Summary
Background
Uncertainties persist about the magnitude of associations of diabetes mellitus and fasting glucose concentration with risk of coronary heart disease and major stroke subtypes. We aimed to quantify these associations for a wide range of circumstances.
Methods
We undertook a meta-analysis of individual records of diabetes, fasting blood glucose concentration, and other risk factors in people without initial vascular disease from studies in the Emerging Risk Factors Collaboration. We combined within-study regressions that were adjusted for age, sex, smoking, systolic blood pressure, and body-mass index to calculate hazard ratios (HRs) for vascular disease.
Findings
Analyses included data for 698 782 people (52 765 non-fatal or fatal vascular outcomes; 8·49 million person-years at risk) from 102 prospective studies. Adjusted HRs with diabetes were: 2·00 (95% CI 1·83–2·19) for coronary heart disease; 2·27 (1·95–2·65) for ischaemic stroke; 1·56 (1·19–2·05) for haemorrhagic stroke; 1·84 (1·59–2·13) for unclassified stroke; and 1·73 (1·51–1·98) for the aggregate of other vascular deaths. HRs did not change appreciably after further adjustment for lipid, inflammatory, or renal markers. HRs for coronary heart disease were higher in women than in men, at 40–59 years than at 70 years and older, and with fatal than with non-fatal disease. At an adult population-wide prevalence of 10%, diabetes was estimated to account for 11% (10–12%) of vascular deaths. Fasting blood glucose concentration was non-linearly related to vascular risk, with no significant associations between 3·90 mmol/L and 5·59 mmol/L. Compared with fasting blood glucose concentrations of 3·90–5·59 mmol/L, HRs for coronary heart disease were: 1·07 (0·97–1·18) for lower than 3·90 mmol/L; 1·11 (1·04–1·18) for 5·60–6·09 mmol/L; and 1·17 (1·08–1·26) for 6·10–6·99 mmol/L. In people without a history of diabetes, information about fasting blood glucose concentration or impaired fasting glucose status did not significantly improve metrics of vascular disease prediction when added to information about several conventional risk factors.
Interpretation
Diabetes confers about a two-fold excess risk for a wide range of vascular diseases, independently from other conventional risk factors. In people without diabetes, fasting blood glucose concentration is modestly and non-linearly associated with risk of vascular disease.
Funding
British Heart Foundation, UK Medical Research Council, and Pfizer.
Introduction
Diabetes mellitus is an established risk factor for coronary heart disease and ischaemic stroke,1,2 but how much its effect varies by age, sex, or levels of conventional risk factors is uncertain.3,4 The extent to which diabetes is associated with fatal versus non-fatal myocardial infarction or ischaemic versus haemorrhagic stroke is also unknown.5,6 Furthermore, how much of the effect of diabetes on vascular risk can be accounted for by conventional vascular risk factors (eg, obesity, lipids, or blood pressure) is unresolved.7 Different uncertainties apply to measures of dysglycaemia in people without diabetes. Fasting blood glucose has been reported to be log-linearly and importantly associated with risk of vascular disease at all concentrations, including below the threshold for diabetes of 7 mmol/L. Available data on this topic are, however, inconclusive.8,9 In 2009, the US Preventive Services Task Force stated that prospective data for fasting blood glucose concentration and coronary heart disease were inconsistent and had serious limitations.10 Furthermore, the value of assessment of fasting blood glucose concentration in vascular risk prediction—beyond measurement of conventional risk factors—is not established.10 We report analyses of individual records of people without initial vascular disease in prospective studies. We aimed to produce reliable estimates of associations of diabetes and fasting blood glucose concentration with fatal or first-ever non-fatal incident ischaemic vascular disease (and deaths from other vascular disorders) for a wide range of circumstances.
Methods
Study design
Details of the Emerging Risk Factors Collaboration have been described previously.11 By May, 2010, 121 prospective studies of vascular risk factors, involving a total of 1·27 million adults, had shared individual records (webappendix p 2). These studies: (1) did not select participants on the basis of previous vascular disease; (2) recorded cause-specific mortality or vascular morbidity, or both, using well defined criteria; and (3) accrued more than 1 year of follow-up. For registration of fatal outcomes, all contributing studies used coding from the International Classification of Diseases (ICD) to at least three digits (or using study-specific classification systems), and ascertainment was based on death certificates. The webappendix (pp 17–20) provides details of the 102 contributing studies that had information at baseline on history of diabetes and/or fasting blood glucose concentration (measured ≥8 h or overnight fasting). Acronyms and references for contributing studies are shown in the webappendix (pp 32–38).
Statistical analyses
We assessed baseline diabetes status (defined by self-report, medication usage, and/or baseline fasting blood glucose concentration ≥7 mmol/L) in relation to coronary heart disease (first-ever myocardial infarction or fatal coronary heart disease); stroke subtypes (fatal or non-fatal ischaemic, haemorrhagic, or unclassified stroke); and deaths attributed to the aggregate of other vascular disorders (consisting of heart failure, cardiac dysrrhythmia, sudden death, hypertensive disease, pulmonary embolism, and aortic aneurysm; webappendix p 21). We used a two-stage approach for analysis, with estimates of association calculated separately within each study, then pooled across studies by random-effects meta-analysis.12,13 Hazard ratios (HRs) were calculated with Cox proportional hazards regression models that were stratified by sex (and, in the few contributing trials, stratified by allocated treatment group). We excluded studies with fewer than 11 cases of an outcome from the analysis of that outcome. In the figures presented, sizes of data markers are proportional to the inverse of the variance of the HRs. Participants contributed only their first non-fatal vascular outcome or death recorded at age 40 years or older (ie, deaths preceded by non-fatal vascular events were not included in the analyses). For the three individually matched nested case-control studies within prospective cohorts, we calculated odds ratios with conditional logistic regression. Since only 60 760 participants had LDL cholesterol concentrations that had been directly measured, we used non-HDL cholesterol as the principal marker of cholesterol content in proatherogenic lipoproteins.
To characterise shapes of associations, study-specific HRs calculated within overall categories of baseline blood glucose concentration were pooled on the log scale, by multivariate random-effects meta-analysis, and plotted against pooled mean concentrations within each category. To restrict potential bias related to having a diagnosis of diabetes (eg, medication use, changes in lifestyle), we assessed separately individuals with and without a history of diabetes at baseline. 95% CIs were estimated from the variances that show the amount of information underlying each group (including the reference group).14 We investigated effect-modification with formal tests of interaction, and calculated p values for interaction using continuous variables, when appropriate. Diversity between studies was investigated by grouping studies with recorded characteristics and meta-regression. We adjusted HRs for baseline age, sex, smoking status, BMI, systolic blood pressure, and lipids (and, in supplementary analyses, for additional factors). Evidence of association was shown by the Wald χ2 statistic and of heterogeneity by the I2 statistic.15 We calculated measures of discrimination for censored time-to-event data (Harrell's C index) and reclassification, with methods described previously.16 We estimated population-attributable fractions with HRs for vascular death,17 and undertook sensitivity analyses allowing for potential misclassification of diabetes. Regression dilution ratios were obtained by regressing serial measurements taken from 307 517 participants (mean interval 2·6 years) on baseline levels of the relevant characteristic and duration of follow-up. In further analyses, we corrected for regression dilution in fasting blood glucose concentration and covariates, with methods described previously.12 We did all analyses using Stata (version 11). This study was approved by the Cambridgeshire ethics review committee.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. NS and JD had full access to all data in the study and had final responsibility to submit for publication.
Results
102 studies had relevant information for this analysis. From these studies, 698 782 participants had no history of myocardial infarction, angina, or stroke at initial examination. 410 299 of these had information recorded about self-reported history of diabetes, but not fasting blood glucose concentration; 195 390 had information on both self-reported diabetes and fasting blood glucose; and 93 093 had information for fasting blood glucose, but not self-reported diabetes (data for participants with information about fasting glucose but not self-reported diabetes contributed to analyses of fasting blood glucose concentration, but did not contribute to analyses of diabetes). 264 353 participants had complete information at baseline for self-reported history of diabetes, age, sex, smoking habits, systolic blood pressure, BMI, high-density lipoprotein (HDL) cholesterol, total cholesterol concentrations, and triglyceride concentration. Deaths were classified by ICD coding, and 70 of 102 contributing studies also used medical records, autopsy findings, and other supplementary sources. 73 studies used standard definitions of myocardial infarction that were based on WHO criteria. For 75 studies, investigators provided information about stroke subtype. For 59 studies, investigators reported diagnosis of strokes on the basis of typical clinical features and brain imaging. Information was generally not available for diabetes type (ie, whether type 1 or 2 diabetes) or diagnosis of diabetes and microvascular disease after baseline.
Overall, the mean age of participants at entry was 52 (SD 13) years, and 300 051 (43%) were women. 669 506 (96%) were in Europe, North America, and Australasia, with the remainder in Japan or the Caribbean. Of the participants with information on self-reported history of diabetes, 38 851 (7%) reported a history of diabetes at baseline. Diabetes prevalence varied across studies, partly affected by differences in sex distribution (webappendix p 3, 22). Baseline mean glucose concentrations were similar across studies (webappendix p 4). In people without known diabetes, glucose concentration was associated with obesity, blood pressure, lipid concentration, and inflammatory markers (webappendix p 5). In this group, serial measurements yielded an age-adjusted and sex-adjusted regression dilution ratio of 0·69 (95% CI 0·66–0·72; webappendix p 6) for fasting blood glucose concentration, 0·64 (0·62–0·65) for non-HDL cholesterol, and 0·51 (0·49–0·53) for systolic blood pressure. During 8·49 million person-years at risk (median 10·8 years to first outcome), 52 765 incident fatal or first-ever non-fatal vascular disease outcomes were recorded (webappendix pp 23–26).
In comparison of people with diabetes versus those without, HRs adjusted for age, sex, smoking status, BMI, and systolic blood pressure (basic covariates) were about two for coronary heart disease, ischaemic stroke, unclassified stroke, and deaths attributed to other vascular diseases (HR for haemorrhagic stroke was somewhat lower). HRs were about a third higher for coronary death than for non-fatal myocardial infarction (figure 1).
Figure 1.
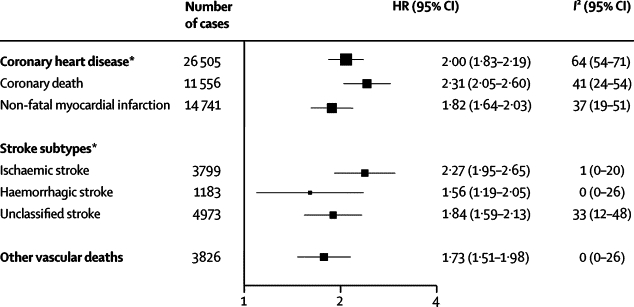
Hazard ratios (HRs) for vascular outcomes in people with versus those without diabetes at baseline
Analyses were based on 530 083 participants. HRs were adjusted for age, smoking status, body-mass index, and systolic blood pressure, and, where appropriate, stratified by sex and trial arm. 208 coronary heart disease outcomes that contributed to the grand total could not contribute to the subtotals of coronary death or non-fatal myocardial infarction because there were fewer than 11 cases of these coronary disease subtypes in some studies. *Includes both fatal and non-fatal events.
HRs for coronary heart disease with diabetes were significantly higher in women than in men, at 40–59 years than at 70 years or older, in non-smokers than in smokers, and at below average BMI or below average systolic blood pressure (figure 2). HRs for coronary heart disease did not vary much by other characteristics (including by geographical location or in people who are of white European ancestry versus those who are not; webappendix p 7). HRs for coronary heart disease did not change substantially after additional adjustment for non-HDL cholesterol, HDL cholesterol, and triglyceride concentration (figure 3). HRs for coronary heart disease were similar when: adjustment was made for apolipoprotein AI and apolipoprotein B instead of HDL cholesterol and non-HDL cholesterol, respectively; waist-to-hip ratio replaced BMI; or additional adjustment was made for fibrinogen, C-reactive protein, or estimated glomerular filtration rate (webappendix p 27). HRs for ischaemic stroke, adjusted for basic covariates, were higher in women, in people aged between 40 and 59 years, and in people with above average BMI (figure 2, webappendix p 7). HRs for ischaemic stroke did not change greatly after additional adjustment for lipids (figure 3).
Figure 2.
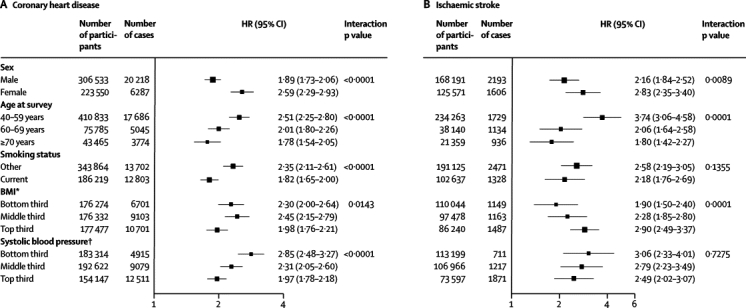
Hazard ratios (HRs) for coronary heart disease and ischaemic stroke in people with versus those without diabetes at baseline, by individual characteristics
HRs were adjusted as described in figure 1. BMI=body-mass index. *Bottom third=<23·8 kg/m2 (mean 21·7 kg/m2); middle third=23·8–<27 kg/m2 (mean 25·3 kg/m2); and top third=≥27 kg/m2 (mean 30·7 kg/m2). †Bottom third=<123 mm Hg (mean 113 mm Hg); middle third=123–<141 mm Hg (mean 132 mm Hg); and top third=≥141 mm Hg (mean 157 mm Hg).
Figure 3.
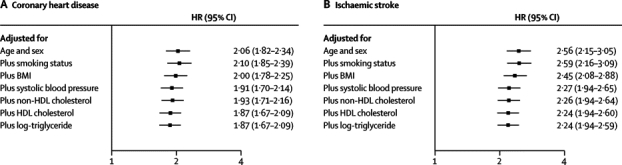
Hazard ratios (HRs) for coronary heart disease and ischaemic stroke in people with versus those without diabetes, progressively adjusted for baseline levels of conventional risk factors
Analyses were based on 264 353 participants (11 848 cases) for coronary heart disease and 157 315 participants (2858 cases) for ischaemic stroke with complete information on all covariates listed. BMI=body-mass index.
In analyses adjusted for basic covariates, fasting blood glucose concentration was non-linearly related to risk of coronary heart disease or ischaemic stroke (figure 4), and unrelated to vascular risk between 3·90 and 5·59 mmol/L (webappendix p 8). In people with no history of diabetes at baseline, compared with people with fasting blood glucose concentrations of 3·90–5·59 mmol/L, risk of coronary heart disease was only modestly higher in those with fasting blood glucose concentrations between 5·60 mmol/L and 6·99 mmol/L (ie, impaired fasting glucose), but substantially higher in those with fasting blood glucose concentrations of 7 mmol/L or higher (figure 5). Compared with the same reference group, risk of coronary heart disease was substantially higher in people with a history of diabetes. HRs were about 50% higher in people with a history of diabetes and with fasting blood glucose concentrations of at least 7 mmol/L than in people with a history of diabetes but fasting glucose concentrations lower than 7 mmol/L (figure 5). HRs for those with impaired fasting blood glucose did not vary materially by age, sex, or other recorded characteristics (webappendix p 9). At fasting blood glucose concentrations higher than 5·6 mmol/L, HR per 1 mmol/L higher concentration was 1·12 (1·08–1·15) for coronary heart disease, assuming existence of log-linear associations above this threshold (although data were insufficient to confirm or refute this assumption: webappendix p 15). Whereas long-term average (usual) concentrations of fasting blood glucose were non-linearly and moderately associated with risk of coronary heart disease, usual levels of total (or non-HDL) cholesterol and systolic blood pressure were nearly log-linearly and more strongly associated with such risk (figure 6). When added to a vascular risk-prediction model containing age, sex, smoking status, systolic blood pressure, and HDL and total cholesterol, information about history of diabetes significantly increased the C index (p<0·0001). By contrast, in people without diabetes at baseline, such addition of information on impaired fasting glucose status (p=0·24) or fasting blood glucose concentration (p=0·26) did not significantly increase the C index (webappendix p 28). Findings based on measures of reclassification for 10-year predicted risk yielded broadly similar results to those with the C index (webappendix p 28).
Figure 4.
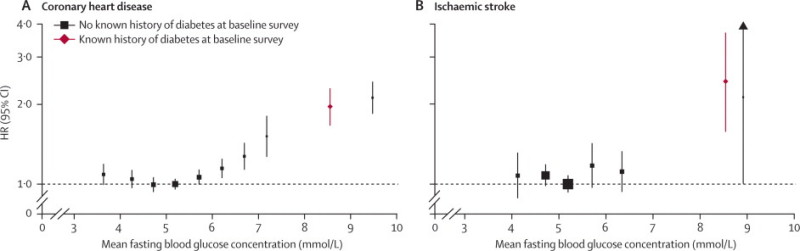
Hazard ratios (HRs) for coronary heart disease and ischaemic stroke by baseline fasting blood glucose concentration
Analyses were based on 279 290 participants (14 814 cases) for coronary heart disease (CHD) and 175 542 participants (1754 cases) for ischaemic stroke. Participants without known diabetes at baseline were classified into groups of fasting glucose (CHD: <4·0, 4·0–4·5, 4·5–5·0, 5·0–5·5, 5·5–6·0, 6·0–6·5, 6·5–7·0, 7·0–7·5, and >7·5 mmol/L; ischaemic stroke: <4·5, 4·5–5·0, 5·0–5·5, 5·5–6·0, 6·0–7·0, and >7·0 mmol/L). HRs were adjusted as described in figure 1 and are plotted against mean fasting blood glucose in each group. Reference group for both outcomes is 5·0–5·5 mmol/L.
Figure 5.
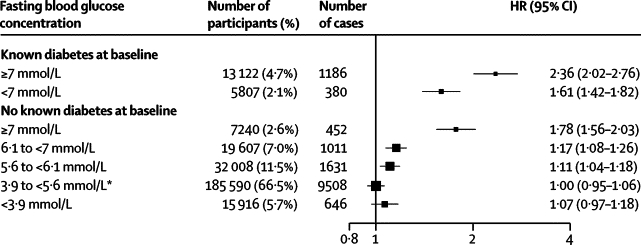
Hazard ratios (HRs) for coronary heart disease by clinically defined categories of baseline fasting blood glucose concentration
Analyses were based on 279 290 participants (14 814 cases). HRs were adjusted as described in figure 1. HR (95% CI) in people with fasting glucose 5·60–6·99 mmol/L was 1·12 (1·06–1·18). *Reference group.
Figure 6.
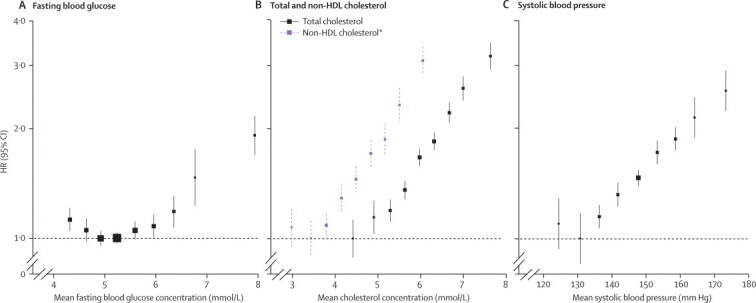
Comparison of hazard ratios (HRs) for coronary heart disease by long-term average concentrations of fasting blood glucose concentration, total (and non-HDL) cholesterol, and systolic blood pressure, in a common set of participants
Analyses were done in participants with no known history of diabetes at baseline. Analyses of fasting blood glucose concentration, total cholesterol, and systolic blood pressure were based on 140 624 participants (10 667 cases). For fasting blood glucose, participants were classified into groups of baseline fasting concentrations, as described in figure 4. For the other factors presented, participants were classified according to baseline values as follows: total cholesterol, <4·5, 4·5–5·1, 5·1–5·7, 5·7–6·3, 6·3–6·9, 6·9–7·5, 7·5–8·1, 8·1–8·7, ≥8·7 mmol/L; non-HDL cholesterol, <3, 3–3·6, 3·6–4·2, 4·2–4·8, 4·8–5·4, 5·4–6·0, 6·0–6·6, 6·6–7·2, ≥7·2 mmol/L; systolic blood pressure: <110, 110–120, 120–130, 130–140, 140–150, 150–160, 160–170, 170–180, ≥180 mm Hg). These categories approximately correspond to those used for fasting blood glucose concentration (ie, increments of half the SD of each factor). HRs were adjusted, where appropriate, for age, smoking status, BMI, systolic blood pressure, total cholesterol and fasting blood glucose, and stratified, where appropriate, by sex and trial arm. HRs were plotted against the mean value in each group. Long-term average values were calculated with information from serial measurements. The reference group for each factor is the category with the lowest HR. *Analyses of non-HDL cholesterol were based on a subset of 71 224 participants (4290 cases).
We noted similar findings in analyses that: used fixed-effect models, fractional polynomials, or spline terms (webappendix pp 10–11); compared larger and smaller studies (webappendix pp 12–13); assessed interactions by sex and age group; excluded initial follow-up (eg, the first 5 years); omitted 71 048 (10%) participants known to be receiving lipid-lowering, blood pressure-lowering, or other cardiovascular drugs at baseline; included fatal outcomes without censoring previous non-fatal outcomes; standardised glucose values in studies that used samples other than plasma;18 and assessed associations with fasting blood glucose concentration, either ignoring history of diabetes at baseline (webappendix p 14) or excluding studies that did not record self-reported history of diabetes.
The overall age-adjusted prevalence of diabetes in adults was 7·0% (6·1–7·9%)—which is lower than some contemporary estimates of about 10% for developed countries.19 Assuming a population-wide diabetes prevalence of 10% (ie, corresponding to a prevalence of 20% in cases of vascular death), 11% (10–12%) of vascular deaths are estimated to be attributable to diabetes (webappendix p 30), or 325 000 vascular deaths per year in the 49 high-income countries defined by WHO.20 For a hypothetical population-wide adult prevalence of diabetes of 20%, we estimated that 22% (20–23%) of vascular disease would be attributable to diabetes. Webappendix p 16 and p 31 provide sensitivity analyses, including estimation of the potential effect on HRs of misclassification of diabetes status.
Discussion
Our analysis has shown that diabetes confers about a two-fold excess risk for coronary heart disease, major stroke subtypes, and deaths attributed to other vascular causes. This pattern of strong associations of diabetes with each of several different vascular diseases contrasts with that of LDL cholesterol (or non-HDL cholesterol), which is strongly related to coronary heart disease, but modestly related to ischaemic stroke, and unrelated to haemorrhagic stroke in prospective observational studies.12 Diabetes is about a third more strongly related to fatal than to non-fatal myocardial infarction, perhaps suggestive of more severe forms of coronary lesions in people with diabetes than in those without, differential response of the myocardium to ischaemia, or possibly in part, differential coding of deaths from coronary heart disease.21–23 Although diabetes is a strong risk factor for coronary heart disease in all clinically relevant subgroups that we assessed, HRs are significantly greater in some groups at lower absolute risk of vascular disease—ie, in women, younger ages, non-smokers, and at lower-than-average blood pressure. Further investigation is needed to establish any implications of such effect modification. Because only a small part of the association between diabetes and ischaemic vascular disease is accounted for by several conventional and emerging risk factors, other mechanisms (including those as yet undiscovered) might be involved.
Our data suggest that in this decade about 10% of vascular deaths in populations in developed countries have been attributable to diabetes in adults, corresponding to an estimated 325 000 deaths per year in high-income countries alone (plus several-fold more people disabled by vascular disease). This burden will increase if the incidence of diabetes continues to rise,19 even if rates of vascular disease continue to fall because of decreases in smoking, improvements in treatment, or other reasons. At a diabetes prevalence of 20% in the general adult population (ie, more than twice the present levels in developed countries), an estimated 20% of vascular deaths would be attributable to diabetes. Increasing rates of obesity worldwide will probably heighten the absolute risk for diabetes and vascular disease.27 However, how these trends will modify the proportional effect of diabetes on risk of vascular disease is unclear. For example, HRs for ischaemic stroke seem somewhat greater at higher than at lower BMI, whereas HRs for coronary heart disease seem greater at lower than at higher BMI.
In contrast with the strong associations observed between diabetes and vascular outcomes, our study shows much more moderate associations of impaired fasting glucose status with coronary heart disease and stroke. Furthermore, there were no material associations with vascular risk at fasting blood glucose concentrations between 3·9 mmol/L and 5·6 mmol/L. By contrast, we have shown in a common set of participants that total (or non-HDL) cholesterol and systolic blood pressure each have much stronger and nearly log-linear associations with vascular risk. Additionally, we identified that, in people without diabetes, assessment of fasting blood glucose concentration or of impaired fasting glucose status does not significantly improve vascular disease prediction beyond the information provided by several conventional risk factors. Fasting blood glucose concentration is, of course, measured for other purposes, such as identification of diabetes.24 Scientific guideline statements, risk assessment strategies, sample sizes for intervention studies, and burden of disease calculations have been premised on the existence of stronger and log-linear associations between fasting blood glucose concentration and vascular disease throughout the range of its values.18,25,26 Review of these efforts might be useful, therefore, given the revised epidemiological estimates provided by our findings.
Our study was powered to characterise reliably several previously uncertain features, including: HRs for ischaemic vascular disease in several clinically relevant subgroups; HRs for vascular disease subtypes (eg, major stroke subtypes); shapes of relations across the range of fasting blood glucose concentrations; and predictive value of diabetes, impaired fasting glucose, and fasting blood glucose concentration for vascular risk assessment. Nevertheless, even more powerful analyses than those reported here are needed to characterise reliably shapes of associations in specific subgroups. For example, the present data seem to suggest the existence of some continuous association between fasting blood glucose concentration and coronary heart disease above an as yet imprecisely defined threshold.
The generalisability of our findings to populations in developed countries is supported by broadly consistent results across 102 cohorts in 25 countries. Because our data derive mostly from high-income countries, however, we could not estimate vascular disease burden attributable to diabetes for low-income and middle-income countries. Sensitivity analyses suggest that plausible degrees of misclassification were unlikely to change reported HRs substantially. Conversely, any preferential diagnosis of vascular disease in people with diabetes would have tended to overestimate HRs. We did not have information about duration or age of onset of diabetes or prevalence of diabetes type (type 1 or 2), although the age distribution suggests that the majority of participants with diabetes would have type 2 diabetes. Future prospective studies should aim to include additional markers of dysglycaemia and insulin resistance.28–30
Acknowledgments
Acknowledgments
The ERFC Coordinating Centre was supported by the British Heart Foundation (RG/08/014), UK Medical Research Council, and a specific grant from Pfizer. RG is supported by a Dorothy Hodgkin Postgraduate Award. SRKS is supported by the Gates Cambridge Trust Scholarship, Overseas Research Studentship Award Scheme, and the Addendrookes Charitable Trust Clinical Research Fellowship. Various sources have supported recruitment, follow-up, and laboratory measurements in the cohorts contributing to the ERFC. Investigators of several of these studies have contributed to a list (http://www.phpc.cam.ac.uk/ceu/erfc) naming relevant funding sources.
Contributors
NS, SRKS, RG, and JD drafted the manuscript. PG and SK undertook the analyses. All members of the writing committee provided critical revisions. All investigators shared individual data and had an opportunity to contribute to interpretation of the results and to redrafting of the report. The data management team collated and standardised the data. All members of the coordinating centre contributed to the collection, standardisation, analysis, and interpretation of the data.
The Emerging Risk Factors Collaboration
Writing Committee N Sarwar*, P Gao*, SR Kondapally Seshasai*, R Gobin*, S Kaptoge, E Di Angelantonio, University of Cambridge, UK; E Ingelsson, Karolinska Institute, Sweden; D A Lawlor, University of Bristol, UK; E Selvin, Johns Hopkins University, USA; M Stampfer, Harvard School of Public Health, USA; C D A Stehouwer, Maastricht University Medical Centre, the Netherlands; S Lewington, University of Oxford, UK; L Pennells, A Thompson, University of Cambridge, UK; N Sattar, University of Glasgow, UK; I R White, MRC Biostatistics Unit, UK; K K Ray, J Danesh, University of Cambridge, UK. *Denotes equal contribution.
Investigators AFTCAPS R W Tipping; ALLHAT CE Ford, S L Pressel; ARIC A R Folsom, L E Chambless, E Selvin, L E Wagenknecht; ATTICA D B Panagiotakos, C Pitsavos, C Chrysohoou, C Stefanadis; BHS M Knuiman; BRHS PH Whincup, S G Wannamethee, R W Morris; BRUN S Kiechl, J Willeit, F Oberhollenzer, A Mayr; BUPA N Wald; BWHHS S Ebrahim, D A Lawlor; CaPS J W Yarnell, J Gallacher; CASTEL E Casiglia, V Tikhonoff; CHARL P J Nietert, S E Sutherland, D L Bachman, J E Keil; CHS I H de Boer, J R Kizer, K J Mukamal (see http://www.chs-nhlbi.org for acknowledgments); COPEN A Tybjærg-Hansen, B G Nordestgaard, M Benn, R Frikke-Schmidt; CUORE S Giampaoli, L Palmieri, S Panico, D Vanuzzo, L Pilotto; DRECE A Gómez de la Cámara, M A Rubio; DUBBO L Simons, J McCallum, Y Friedlander; EAS F G R Fowkes, A J Lee; EPESEBOS J Taylor, J M Guralnik, C L Phillips; EPESEIOW R Wallace, J M Guralnik, C L Phillips; EPESENCA D G Blazer, J M Guralnik, C L Phillips; EPESENHA C L Phillips, J M Guralnik; EPICNOR K-T Khaw; ESTHER: H Brenner, E Raum, H Müller, D Rothenbacher; FIA J H Jansson, P Wennberg; FINE-FIN A Nissinen; FINE-IT C Donfrancesco, S Giampaoli; FINRISK-92, FINRISK-97 V Salomaa, K Harald, P Jousilahti, E Vartiainen; FLETCHER M Woodward; FRAMOFF R B D'Agostino, R S Vasan, C S Fox, M J Pencina; GLOSTRUP E Bladbjerg, T Jørgensen, L Møller, J Jespersen; GOH R Dankner, A Chetrit, F Lubin; GOTO13 L Wilhelmsen, H Eriksson, K Svärdsudd, L Welin; GOTO33, GOTO43 A Rosengren, L Wilhelmsen, G Lappas, H Eriksson; GOTOW C Björkelund, L Lissner, C Bengtsson; GRIPS P Cremer, D Nagel; HBS T E Strandberg, V Salomaa, R S Tilvis, T A Miettinen; HELSINAG R S Tilvis, T E Strandberg; HISAYAMA Y Kiyohara, H Arima, Y Doi, T Ninomiya; HONOL B Rodriguez; HOORN J M Dekker, G Nijpels, C D A Stehouwer; HPFS E Rimm, J K Pai; IKNS S Sato, H Iso, A Kitamura, H Noda; ISRAEL U Goldbourt; KIHD K Nyyssönen, T-P Tuomainen, J T Salonen; LASA D Deeg, J L Poppelaars; LEADER T W Meade, J A Cooper; MALMO B Hedblad, G Berglund, G Engström; MCVDRFP W M M Verschuren, A Blokstra; MESA M Cushman, A R Folsom, B M Psaty, S Shea; MOGERAUG1, MOGERAUG2, MOGERAUG3 A Döring, W Koenig, C Meisinger, W Mraz; MORGEN W M M Verschuren, A Blokstra, H Bas Bueno-de-Mesquita; MOSWEGOT L Wilhelmsen, A Rosengren, G Lappas; MRCOLD A Fletcher; MRFIT L H Kuller, G Grandits; NCS R Selmer, A Tverdal, W Nystad; NHANES I, NHANES III R Gillum, M Mussolino; NHS E Rimm, S Hankinson, J E Manson, J K Pai; NORTH KARELIA V Salomaa, K Harald, P Jousilahti, E Vartiainen; NPHS II J A Cooper, K A Bauer; NSHS K W Davidson, S Kirkland, J Shaffer, M R Korin; OSAKA A Kitamura, S Sato, H Iso; OSLO I Holme, R Selmer, A Tverdal, W Nystad; PARIS1 P Ducimetiere, X Jouven; PREVEND S J L Bakker, R T Gansevoort, H L Hillage; PRHHP C J Crespo, M R Garcia Palmieri; PRIME P Amouyel, D Arveiler, A Evans, J Ferrières; PROCAM H Schulte, G Assmann; PROSPER R G Westendorp, B M Buckley, C J Packard, N Sattar; QUEBEC B Cantin, B Lamarche, J-P Després, G R Dagenais; RANCHO E Barrett-Connor, D L Wingard, R Bettencourt; REYK V Gudnason, T Aspelund, G Sigurdsson, B Thorsson; RIFLE M Trevisan; ROTT J Witteman, I Kardys, M Breteler, A Hofman; SHHEC H Tunstall-Pedoe, R Tavendale, G D O Lowe, M Woodward; SHS B V Howard, Y Zhang, L Best, J Umans; SPEED Y Ben-Shlomo, G Davey-Smith; TARFS A Onat, G Hergenç, G Can; TROMSØ I Njølstad, E B Mathiesen, M L Løchen, T Wilsgaard; ULSAM B Zethelius, U Risérus, C Berne, E Ingelsson; USPHS J M Gaziano, M Stampfer, P Ridker; USPHS2 J M Gaziano, P Ridker; VHMPP H Ulmer, G Diem, H Concin; VITA A Tosetto, F Rodeghiero; WHI-HaBPS J E Manson, L Tinker, S Liu, B V Howard; WHITE I M Marmot, R Clarke, R Collins, A Fletcher; WHITE II E Brunner, M Shipley; WHS P Ridker, J Buring; WOSCOPS J Shepherd, S M Cobbe, I Ford, M Robertson; ZARAGOZA A Marin Ibañez; ZUTE E J M Feskens, D Kromhout.
Data Management Team M Walker, S Watson.
Coordinating Centre M Alexander, E Di Angelantonio, S Erqou, P Gao, R Gobin, P Haycock, S Kaptoge, S R Kondapally Seshasai, S Lewington, L Pennells, P L Perry, K K Ray, N Sarwar, A Thompson, S G Thompson, M Walker, S Watson, I R White, A M Wood, D Wormser, J Danesh (principal investigator).
Conflicts of interest
JD has received research funding from the British Heart Foundation, BUPA Foundation, Denka, diaDexus, European Union, Evelyn Trust, Fogarty International Centre, GlaxoSmithKline, Medical Research Council, Merck Sharp and Dohme, National Heart, Lung and Blood Institute, National Institute of Neurological Disorders and Stroke, Novartis, Pfizer, Roche, Wellcome Trust, and UK Biobank, and has served on advisory boards for Merck and Novartis, for which he has received compensation. AT has received honoraria and reimbursement of costs for speaking at scientific meetings from GlaxoSmithKline. All other members of the writing committee declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Spencer EA, Pirie KL, Stevens RJ. Diabetes and modifiable risk factors for cardiovascular disease: the prospective Million Women Study. Eur J Epidemiol. 2008;23:793–799. doi: 10.1007/s10654-008-9298-3. [DOI] [PubMed] [Google Scholar]
- 2.Schramm TK, Gislason GH, Kober L. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117:1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2002;162:1737–1745. doi: 10.1001/archinte.162.15.1737. [DOI] [PubMed] [Google Scholar]
- 5.Woodward M, Zhang X, Barzi F. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003;26:360–366. doi: 10.2337/diacare.26.2.360. [DOI] [PubMed] [Google Scholar]
- 6.Janghorbani M, Hu FB, Willett WC. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses' Health Study. Diabetes Care. 2007;30:1730–1735. doi: 10.2337/dc06-2363. [DOI] [PubMed] [Google Scholar]
- 7.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 9.Lawes CM, Parag V, Bennett DA. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27:2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 10.Helfand M, Buckley DI, Freeman M. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- 11.Emerging Risk Factors Collaboration Analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22:839–869. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 12.Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerging Risk Factors Collaboration Statistical methods for time-to-event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010 doi: 10.1093/ije/dyq063. published online May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Fibrinogen Studies Collaboration Measures to assess the prognostic ability of the stratified Cox proportional hazards model. Stat Med. 2009;28:389–411. doi: 10.1002/sim.3378. [DOI] [PubMed] [Google Scholar]
- 17.Graubard BI, Flegal KM, Williamson DF. Estimation of attributable number of deaths and standard errors from simple and complex sampled cohorts. Stat Med. 2007;26:2639–2649. doi: 10.1002/sim.2734. [DOI] [PubMed] [Google Scholar]
- 18.Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 19.Gan D, editor. Diabetes atlas. 3rd edn. International Diabetes Federation; Brussels: 2007. [Google Scholar]
- 20.Lopez A, Mathers C, Ezatt M. Global burden of disease and risk factors. World Bank and Oxford University Press; New York: 2006. [Google Scholar]
- 21.Creager MA, Luscher TF, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 22.Natali A, Vichi S, Landi P. Coronary atherosclerosis in Type II diabetes: angiographic findings and clinical outcome. Diabetologia. 2000;43:632–641. doi: 10.1007/s001250051352. [DOI] [PubMed] [Google Scholar]
- 23.Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554–560. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(supp 1):62–69. [Google Scholar]
- 25.Avendano M, Mackenbach JP. Blood glucose levels: facing a global crisis. Lancet. 2006;368:1631–1632. doi: 10.1016/S0140-6736(06)69675-X. [DOI] [PubMed] [Google Scholar]
- 26.Danaei G, Lawes CM, Vander HS. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368:1651–1659. doi: 10.1016/S0140-6736(06)69700-6. [DOI] [PubMed] [Google Scholar]
- 27.Kelly T, Yang W, Chen C-S. Global burden of obesity in 2005 and projections to 2030. Intl J Obes. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 28.Gao W, Qiao Q, Tuomilehto J. Post-challenge hyperglycaemia rather than fasting hyperglycaemia is an independent risk factor of cardiovascular disease events. Clin Lab. 2004;50:609–615. [PubMed] [Google Scholar]
- 29.Sarwar N, Aspelund T, Eiriksdottir G. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PloS Med. 2010;7:e1000278. doi: 10.1371/journal.pmed.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarwar N, Sattar N, Gudnason V, Danesh J. Circulating concentrations of insulin markers and coronary heart disease: a quantitative review of 19 Western prospective studies. Eur Heart J. 2007;249:1–7. doi: 10.1093/eurheartj/ehm115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


