These predictions raise the interesting prospect that continued psychological testing and imaging studies in depressed individuals may be ways to test the function of neurogenesis, and related theories, in humans.
While behavioral tasks on humans and rodents may increasingly be able to test the theories presented by the models described here; there are still many aspects in which these models need to be further developed. Modeling biologically realistic sized networks is increasingly possible given advances in computational hardware[92]. It will be important to determine whether arguments that neurogenesis is necessary for memory capacity, which have been described using relatively small networks, apply to a rat-sized DG with over a million GCs. Another question is whether young GCs have a processing role beyond capacity or temporal encoding. For instance, hippocampal place field formation has been extensively studied by in vivo physiology and models of other hippocampal regions, but how new neurons affect the representation of spatial information is unclear. Such an informatic description of neurogenesis function would complement its emerging role in memory formation, and may help in future endeavors to translate the novel dynamic introduced by neurogenesis to other computational domains, such as robotic implementations of a hippocampal spatial processing circuit[93, 94]. Finally, while most models of the DG and neurogenesis have focused on a role in memory encoding, their roles in other cognitive states, such as consolidation and retrieval, should be explored (Box 3). These other roles not only require formulating new perspectives on hippocampal function, but also an improved understanding of how the DG and neurogenesis are affected by ascending aminergic and cholinergic modulatory systems. Given the recent emphasis on the relationship of neurogenesis to antidepressants that target serotonin and other neurotransmitters (Box 5), this is an area where future modeling will be invaluable.
Acknowledgments
We would like to thank M.L. Gage for editorial comments on the manuscript. This work is funded by the James S. McDonnell Foundation, the Lookout Fund, the Kavli Institute for Brain and Mind, the NSF Temporal Dynamics of Learning Center, and the National Institutes of Health (MH-090258).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James B. Aimone, Email: aimone@salk.edu.
Wei Deng, Email: deng@salk.edu.
Fred H. Gage, Email: gage@salk.edu.
References
- 1.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zupanc GK. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Brain Behav Evol. 2001;58:250–275. doi: 10.1159/000057569. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Mouret A, et al. Centrifugal drive onto local inhibitory interneurons of the olfactory bulb. Ann N Y Acad Sci. 2009;1170:239–254. doi: 10.1111/j.1749-6632.2009.03913.x. [DOI] [PubMed] [Google Scholar]
- 5.Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog Neurobiol. 2009;89:162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng W, et al. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nature Reviews Neuroscience. 2010 doi: 10.1038/nrn2822. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabel K, et al. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogeneis in mice. Frontiers in Neurogenesis. 2009;1:1–7. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupret D, et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould E, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 11.Jessberger S, Gage FH. Stem-cell-associated structural and functional plasticity in the aging hippocampus. Psychol Aging. 2008;23:684–691. doi: 10.1037/a0014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 13.Shors TJ, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treves A, et al. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 16.Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- 17.Blumenfeld B, et al. Dynamics of memory representations in networks with novelty-facilitated synaptic plasticity. Neuron. 2006;52:383–394. doi: 10.1016/j.neuron.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaral DG, et al. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- 21.Henze DA, et al. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 23.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987;10:408–415. [Google Scholar]
- 24.Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert PE, et al. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 27.Lee I, Kesner RP. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- 28.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 29.Bakker A, et al. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leutgeb JK, Moser EI. Enigmas of the dentate gyrus. Neuron. 2007;55:176–178. doi: 10.1016/j.neuron.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Leutgeb JK, et al. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 32.Nakashiba T, et al. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 33.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh H, et al. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 35.Esposito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overstreet-Wadiche LS, et al. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambrogini P, et al. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, et al. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- 39.Zhao C, et al. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 41.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 42.Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mongiat LA, et al. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markwardt SJ, et al. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci. 2009;29:15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laplagne DA, et al. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laplagne DA, et al. Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci. 2007;25:2973–2981. doi: 10.1111/j.1460-9568.2007.05549.x. [DOI] [PubMed] [Google Scholar]
- 48.Ge S, et al. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt-Hieber C, et al. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 50.O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 51.Myers CE, Scharfman HE. A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus. 2009;19:321–337. doi: 10.1002/hipo.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aimone JB, et al. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weisz VI, Argibay PF. A putative role for neurogenesis in neuro-computational terms: inferences from a hippocampal model. Cognition. 2009;112:229–240. doi: 10.1016/j.cognition.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Aimone JB, et al. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 55.Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 56.Friedman WJ. Comment on "Potential role for adult neurogenesis in the encoding of time in new memories". Hippocampus. 2007;17:503–504. doi: 10.1002/hipo.20280. [DOI] [PubMed] [Google Scholar]
- 57.Saxe MD, et al. Paradoxical Influence of hippocampal neurogenesis on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Creer DJ, et al. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jessberger S, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang CL, et al. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 62.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 63.Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snyder JS, et al. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Warner-Schmidt JL, et al. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur J Neurosci. 2008;27:1485–1493. doi: 10.1111/j.1460-9568.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- 66.Winocur G, et al. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 67.Deng W, et al. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 69.Snyder JS, et al. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 70.Kempermann G, et al. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 71.Tashiro A, et al. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chambers RA, Conroy SK. Network modeling of adult neurogenesis: shifting rates of neuronal turnover optimally gears network learning according to novelty gradient. J Cogn Neurosci. 2007;19:1–12. doi: 10.1162/jocn.2007.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chambers RA, et al. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology. 2004;29:747–758. doi: 10.1038/sj.npp.1300358. [DOI] [PubMed] [Google Scholar]
- 74.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 75.Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- 76.Appleby PA, Wiskott L. Additive neurogenesis as a strategy for avoiding interference in a sparsely-coding dentate gyrus. Network. 2009;20:137–161. doi: 10.1080/09548980902993156. [DOI] [PubMed] [Google Scholar]
- 77.Wiskott L, et al. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- 78.Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- 79.Bayer SA, et al. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 80.Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical transactions of the Royal Society of London. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical transactions of the Royal Society of London. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guzowski JF, et al. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 83.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 84.Snyder JS, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramirez-Amaya V, et al. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kee N, et al. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature Neuroscience. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 87.Trouche S, et al. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci U S A. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 89.Brun VH, et al. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 90.Brun VH, et al. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- 91.Colgin LL, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 92.Izhikevich EM, Edelman GM. Large-scale model of mammalian thalamocortical systems. Proc Natl Acad Sci U S A. 2008;105:3593–3598. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fleischer JG, et al. Retrospective and prospective responses arising in a modeled hippocampus during maze navigation by a brain-based device. Proc Natl Acad Sci U S A. 2007;104:3556–3561. doi: 10.1073/pnas.0611571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krichmar JL, et al. Characterizing functional hippocampal pathways in a brain-based device as it solves a spatial memory task. Proc Natl Acad Sci U S A. 2005;102:2111–2116. doi: 10.1073/pnas.0409792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gould E, et al. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 96.Snyder JS, et al. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Praag H, et al. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 98.Jagasia R, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tashiro A, et al. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 100.Nakagawa S, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bergami M, et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee KJ, et al. Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus. Experimental & molecular medicine. 2006;38:44–54. doi: 10.1038/emm.2006.6. [DOI] [PubMed] [Google Scholar]
- 104.Wong EY, Herbert J. The corticoid environment: a determining factor for neural progenitors’ survival in the adult hippocampus. Eur J Neurosci. 2004;20:2491–2498. doi: 10.1111/j.1460-9568.2004.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garthe A, et al. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 107.Dupret D, et al. Methylazoxymethanol acetate does not fully block cell genesis in the young and aged dentate gyrus. Eur J Neurosci. 2005;22:778–783. doi: 10.1111/j.1460-9568.2005.04262.x. [DOI] [PubMed] [Google Scholar]
- 108.Merrill DA, et al. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- 109.Hasselmo ME, et al. Encoding and retrieval of episodic memories: role of cholinergic and GABAergic modulation in the hippocampus. Hippocampus. 1996;6:693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 110.Kitamura T, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 111.Nakashiba T, et al. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62:781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- 113.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 115.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 116.Bhardwaj RD, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winner B, et al. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- 120.Hoglinger GU, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 121.Zhang C, et al. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol. 2007;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rockenstein E, et al. Effects of Cerebrolysin on neurogenesis in an APP transgenic model of Alzheimer’s disease. Acta Neuropathol. 2007;113:265–275. doi: 10.1007/s00401-006-0166-5. [DOI] [PubMed] [Google Scholar]
- 123.Boekhoorn K, et al. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 124.Jin K, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 127.Sheline YI, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bremner JD, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 129.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Surget A, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biological Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 131.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 132.Becker S, et al. Computational modeling and empirical studies of hippocampal neurogenesis-dependent memory: Effects of interference, stress and depression. Brain Res. 2009;1299:45–54. doi: 10.1016/j.brainres.2009.07.095. [DOI] [PubMed] [Google Scholar]