Abstract
Proper regulation of cell death is essential for metazoan development and functions. Unlike apoptosis, necrosis is a more inflammatory form of cell death that might contribute to anti-viral immunity. Indeed, necrotic cell injury is distinguished from apoptosis by extensive organelle and cell swelling and plasma membrane rupture. Recent evidence indicates that an elaborate biochemical network emanating from receptors in the TNF superfamily can induce apoptosis as well as necrotic cell death. The induction of necrosis by TNF-like cytokines requires biochemical components that are distinct from those involved in apoptosis. Specifically, serine/threonine protein kinases in the receptor interacting protein (RIP) family are required for “programmed” necrotic cell injury. In this review, we discuss the molecular crosstalk between apoptosis and programmed necrosis, with a special emphasis on how caspases, protein ubiquitylation and phosphorylation regulate the induction of necrotic cell injury.
Keywords: TNF, programmed necrosis, RIP1, RIP3, reactive oxygen species (ROS), NADPH oxidase
No accident: necrotic cell death is a programmed event
The balance between cellular proliferation and cell death is critical for homeostasis of higher organisms. Pathologists have long relied on morphology to distinguish different forms of cell death. The advent of molecular biology greatly enhanced our knowledge of the biochemical regulation of apoptosis. By comparison, our understanding of the biochemical pathways that regulate non-apoptotic cell death programs such as necrosis remained scarce. Until recently, the prevalent view was that cellular necrosis is the consequence of non-specific cell injury from trauma. However, with the identification of dedicated molecular machinery regulating the process, the study of necrosis has experienced a renaissance lately. Owing to the requirement for a dedicated molecular circuitry, we have coined the term “programmed necrosis” to distinguish necrosis induced by tumor necrosis factor (TNF) family death cytokines from those induced by non-specific trauma or injury (e.g. heat shock). Other terms, including “necroptosis”, have also been used to describe receptor interacting protein 1 (RIP1)-dependent, death cytokine-induced programmed necrosis [1, 2]. Although the term “programmed necrosis” has also been used to describe poly (ADP-ribose) polymerase 1 (PARP1)-mediated cell death induced by DNA damaging agents [3], it remains unclear if the underlying molecular mechanisms are the same. In this review, we will focus on the recent advances in understanding the molecular regulation of programmed necrosis induced by TNF-like cytokines.
Crosstalk between apoptosis and programmed necrosis
It is now clear that signaling by TNF-like death cytokines can result in at least one of three outcomes: nuclear factor kappa-B (NF-κB) activation, apoptosis or programmed necrosis. Evidence indicates that the activation of one response often opposes the others. For example, under most circumstances, TNF stimulation results in NF-κB activation rather than cell death. However, when NF-κB activation is inhibited, either by macro-molecular synthesis inhibitors or by expression of a dominant negative mutant of the negative regulator IκBα, TNF induces apoptosis (reviewed in [4]). These systems were widely used to study the mechanism of TNF-induced apoptosis and contributed to the perception that TNF predominantly induces apoptotic cell death. However, a very early study of TNF showed that it causes solid tumor regression in the form of necrotic cell death [5]. In the late 1990s, it was realized that caspase inhibition does not always inhibit TNF or Fas ligand (FasL)-induced cell death in certain cell types. Rather, caspase inhibition by pan-caspase inhibitors or expression of the viral caspase 1/8 inhibitor CrmA often led to necrosis marked by cell/organelle swelling and rupture of the plasma membrane (reviewed in [6]). In addition, expression of a dimerized FADD death domain (FADD-DD), which inhibits FasL- and TNF-induced apoptosis, triggered caspase-independent cell death with characteristic “necrotic” morphology [7, 8]. A comparison between wild type and mutant Jurkat T-cells reveals that FADD and caspase 8 deficiencies potently sensitize cells to programmed necrosis [9]. Similarly, primary T-cells deficient in caspase 8 or transgenic T-cells expressing FADD-DD are also highly sensitive to programmed necrosis [10, 11]. These results clearly illustrate that the molecular pathways regulating death ligand-induced apoptosis and programmed necrosis are intimately intertwined. They also firmly establish the paradigm that inhibition of caspase-dependent apoptosis primes cells towards programmed necrosis.
RIP1: a pleiotropic kinase controlling cell survival and cell death signals
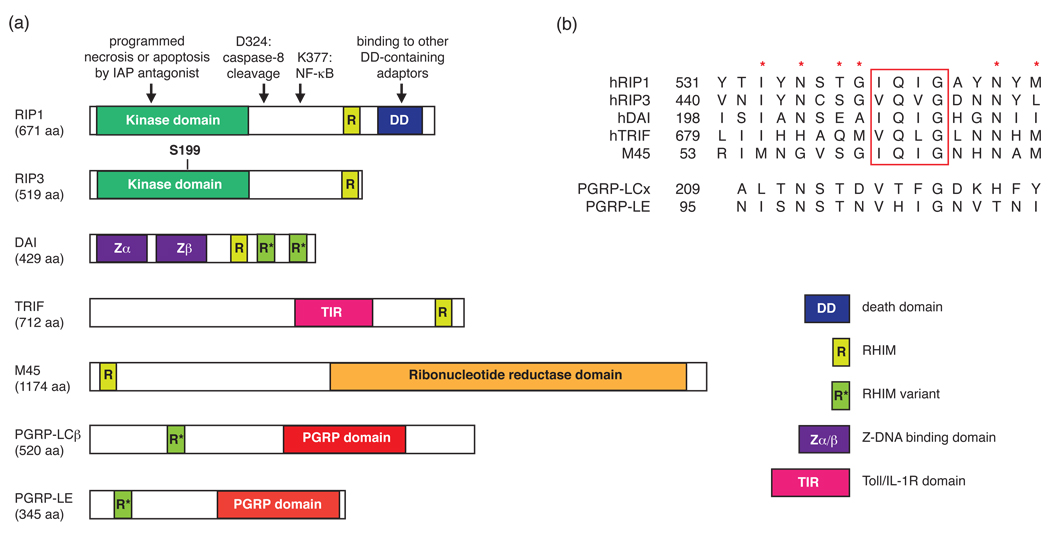
A breakthrough in the study of programmed necrosis came when several groups described that the serine/threonine kinase RIP1 plays an obligate role in mediating programmed necrotic cell death induced by FasL, TNF, TRAIL (TNF-related apoptosis-inducing ligand), and the combination of interferon and double stranded RNA [9, 12–14]. Early studies indicated that RIP1 plays an obligate role in the activation of NF-κB (reviewed in [15, 16]). For example, Abelson-transformed Rip1−/− pre-B cells were hypersensitive to TNF-induced apoptosis. However, a recent study shows that Rip1−/− mouse embryonic fibroblasts (MEFs) activate NF-κB normally in response to TNF [17]. The discrepant results might be due to the differential requirement for RIP1 in the different cell types used in these studies. Nonetheless, RIP1 polyubiquitylation at lysine 377, which lies in the intermediate domain (Fig. 1), appears to be essential for NF-κB activation [18–20]. By contrast, an intact kinase domain is crucial for RIP1-mediated programmed necrosis, but dispensable for NF-κB activation [9, 12]. Thus, RIP1 utilizes distinct domains to activate NF-κB and programmed necrosis (Fig. 1).
Figure 1. The emerging family of RHIM-containing proteins.
(a) Schematic diagram of human, viral and Drosophila adaptor proteins containing RHIMs. The arrows indicate the amino acid residues and domains important for RIP1 function. S199 is a reported necrosis-specific phosphorylation target site on RIP3 [28]. (b) Sequence alignment of the RHIM or RHIM-like domains. The red rectangle denotes the highly conserved (I/V)Q(I/V/L)G tetrapeptide found in the RHIM. The red asterisks represent other highly conserved residues within the RHIM domain. The numbers represent the amino-terminal boundary of the listed sequences.
Although RIP1 is normally dispensable for death cytokine-induced apoptosis, recent evidence indicates that RIP1 can facilitate apoptosis under certain circumstances. For instance, although RIP1 is not required for apoptosis induced by agonistic anti-Fas antibody or crosslinked FasL, it promotes caspase 8 activation within the receptor-associated death inducing signal complex (DISC) and apoptosis in response to membrane-bound FasL [21]. In addition, RIP1 is required for detachment-induced, Fas-mediated anoikis [22]. Inhibitor of apoptosis (IAP) antagonists are small molecule mimics that sensitize cells to apoptosis by inducing autoubiquitylation and proteasomal degradation of cIAP1 and cIAP2, and the autocrine production of TNF [23, 24]. They potentiate RIP1 binding to the Fas DISC and assembly of an alternative caspase 8 activating complex containing FADD and RIP1 [25, 26]. Importantly, RIP1 kinase activity is crucial for the assembly and function of this alternative caspase 8 activating complex [25]. Thus, in addition to programmed necrosis, RIP1 kinase activity is also required for apoptosis induced by IAP antagonists (Fig. 1).
A RIP1–RIP3 pro-necrotic complex regulates programmed necrosis
The fact that RIP1 activates signaling pathways other than programmed necrosis suggests that additional mechanisms must exist to specifically regulate or mediate its pro-necrotic function. Recently, two separate RNA interference (RNAi) screens identified another RIP family kinase, RIP3, as an essential mediator for TNF-, FasL- and TRAIL-mediated programmed necrosis [27, 28]. Rip3−/− primary MEFs respond normally to TNF-induced apoptosis and NF-κB activation, but are resistant to programmed necrosis [27, 28]. The requirement for RIP3 in necrosis was further corroborated by a third report [29]. These new studies demonstrate the importance of RIP3 in programmed necrosis and are consistent with an early report which showed that RIP3 over-expression induces apoptotic as well as necrotic cell death [30]. Thus, RIP3 is an essential inducer of programmed necrosis.
TNFR1 (TNF receptor 1) signaling is mediated through two spatially and temporally separate signaling complexes: a transient and unstable receptor-associated signaling complex termed “Complex I”, and a slow-forming receptor-independent cytoplasmic complex termed “Complex II” [31]. Whereas RIP1 is recruited to both complexes, RIP3 only binds RIP1 within a “pro-necrotic” Complex II. The assembly of the pro-necrotic RIP1–RIP3 complex is specifically induced during TNF-induced programmed necrosis, but not during apoptosis or NF-κB activation. The RIP1–RIP3 interaction results in the induction of their kinase activities [27]. Although intact RIP1 and RIP3 kinase activities are critical for TNF-, FasL- and TRAIL-induced programmed necrosis in most cells, small interfering RNA (siRNA)-mediated silencing of Rip1 expression conferred no protection against TNF-induced programmed necrosis in L929 cells [32]. In addition, a mutant murine cytomegalovirus (MCMV) expressing a defective programmed necrosis inhibitor M45 triggers programmed necrosis in a RIP3-dependent, but RIP1-independent, manner [33]. These results emphasize the central role of RIP3 in the necrotic signaling pathway and indicate that programmed necrosis can proceed in a RIP1-independent manner. By contrast, RIP1-driven necrosis that is independent of RIP3 has not been observed. Strikingly, Rip3−/− macrophages are also resistant to programmed necrosis induced by the Toll-like receptor 4 (TLR4) agonist LPS and the broad caspase inhibitor zVAD-fmk [29]. Although it is not clear if this cell death results from direct TLR4 signaling or is indirectly mediated by LPS-induced autocrine TNF expression, this result nonetheless raises the possibility that the RIP1–RIP3 complex might regulate necrotic cell death emanating from receptors beyond the TNF receptor superfamily.
The assembly of the pro-necrotic RIP1–RIP3 complex is mediated through the “RIP homotypic interaction motif” (RHIM) [34] (Fig. 1). The RHIM represents an emerging protein–protein interaction motif whose structure is undefined at present. In addition to RIP1 and RIP3, the RHIM is also found in the TLR3 adaptor Toll–interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (IFN-β) (TRIF), the DNA-dependent activator of interferon regulatory factors (DAI) [35, 36], and the MCMV cell death inhibitor protein M45 [37, 38] (Fig. 1). In addition, the Drosophila melanogaster innate immune receptors PGRP-LC and PGRP-LE contain a “RHIM-like” motif that is important for downstream signal transduction [39] (Fig. 1). The PGRP receptors signal via the immune deficiency (IMD) protein, an adaptor that shares homology with the death domain of mammalian RIP1 [40]. Furthermore, RHIM-mediated RIP1–TRIF and RIP1–DAI interactions regulate NF-κB activation by TLR3/TLR4 and DAI, respectively [35, 41, 42]. TRIF can also induce cell death through a type I interferon-dependent RHIM-mediated interaction with RIP1 [43, 44]. Because Rip3−/− macrophages are resistant to TLR4-induced programmed necrosis [29], it is tempting to speculate that TRIF might regulate programmed necrosis through the RHIM-mediated RIP1–RIP3 interaction. The requirement of RHIM-containing adaptors in necrotic and innate immune signaling pathways suggests that the two pathways might have co-evolved to control innate inflammatory responses.
The identification of a RHIM in the MCMV M45 protein suggests that this virus might target cellular responses mediated by RHIM-containing proteins. Indeed, an intact RHIM is essential for both the M45-mediated inhibition of NF-κB activation by DAI [35, 36] and RIP1-induced cell death [37]. A recombinant MCMV virus encoding an M45 RHIM mutant failed to establish a productive infection in tissue culture or in wild type mice due to the pre-mature induction of RIP3-dependent programmed necrosis. Strikingly, productive infection was not restored by necrostatin-1, a RIP1-specific kinase inhibitor [1, 2], or siRNA-mediated silencing of RIP1. By contrast, productive infection by the M45 mutant virus was restored in Rip3−/− cells and Rip3−/− mice [33]. These results demonstrate that viral inhibition of RIP3 and programmed necrosis is an important innate immune evasion strategy employed by certain pathogens (Box 1).
Box 1 Viral inhibition of programmed necrosis
Many viruses encode gene products that inhibit the host apoptosis machinery (reviewed in [65]). In these scenarios, programmed necrosis can serve as an alternative host cell death mechanism that circumvents viral inhibition of caspases and apoptosis. Moreover, the pro-inflammatory nature of programmed necrotic cell death might further stimulate anti-viral immune responses. In support of an anti-viral role for programmed necrosis, Rip3−/− mice are highly susceptible to vaccinia virus infections [27].
The anti-viral function of programmed necrosis suggests that viruses might have developed strategies to interfere with this pathway. Indeed, several viral FLICE (caspase 8)-like inhibitor proteins (FLIPs) such as MC159 from the poxvirus Molluscum contagiosum and E8 from equine herpevirus potently inhibit programmed necrosis [27]; however the underlying mechanisms remain unknown. Viral FLIPs (v-FLIPs) were first identified as apoptosis inhibitors. They share the amino-terminal death effector domains (DEDs) with the initiator caspases caspase 8 and caspase 10, but lack an intact caspase enzyme domain. Binding of the v-FLIP MC159 to FADD, caspase 8, or TRAF3 contributes to inhibition of death cytokine-induced apoptosis [66, 67]. Transgenic expression of MC159 leads to autoimmune symptoms and impairment of immune functions similar to those caused by mutations in the Fas death receptor [68, 69]. These results further support an immuno-modulatory role for viral inhibitors of programmed necrosis. Thus, the v-FLIPs and RHIM-containing inhibitors like M45 represent two distinct classes of viral programmed necrosis inhibitors.
Questions remain regarding whether RIP1 or RIP3 is the upstream activator in the necrotic signaling cascade, but several lines of evidence favor RIP1 as the upstream kinase. For example, RIP3-dependent and RIP1-independent programmed necrosis has been observed during MCMV infection and in L929 cells [32, 45]. Necrosis-specific RIP3 phosphorylation, but not RIP1 phosphorylation, is inhibited by necrostatin-1 [27]. Moreover, a kinase defective RIP3 mutant binds RIP1 normally, indicating that an active RIP3 kinase is not required for the assembly of the RIP1–RIP3 complex. Because necrostatin-1 abolished the assembly of the RIP1–RIP3 pro-necrotic complex [27, 28], these results are consistent with the notion that RIP1 acts upstream of RIP3. However, RIP1 expressed in 293T cells is unable to phosphorylate RIP3 in vitro, whereas RIP3 expressed in 293T cells weakly phosphorylates RIP1 [27] . Furthermore, necrosis-specific RIP1 phosphorylation is absent in Rip3−/− MEFs [27] . These results argue that RIP3 also regulates RIP1 function. Additional experiments will be needed to unequivocally determine the order in which RIP1 and RIP3 are activated. Importantly, several putative phosphorylation sites on RIP1 and RIP3 were recently identified [1, 28]. Reconstitution of Rip1−/− and Rip3−/− cells with these phosphorylation site mutants could help to determine the hierarchy of activation for RIP1 and RIP3 during programmed necrosis.
The role of FADD and caspases: friend or foe?
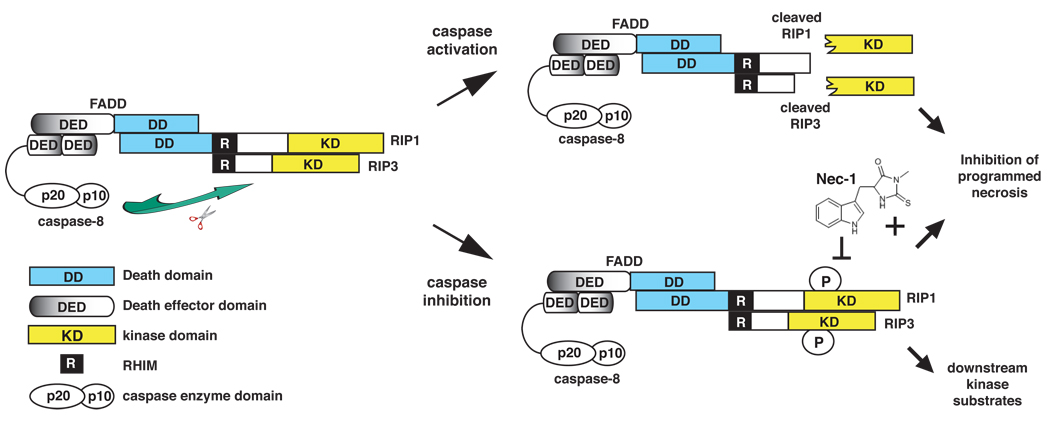
Caspase inhibition has been observed in malignant diseases and during certain viral infections [9, 46]. Under these conditions, TNF-like cytokines might preferentially induce programmed necrosis. However, it is important to remember that programmed necrosis can proceed in the absence of caspase inhibition. For instance, in Jurkat cells expressing both TNFR1 and TNFR2, TNF stimulation alone is sufficient to induce RIP1 and RIP3 recruitment to the caspase 8 associated complex and programmed necrosis, although apoptosis remained the dominant form of cell death under these conditions [9, 47]. Caspase inhibition further enhanced the kinetics of RIP1/RIP3 binding to caspase 8 and programmed necrosis [27], in part through inhibition of caspase 8-mediated RIP1 cleavage [9, 27, 48]. Cleavage of RIP1 at D324 results in the separation of the kinase domain from the carboxyl terminal fragment containing the RHIM and DD. Thus, inhibition of caspase 8-mediated RIP1 cleavage is crucial to ensure the phosphorylation and activation of downstream RIP1/RIP3 substrates (Fig. 2). In addition to RIP1, RIP3 has also been reported to be a caspase 8 substrate [29, 30]. For instance, RIP3 cleavage could be detected in response to different apoptotic stimuli [30]. Furthermore, substitution of the putative caspase cleavage site on RIP3 to alanine (D333A) abolishes the sensitizing effect of zVAD-fmk on programmed necrosis [29]. Thus, although further work is needed to validate whether RIP3 is similarly cleaved and inactivated by caspase 8 within Complex II, these results are in agreement with the model that preservation of the integrity of both RIP1 and RIP3 is important for optimal induction of programmed necrosis.
Figure 2. Caspase 8-mediated cleavage of RIP1 and RIP3 inhibits programmed necrosis.
The assembly of a cytoplasmic RIP1–RIP3 complex via the RHIM is critical for programmed necrosis. This complex is further stabilized by phosphorylation of RIP1 and RIP3. FADD and caspase 8 inhibit TNF-induced programmed necrosis by cleaving RIP1 (at D324) and RIP3 (at D333) within this complex. Cleavage results in the release of amino-terminal fragments containing the kinase domains, thereby preventing phosphorylation of RIP1, RIP3 and other possible downstream substrates. Necrostatin-1 (Nec-1), which inhibits RIP1 kinase activity, blocks programmed necrosis by preventing the stable association of the RIP1–RIP3 complex.
When compared to caspase 8, the role of the adaptor protein FADD in programmed necrosis is enigmatic. FADD is required for programmed necrosis induced by FasL and TRAIL [12], but dispensable for TNF-induced programmed necrosis [9]. In fact, TNF-induced programmed necrosis is exacerbated in Jurkat cell variants that lack FADD expression [9]. It is not clear why programmed necrosis induced by different TNF-like death cytokines exhibits differential requirements for FADD. One possible explanation is that FADD is required for assembly of the Fas- and TRAIL-R-associated DISC, but not the TNFR1-associated Complex I. Because the pro-necrotic RIP1–RIP3 complex is formed as a consequence of the receptor-associated complex, FADD deficiency might preferentially inhibit necrotic signaling by Fas and TRAIL receptors.
Similar to the Jurkat cell variants that lack FADD expression, expression of FADD death domain alone (FADD-DD), which dominantly inhibits caspase-dependent apoptosis, also sensitizes cells to programmed necrosis. Primary T-cells expressing FADD-DD were recently shown to undergo programmed necrosis in response to T-cell receptor stimulation [11]. This finding is reminiscent of early results showing that expression of an FK506 binding protein (FKBP)-dimerized FADD-DD led to spontaneous programmed necrosis [7, 8]. FADD-DD might facilitate programmed necrosis by binding to and aggregating RIP1 through a DD–DD interaction [8]. In addition, because it lacks the death effector domain (DED) that recruits caspase 8, FADD-DD might promote programmed necrosis by inhibiting caspase 8-mediated RIP1/RIP3 cleavage (Fig. 2). This model explains why full-length FADD and FADD-DD exhibit differential effects on TNF-induced programmed necrosis. Strikingly, a fraction of cellular RIP3 constitutively associates with FADD. This interaction appears to be indirect, because RIP3 and FADD expressed in 293T cells failed to interact with each other [27]. The functional significance of this interaction will require further investigation.
Does protein ubiquitylation regulate programmed necrosis?
Protein ubiquitylation is an important process that regulates numerous signal transduction pathways. Many proteins in the TNF signaling pathway are targets of ubiquitylation. For instance, TNFR1-bound RIP1 is heavily modified through K63-specific polyubiquitylation, although more recent results indicate that RIP1 can also undergo non-K63-mediated ubiquitylation [49]. Polyubiquitylated RIP1 mediates activation of the pro-survival transcription factor NF-κB by binding NEMO, the regulatory subunit of the IKK (I Kappa B kinase) complex. Several interesting recent reports indicate that polyubiquitylated RIP1–NEMO binding provides an early anti-death signal that is independent of NF-κB activation [18, 19, 50, 51]. The protection conferred by polyubiquitylated RIP1 appears to act through preventing the RIP1–caspase 8 interaction [50, 51].
Could polyubiquitylated RIP1 similarly protect cells against programmed necrosis? Interestingly, the K63-specific deubiquitylase (DUB) CYLD was recently identified in a genome-wide RNAi screen as an important mediator for TNF-induced programmed necrosis [32]. CYLD encodes a gene that is mutated in familial cylindromatosis, Brooke-Spiegler syndrome, and multiple familial trichoepithelioma, an overlapping set of tumors affecting the head, the neck and the skin appendages (reviewed in [52]). Consistent with a role in programmed necrosis, CYLD promotes the assembly of the RIP1–FADD–caspase 8 complex in response to TNF, IAP antagonist, and zVAD-fmk treatment [25]. Importantly, polyubiquitylated RIP1 is a CYLD substrate [53]. Furthermore, programmed necrosis induced by IAP antagonists and TNFR-2 signaling both led to degradation of RIP1 targeting E3 ligases such as cIAP1/2 and TRAF2 [23, 24, 54]. It is tempting to speculate that RIP1 polyubiquitylation sterically hinders the recruitment of downstream pro-necrotic proteins such as RIP3. In this scenario, CYLD might facilitate programmed necrosis by removal of the polyubiquitin chains on RIP1. Interestingly, in necrotic MEFs, FADD-associated RIP3 exhibits a partial “laddering” pattern that resembles polyubiquitylation [27]. However, it remains unknown whether RIP3 polyubiquitylation is an important regulatory mechanism in programmed necrosis.
Effector mechanisms of programmed necrosis
Although it is clear that caspase-mediated cleavage of cellular proteins causes apoptotic death, much less is known about the mechanisms by which programmed necrosis kills cells. The most remarkable morphological feature of programmed necrosis is the organelle and cell swelling that culminates in rupture of the plasma membrane. The increase in cell volume and extensive intracellular vacuole formation implies an imbalance in osmotic pressure. Although the details remain fuzzy, the prevailing view is that reactive oxygen species (ROS) production is an important effector killing mechanism for programmed necrosis. ROS can induce lipid peroxidation or alter the function of certain channel proteins, both of which can lead to necrotic cell injury. However, ROS are not specifically required for programmed necrosis, as ROS also regulate apoptosis under certain conditions [55, 56]. Factors such as the concentration of ROS produced and the cellular ATP level could determine whether ROS triggers apoptosis or programmed necrosis [55, 57].
The mitochondria are major producers of ROS. Consistent with a role for mitochondrial ROS in programmed necrosis, inhibition of the mitochondrial complex I and complex II (distinct from the Complex I and Complex II in TNF signaling pathway) protects the fibrosarcoma L929 cell line against TNF-induced programmed necrosis [58]. RIP1 and RIP3 act upstream to regulate ROS production during programmed necrosis [27, 29]. Although the results are controversial, both RIP1 and RIP3 were reported to localize to the mitochondria [59, 60]. Indeed, a recent report shows that RIP3 interacts with several mitochondrial enzymes including glycogen phosphorylase (PYGL), glatamate-ammonia ligase (GLUL) and glutamate dehydrogenase 1 (GLUD1) [29]. RIP3 overexpression activates the activities of these enzymes and enhances mitochondrial energy metabolism, which correlates with enhanced ROS production and programmed necrosis [29]. Thus, the RIP1–RIP3 complex might directly regulate ROS production from the mitochondria by engaging the mitochondrial metabolism machinery.
In contrast to the evidence supporting mitochondrial production of ROS, several recent reports indicate that the plasma membrane-associated NADPH oxidase 1 (NOX1) forms a complex with TRADD, RIP1 and the small GTPase RAC1 to regulate ROS production at the plasma membrane [61]. The disparate results might be reconciled by the TNF-induced shuttling of NADPH oxidases between the cytoplasm and the plasma membrane [62]. Alternatively, the pro-necrotic signal generated by NOX1-induced ROS might be amplified through mitochondrial ROS production. NOX1 recruitment requires riboflavin kinase (RFK), which physically couples NOX1 to the TNFR1 signaling complex [63]. However, RFK is more than just an adaptor linking NOX1 to the receptor, as products of the riboflavin kinase reaction such as flavin mononucleotide and flavin adenine dinucleotide (FAD) rescue the ROS production deficiency of Rfk−/− cells [63]. These results suggest that FAD might be an unexpected co-factor in the assembly of a functional ROS-producing complex at the plasma membrane.
It is noteworthy that ROS are not required for programmed necrosis in all cell types. For example, ROS scavengers do not inhibit programmed necrosis in the monocytic lymphoma U937 cell line, the colon carcinoma HT-29 cell line, or in Jurkat T-cells [28, 59]. Rather, a RIP1-dependent loss of cellular ATP was reported to precede programmed necrosis in U937 cells [59]. Through a poorly defined mechanism, RIP1 disrupts the interaction between adenine nucleotide translocase (ANT) and cyclophilin D (CYPD) [59]. ANT and CYPD are components of the mitochondrial permeability transition pore (mPTP) that regulates the exchange of ADP and ATP between the cytosol and the mitochondria (reviewed in [64]). During programmed necrosis, this ADP-ATP exchange function of the mPTP is compromised [59]. Consistent with this observation, Cypd−/− cells are partially resistant to TNF-, IAP antagonist-, and zVAD-fmk induced programmed necrosis [28]. Thus, the cellular metabolic “fitness level” might greatly influence the outcome of pro-necrotic cell signaling, a notion that seems to be supported by the correlation between reduced cellular ATP level and increased susceptibility to necrotic cell injury in older individuals [57]. These recent findings suggest that different effector mechanisms operate in different cell types to mediate programmed necrosis (Box 2).
Box 2 Distinct effector mechanisms in programmed necrosis
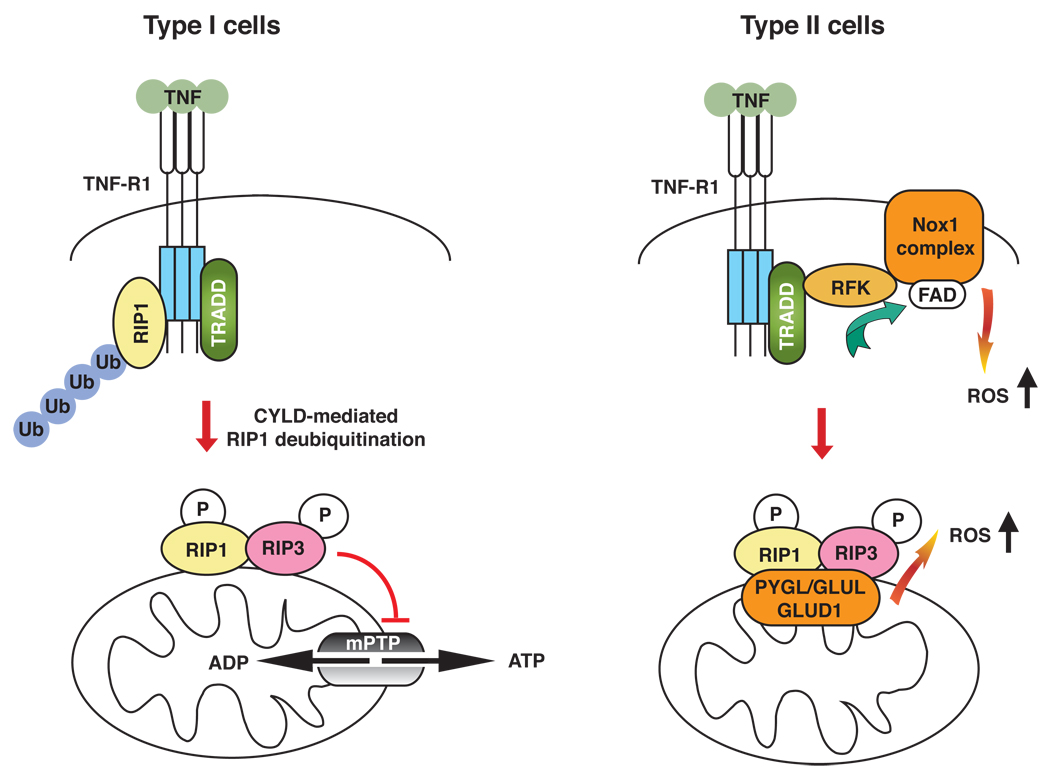
ROS are essential for programmed necrosis in many transformed (e.g. L929) and primary cells (e.g. embryonic fibroblasts). However, in cells of hematopoeitic origin (e.g. U937 and Jurkat) and the colon carcinoma HT-29 cell line, programmed necrosis proceeds in the presence of ROS scavengers. Based on these observations, we propose that programmed necrosis can be sub-divided into two classes (Figure I). In Type I cells such as U937, disruption of mPTP function leads to bioenergetic breakdown and the eventual demise of the cell. By contrast, Type II cells such as fibroblasts require high ROS levels to trigger programmed necrosis. This scenario is akin to that of Fas-induced apoptosis, where different cell types exhibit differential requirement for mitochondrial amplification of the caspase-driven apoptotic signal [70]. Although Type I and Type II cells exhibit differential requirements for ROS, the disruption of mPTP function can lead to dysregulated electron transport chain and further ROS production. Thus, the mitochondria might have important functions in programmed necrosis in ROS-dependent, as well as ROS-independent, necrotic cell injury.
Concluding remarks
Recent studies have defined a RIP1–RIP3 kinase complex that regulates death cytokine-induced programmed necrosis. Rip3−/− mice have provided a valuable model to examine the role of programmed necrosis in anti-viral inflammatory responses. RIP1/RIP3-dependent programmed necrosis might be important in other inflammatory diseases including drug-induced tissue inflammation, auto-inflammatory diseases and cancers. Indeed, RIP3 is required for cerulein-induced pancreatitis [28, 29]. Drugs that target the RIP1 or RIP3 kinase, such as necrostatins, are likely to be useful in the treatment of these diseases.
Despite the recent development, many questions remain about the molecular mechanisms that regulate programmed necrosis. For example, what are the downstream substrates for the RIP1–RIP3 kinase complex? What is the role of the mitochondria? Are proteases involved as in apoptosis? What are the cellular targets affected by ROS or loss of ATP during programmed necrosis? With a growing interest in studying this cell death pathway, one can realistically hope that these questions will be answered in the near future.
Box 2, Figure 1. ROS-dependent and independent programmed necrosis in different cell types.
Assembly of the RIP1–RIP3 (pink–yellow) complex involves deubiquitylation of RIP1, possibly by CYLD. The assembled RIP1–RIP3 complex might act on the mitochondria to trigger downstream effector functions. In Type I cells such as the U937 cell line, Jurkat T-cells, and the HT-29 cell line, ROS scavengers such as butylated hydroxyanisole (BHA) do not rescue programmed necrosis. In these cells, programmed necrosis might be mediated by loss of function of the mPTP and cellular ATP. By contrast, in Type II cells such as the L929 cell line or MEFs, programmed necrosis is ROS-dependent as BHA rescues the cell death. Evidence indicates that ROS can be produced at the RFK–NOX1–FAD plasma membrane complex or within the mitochondria through RIP3-mediated interaction with mitochondrial metabolic enzymes (PYGL, GLUL, GLUD1; orange). It is unclear if the two sources of ROS might synergize with each other to facilitate programmed necrosis. Ubiquitin (Ub): blue.
Acknowledgement
The authors would like to thank Tia Bumpus for critical reading of the manuscript. DM is supported by NIH pre-doctoral training grant (T32 AI07349).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 3.Tu HC, et al. The p53-cathepsin axis cooperates with ROS to activate programmed necrotic death upon DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1093–1098. doi: 10.1073/pnas.0808173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papa S, et al. Linking JNK signaling to NF-kappaB: a key to survival. Journal of cell science. 2004;117:5197–5208. doi: 10.1242/jcs.01483. [DOI] [PubMed] [Google Scholar]
- 5.Carswell EA, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Festjens N, et al. Necrosis, a well-orchestrated form of cell demise: signaling cascades, important mediators and concomitant immune response. Biochimica et biophysica acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Kawahara A, et al. Caspase-independent cell killing by Fas-associated protein with death domain. The Journal of cell biology. 1998;143:1353–1360. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanden Berghe T, et al. Differential signaling to apoptotic and necrotic cell death by Fas-associated death domain protein FADD. The Journal of biological chemistry. 2004;279:7925–7933. doi: 10.1074/jbc.M307807200. [DOI] [PubMed] [Google Scholar]
- 9.Chan FK, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. The Journal of biological chemistry. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 10.Ch'en IL, et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell BD, et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 13.Kalai M, et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell death and differentiation. 2002;9:981–994. doi: 10.1038/sj.cdd.4401051. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. The Journal of biological chemistry. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 15.Meylan E, Tschopp J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci. 2005;30:151–159. doi: 10.1016/j.tibs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Festjens N, et al. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell death and differentiation. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 17.Wong WW, et al. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell death and differentiation. 17:482–487. doi: 10.1038/cdd.2009.178. [DOI] [PubMed] [Google Scholar]
- 18.Ea CK, et al. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Wu CJ, et al. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 20.Li H, et al. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. The Journal of biological chemistry. 2006;281:13636–13643. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- 21.Morgan MJ, et al. Membrane-bound Fas ligand requires RIP1 for efficient activation of caspase-8 within the death-inducing signaling complex. J Immunol. 2009;183:3278–3284. doi: 10.4049/jimmunol.0803428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamarajan P, et al. Receptor-Interacting Protein (RIP) Shuttles between Cell Death and Survival Signaling Pathways. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 24.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, et al. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Geserick P, et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. The Journal of cell biology. 2009;187:1037–1054. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Zhang DW, et al. Science. Vol. 325. New York, N.Y: 2009. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis; pp. 332–336. [DOI] [PubMed] [Google Scholar]
- 30.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 32.Hitomi J, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Upton JW, et al. Pathogen Subversion of RIP3-dependent Necrosis. Cell Host and Microbes. 2010 doi: 10.1016/j.chom.2010.03.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, et al. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. The Journal of biological chemistry. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 35.Kaiser WJ, et al. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebsamen M, et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upton JW, et al. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. The Journal of biological chemistry. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mack C, et al. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3094–3099. doi: 10.1073/pnas.0800168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneko T, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- 40.Georgel P, et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Developmental cell. 2001;1:503–514. doi: 10.1016/s1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 41.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 42.Cusson-Hermance N, et al. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. The Journal of biological chemistry. 2005;280:36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 43.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 44.Rasschaert J, et al. Toll-like receptor 3 and STAT-1 contribute to double-stranded RNA+ interferon-gamma-induced apoptosis in primary pancreatic beta-cells. The Journal of biological chemistry. 2005;280:33984–33991. doi: 10.1074/jbc.M502213200. [DOI] [PubMed] [Google Scholar]
- 45.Upton JW, et al. Cytomegalovirus M45 cell death suppression requires RHIM-dependent interaction with receptor-interacting protein 1 (RIP1) The Journal of biological chemistry. 2008 doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stupack DG, et al. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–99. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- 47.Zheng L, et al. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol Cell Biol. 2006;26:3505–3513. doi: 10.1128/MCB.26.9.3505-3513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y, et al. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu M, et al. A ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFalpha and IL-1beta. Mol Cell. 2009;36:302–314. doi: 10.1016/j.molcel.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Donnell MA, et al. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Legarda-Addison D, et al. NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling. Cell death and differentiation. 2009;16:1279–1288. doi: 10.1038/cdd.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massoumi R, Paus R. Cylindromatosis and the CYLD gene: new lessons on the molecular principles of epithelial growth control. Bioessays. 2007;29:1203–1214. doi: 10.1002/bies.20677. [DOI] [PubMed] [Google Scholar]
- 53.Wright A, et al. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Developmental cell. 2007;13:705–716. doi: 10.1016/j.devcel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 55.Hildeman DA, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 56.Sidoti-de Fraisse C, et al. TNF-alpha activates at least two apoptotic signaling cascades. Oncogene. 1998;17:1639–1651. doi: 10.1038/sj.onc.1202094. [DOI] [PubMed] [Google Scholar]
- 57.Miyoshi N, et al. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1727–1731. doi: 10.1073/pnas.0510346103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze-Osthoff K, et al. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. The Journal of biological chemistry. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 59.Temkin V, et al. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol. 2006;26:2215–2225. doi: 10.1128/MCB.26.6.2215-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasof GM, et al. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473:285–291. doi: 10.1016/s0014-5793(00)01473-3. [DOI] [PubMed] [Google Scholar]
- 61.Kim YS, et al. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 62.Dusi S, et al. Mechanisms of stimulation of the respiratory burst by TNF in nonadherent neutrophils: its independence of lipidic transmembrane signaling and dependence on protein tyrosine phosphorylation and cytoskeleton. J Immunol. 1996;157:4615–4623. [PubMed] [Google Scholar]
- 63.Yazdanpanah B, et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460:1159–1163. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- 64.Halestrap AP, Brennerb C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 65.Benedict CA, et al. To kill or be killed: viral evasion of apoptosis. Nat Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 66.Garvey TL, et al. Binding of FADD and caspase-8 to molluscum contagiosum virus MC159 v-FLIP is not sufficient for its antiapoptotic function. Journal of virology. 2002;76:697–706. doi: 10.1128/JVI.76.2.697-706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thurau M, et al. The TRAF3-binding site of human molluscipox virus FLIP molecule MC159 is critical for its capacity to inhibit Fas-induced apoptosis. Cell death and differentiation. 2006;13:1577–1585. doi: 10.1038/sj.cdd.4401847. [DOI] [PubMed] [Google Scholar]
- 68.Woelfel M, et al. Transgenic expression of the viral FLIP MC159 causes lpr/gld-like lymphoproliferation and autoimmunity. J Immunol. 2006;177:3814–3820. doi: 10.4049/jimmunol.177.6.3814. [DOI] [PubMed] [Google Scholar]
- 69.Wu Z, et al. Viral FLIP impairs survival of activated T cells and generation of CD8+ T cell memory. J Immunol. 2004;172:6313–6323. doi: 10.4049/jimmunol.172.10.6313. [DOI] [PubMed] [Google Scholar]
- 70.Barnhart BC, et al. The CD95 type I/type II model. Seminars in immunology. 2003;15:185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]