Abstract
AIM: To study the activation of pancreatic and pulmonary mast cells and the effect of mast cell inhibition on the activation of peritoneal and alveolar macrophages during acute pancreatitis.
METHODS: Pancreatitis was induced by intraductal infusion of 5% sodium taurodeoxycholate in rats. The mast cell inhibitor cromolyn was administered intraperitoneally (i.p.) 30 min before pancreatitis induction. The pancreatic and pulmonary tissue damage was evaluated histologically and mast cells and their state of activation were evaluated. Peritoneal and alveolar macrophages were obtained and the expression of tumor necrosis factor α was determined. Myeloperoxidase activity was measured to evaluate the effect of mast cell inhibition on the progression of the inflammatory process. Finally, the effect of plasma on cultured mast cells or macrophages was evaluated in vitro.
RESULTS: The mast cell stabilizer significantly reduced inflammation in the pancreas and lung and the activation of alveolar macrophages but had no effect on peritoneal macrophages. Mast cell degranulation was observed in the pancreas during pancreatitis but no changes were observed in the lung. Plasma from rats with pancreatitis could activate alveolar macrophages but did not induce degranulation of mast cells in vitro.
CONCLUSION: Pancreatic mast cells play an important role in triggering the local and systemic inflammatory response in the early stages of acute pancreatitis. In contrast, lung mast cells are not directly involved in the inflammatory response related to pancreatic damage.
Keywords: Cytokines, Inflammation, Macrophages, Mast cells, Pancreatitis
INTRODUCTION
Acute pancreatitis represents a substantial clinical problem with increasing incidence and it is associated with high morbidity and mortality[1]. The most important predictor of mortality is the development of persistent or multiple organ failure and the commonest affected organ is the lung[2,3]. In these cases, acute lung injury is frequently related to early deaths in the first week of the disease[4]. The mechanisms involved in triggering distant organ inflammation are unclear, however, in addition to the release of activated hydrolytic enzymes, different pathways have been reported, including cytokines[5], oxygen-derived free radicals[6] or activated complement[7].
Among these mechanisms, mast cells have been reported to contribute to several aspects of pancreatitis-associated lung injury. These cells release a number of mediators, including histamine, tumor necrosis factor (TNF)α or monocyte chemotactic protein-1 (MCP-1) which could have a strong effect on pulmonary endothelial cells, thus potentiating the progression of inflammation[8,9]. The expression of different adhesion molecules increases early after pancreatitis induction, and in some of these molecules this increase could be prevented by administering mast cell degranulation inhibitors such as sodium cromolyn[10,11]. These observations suggest that masts cells are responding to mediators released during pancreatitis and, when activated, play a role in the induction of endothelial lung dysfunction and in the progression of the local and systemic inflammatory process.
Mast cells are usually located close to endothelial cells and this explains their effect on endothelial dysfunction when activated during pancreatitis. However, tissue-related variability on the number, phenotype and distribution of mast cell populations have been reported, resulting in different activation during inflammatory processes[12]. In the case of acute pancreatitis, the use of mast cell stabilizers prevented changes in systemic inflammation and in endothelial permeability in different organs[10], however, the involvement of the particular mast cell populations remains unclear.
In this work we have evaluated the effect of mast cell inhibition on the activation of peritoneal and alveolar macrophages in an experimental model of acute pancreatitis.
MATERIALS AND METHODS
Animal model of acute pancreatitis
Male Wistar rats (250-300 g b/w) (n = 6 each group) were anaesthetized with 10% urethane (1 mL/100 g, i.p.). The biliopancreatic duct was cannulated through the duodenum and the hepatic duct was closed by a small bulldog clamp. Severe acute pancreatitis was induced by retrograde infusion into the biliopancreatic duct of 5% sodium taurocholate (Sigma Chemical, St. Louis, MO, USA) in a volume of 0.1 mL/100 g b/w using a Harvard ‘22’ infusion pump (Harvard Instruments, Edenbridge, UK)[13]. Control animals received an intraductal infusion of saline solution (0.9% NaCl). In a group of animals, cromolyn (Sigma, St Louis, MO, USA) (5 mg/kg b/w) was administered i.p. 30 min before pancreatitis induction. Three hours after induction, tissue samples of pancreas and lung were obtained, immediately frozen and maintained at -80°C until processed. This time point was selected because we previously reported that, in this model, a significant systemic inflammation is initiated three hours after the induction of pancreatitis[14]. Plasma samples were pelleted and the supernatant was stored at -40°C until use. Pancreas and lung samples were also obtained and stored for histological analysis.
Histological analysis
Tissue samples were fixed in 10% neutral buffered formalin, embedded in paraplast, sectioned in 5 μm slices and stained with toluidine blue (0.1%). The dye was allowed to dry on the slide for a few seconds; the slides were then rinsed in xylene for 5-10 min. and rinsed twice with acetone. Finally, the slides were cleared in xylene and mounted in diphenylphthalein xylene. Sections were evaluated by light microscopic examination.
Cell culture
Peritoneal macrophages were harvested by 5 peritoneal washes with 10 mL of phosphate buffered saline (PBS) containing 3 units/mL heparin. The obtained cell suspension was centrifuged (300 × g, 7 min). Cells were suspended in the RPMI1640 culture medium containing 10% fetal calf serum, 2 mmol/L glutamine, penicillin (100 U/mL) and streptomycin (100 μg/mL). Aliquots of about 3 × 106 cells were plated in 6 well plates and cultured at 37°C under a gas phase of air/CO2 (95:5). After an attachment period of 4 h, the non-adhered cells were removed by shaking. The resulting adherent population consisted of > 92% peritoneal macrophages.
Alveolar macrophages were obtained by bronchoalveolar wash. After exsanguinations, lung and trachea were excised en bloc and washed 5 times with 10 mL cold (4°C) saline solution. The supernatant was centrifuged at 300 × g for 7 min and the cells were resuspended in RPMI1640 culture medium containing 10% fetal calf serum, 2 mmol/L glutamine, penicillin (100 U/mL) and streptomycin (100 μg/mL). Aliquots of about 3 × 106 cells were plated in 6 well plates and cultured at 37°C under a gas phase of air/CO2 (95:5). After an attachment period of 4 h, the non-adhered cells were removed by shaking. The resulting adherent population consisted of > 95% alveolar macrophages.
The rat mast cell line RBL-2H3 was maintained as a monolayer culture in RPMI-1640 medium supplemented with 10% fetal calf serum, penicillin (100 U/mL) and streptomycin (100 μg/mL) in an incubator with 5% CO2 at 37°C.
In vitro effect of plasma
The effects of circulating mediators on mast cells or macrophages were evaluated by incubating alveolar macrophages or the mast cell line RBL-2H3 with plasma obtained from controls, animals with pancreatitis or with cromolyn treated pancreatitis. Cells were cultured in 12 well plates in the presence of 20% plasma in the culture media. One hour after culture at 37°C, the levels of histamine were measured in RBL-2H3 supernatants. For macrophages, RNA was obtained and the expression of TNFα was evaluated by quantitative real-time polymerase chain reaction (RT-PCR).
RNA isolation and RT-PCR
Total RNA from cells was extracted using the TRizol® reagent (Invitrogen, Carlsbad, CA, USA). The RNA was quantified by measurement of the absorbance at 260 and 280 nm using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA).
cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA), and reverse transcription was then performed on 1 μg RNA sample by adding iScript reagents. The reaction was incubated at 25°C for 5 min, 42°C for 30 min, and 85°C for 5 min, and then stored at -80°C.
Subsequent PCR amplification was performed in a DNA Engine, Peltier Thermal Cycler (Bio-Rad Laboratories, CA, USA) using IQTM SYBR Green Super mix and the correspondent rat primers: TNFα forward: 5'-AACTCCCAGAAAAGCAAGCA-3' reverse: 5'-CGAGCAGGAATGAGAAGAGG-3'; GAPDH forward: 5'-CTGTGTCTTTCCGCTGTTTTC-3', reverse: 5'-TGTGCTGTGCTTATGGTCTCA-3'.
Initial denaturation was followed by 40 cycles of DNA amplification with fluorescence detection at the end of the elongation step (SYBR Green format). Reactions were performed in duplicate and threshold cycle values were normalized to GAPDH gene expression. The specificity of the products was determined by melting curve analysis. The ratio of the relative expression of target genes to GAPDH was calculated by using the ΔC(t) formula.
Lipase
Plasma lipase was determined using commercial turbidimetric assay kits from Randox (Antrim, UK), according to the supplier’s specifications.
Histamine analysis
Histamine levels in plasma and in cell culture supernatants were evaluated using a commercial ELISA assay from Labor Diagnostika Nort (Nordhorn, Germany) according to the supplier’s specifications.
TNFα
TNFα concentration in the cell culture medium was measured using a commercial kit for rat TNFα from BLK International (Badalona, Spain), according to the supplier’s specifications.
Myeloperoxidase
Neutrophilic infiltration was assessed by measuring myeloperoxidase (MPO) activity. MPO was determined photometrically with 3,3’,5,5’-tetramethylbenzidine as substrate. Tissue samples were homogenized with 0.5% hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer at pH 6.0. Homogenates were disrupted for 30 s using a Labsonic sonicator (Braun Biotech, Inc., Allentown, PA, USA) at 20% power and submitted to three cycles of snap freezing in dry ice and thawing before a final 30 s sonication. Samples were incubated at 60°C for 2 h and then spun down at 4000 × g for 12 min. The supernatants were collected for MPO assay. Enzyme activity was assessed photometrically using 630 nm wavelength. The assay mixture consisted of 20 μL supernatant, 10 μL tetramethylbenzidine (final concentration 1.6 mmol/L) dissolved in DMSO, and 70 μL H2O2 (final concentration 3.0 mmol/L) diluted in 80 mmol/L phosphate buffer, pH 5.4. The results are expressed as units (U) MPO activity per g protein.
Protein measurement: Total protein concentration in homogenates was determined using a commercial kit from BioRad (Munich, Germany).
Statistical analysis
Data are expressed as mean ± SE. Means of different groups were compared using a one-way analysis of variance. Tukey’s multiple comparison test was performed to evaluate significant differences between groups. Differences were assumed to be significant when P < 0.05.
RESULTS
Mast cell activation during pancreatitis
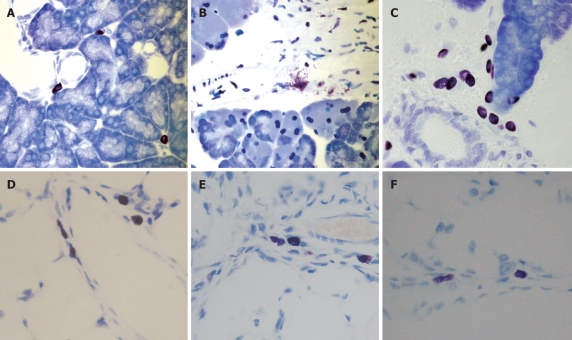
Histological analysis revealed the presence of mast cells in the interlobular areas of control pancreas (Figure 1A) and a general pancreatic mast cell degranulation after induction of pancreatitis (Figure 1B). This degranulation was prevented by cromolyn treatment (Figure 1C). In lungs, mast cells could also be observed (Figure 1D), however, no apparent degranulation was detected in histological samples of the lung 3 h after induction of pancreatitis (Figure 1E). Cromolyn treatment had no effect on lung mast cells (Figure 1F).
Figure 1.
Presence of mast cells in pancreas (A-C) and lung (D-F), in control (A and D). Three hours after induction of pancreatitis (B and E) and under cromolyn treatment (C and F). Degranulating mast cells were observed in the pancreas after pancreatitis induction (B). Cromolyn treatment prevented mast cell degranulation (C). In contrast, no evident degranulation was observed in lung after induction of pancreatitis. Toluidine blue, × 40.
Effects of mast cell inhibition
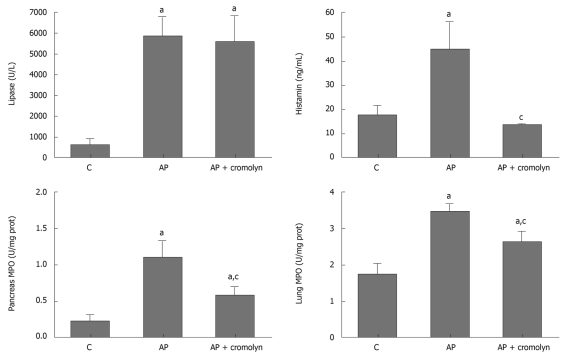
Pancreatitis resulted in increased levels of circulating lipase and histamine in plasma as well as enhanced MPO activity in both pancreas and lung (Figure 2). The inhibition of mast cell degranulation with cromolyn did not modify the lipase levels, but resulted in a reduction in pancreatic MPO activity, indicating that this treatment had no effect on acinar cell damage but reduced pancreatic inflammation. Inhibition of the inflammatory process in lung was also observed. Nevertheless, these inhibitions did not achieve the control values and MPO activity remained significantly increased with respect to the control group in both pancreas and lung.
Figure 2.
Effect of mast cell inhibitor, cromolyn. Three hours after pancreatitis induction, increased levels of lipase and histamine were detected in plasma. Cromolyn treatment had no effect on lipase, which is related to acinar cell damage, but prevented the increase in histamine. In tissue, leukocyte infiltration was evaluated by measuring myeloperoxidase (MPO) activity. Pancreatitis resulted in increased MPO activity in both pancreas and lung. Cromolyn treatment partially prevented these increases. aP < 0.05 vs C; cP < 0.05 vs AP. C: Control; AP: Acute pancreatitis.
Changes in peritoneal and alveolar macrophages
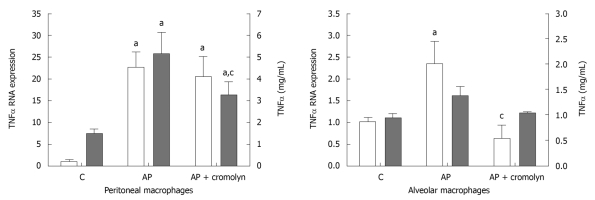
Pancreatitis resulted in the induction of TNFα expression in both peritoneal and alveolar macrophages (Figure 3). However, at this time point the activation observed in peritoneal macrophages was one order of magnitude greater than that observed in alveolar macrophages. This was reflected in the release of TNFα which only showed a significant increase in peritoneal macrophages after pancreatitis induction, while the increase observed in alveolar macrophages was not statistically significant (Figure 3). Inhibition of mast cell degranulation had no effect on peritoneal macrophage activation, but completely prevented the increase observed in TNFα expression in alveolar macrophages.
Figure 3.
Both peritoneal and alveolar macrophages were activated after induction of pancreatitis, but the expression of tumor necrosis factor α mRNA in peritoneal macrophages was one order of magnitude higher than that observed in alveolar macrophages. Tumor necrosis factor (TNF)α release was induced in peritoneal cells, while in alveolar cells the observed increase was not statistically significant. Cromolyn treatment completely prevented the activation of alveolar macrophages. In contrast, peritoneal macrophages remained activated under cromolyn treatment. aP < 0.05 vs C; cP < 0.05 vs AP. C: Control; AP: Acute pancreatitis.
Effect of plasma on cultured mast cells and macrophages
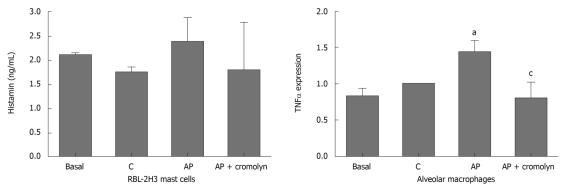
Incubation of the RBL-2H3 cell line with plasma obtained from animals with pancreatitis did not result in increased histamine release (Figure 4). In contrast, this plasma was able to induce the activation of alveolar macrophages, reflected in an increase in the expression of TNFα. This induction was not observed when plasma was obtained from animals treated with cromolyn (Figure 4).
Figure 4.
The effect of plasma on cultured mast cell line RBL-2H3 and alveolar macrophages. Mast cells were not activated by plasma from animals with pancreatitis. In contrast, the expression of tumor necrosis factor (TNF)α in alveolar macrophages was induced by plasma from animals with pancreatitis. This induction was not observed when animals were treated with cromolyn. aP < 0.05 vs C; cP < 0.05 vs AP. C: Control; AP: Acute pancreatitis.
DISCUSSION
The development of systemic inflammation during the progression of severe acute pancreatitis involves multiple pathways and cell systems. Among them, mast cells appear to play a pivotal role between the hydrolytic enzymes released by damaged pancreatic acinar cells and the stress-induced response mediated by free radicals and inflammatory mediators.
In this sense, mast cells seem to play a role amplifying the acute inflammatory response in different organs early after the onset of pancreatitis. Several mediators known to be released by mast cells, including platelet activating factor (PAF), histamine or prostaglandin D2 (PGD2), have been shown to be increased a few minutes after the induction of pancreatitis in experimental models[15,16]. In addition, the administration of mast cell inhibitors results in a reduction of the local and systemic inflammatory response and, in particular, prevents changes in endothelial cells and vascular permeability[10].
However, it is important to evaluate the particular role of the different mast cell populations in this process, in order to design therapeutic strategies centered on these cells. Due to the rapid activation of pancreatic mast cells in the onset of pancreatitis, the obvious therapeutic target may be pulmonary mast cells that are suspected of being activated in the later stages of the disease.
In the present study, we evaluated the degranulation of mast cells in pancreas and lung and found a different response during pancreatitis. Histological evaluation showed a clear and extensive degranulation of mast cells located in pancreatic tissue (Figure 1B). As expected, this degranulation was prevented by cromolyn administration (Figure 1C). In contrast, no clear evidence of mast cell degranulation was observed in lung tissue (Figure 1E).
This was a surprising result, taking into account that cromolyn administration resulted in a clear reduction in lung inflammation revealed by MPO activity and by a lower activation of alveolar macrophages (Figure 3). In addition, these results are in line with other authors who reported on the critical role of mast cells in the inflammatory response in lung during pancreatitis.
An explanation for this apparent contradictory result is an indirect effect of pancreatic mast-cell derived mediators on distant organs. Activation of mast cells results in the immediate release of mediators that play a role in the activation of circulating leukocytes as demonstrated by Zhao et al[11]. On the other hand, the progression of inflammation in pancreatic tissue is modified by cromolyn treatment (Figures 1 and 2). Consequently, it is suspected that the profile of pro-inflammatory mediators released to the bloodstream by pancreatic tissue and their ability to induce lung endothelial dysfunction could be modified by pancreatic mast cell inhibition.
To evaluate this possibility we treated alveolar macrophages as well as the mast cell line RBL-2H3 in vitro with plasma obtain from the different experimental groups. Our results indicate that plasma from the pancreatitis-induced group did not stimulate the production of significant amounts of histamine in culture (Figure 4). This result suggests that while pancreatic damage could enhance the activation and degranulation of mast cells in the pancreas a few minutes after pancreatitis induction[8], mediators present in plasma are not sufficient to activate these cells in distant organs.
However, plasma from pancreatitis animals was able to induce the activation of macrophages in vitro, reflected in the increased expression of TNFα. This effect was clearly reduced when animals were treated with cromolyn (Figure 4). Together, these results indicate that pancreatic mast cells play an important role in triggering the local and systemic inflammatory response in the early stages of acute pancreatitis. In contrast, lung mast cells are not directly involved in the inflammatory response related to pancreatic damage. The early activation reported in pancreatic mast cells may make the use of these cells as a pharmacological target difficult due to the short therapeutic window.
COMMENTS
Background
Mast cells have been reported to contribute to several aspects of pancreatitis associated lung injury. However, the involvement of particular mast cell populations remains unclear.
Research frontiers
Using an experimental model of acute pancreatitis in rats, the authors evaluated the activation of mast cells from pancreas and lung as well as the effect of mast cell inhibitors on progression of the inflammatory reaction.
Innovations and breakthroughs
Pancreatic mast cells play an important role in triggering the local and systemic inflammatory response in the early stages of acute pancreatitis. In contrast, lung mast cells are not directly involved in the systemic inflammatory response related to pancreatic damage.
Applications
The identification of active mast cells in the early stages of pancreatitis may improve our understanding of their role in this disease and the possible therapeutic strategies focussed on these cells.
Terminology
Cromolyn is a mast cell stabilizer that prevents the release of inflammatory mediators, such as histamine, from these cells.
Peer review
In this work, the authors have evaluated the effect of mast cell inhibition on the activation of peritoneal and alveolar macrophages in an experimental model of acute pancreatitis. The manuscript portrays a good effort by its authors.
Footnotes
Supported by The Project SAF2006-08449 and a grant from Fundación para la Investigación del Hospital General Universitario de Alicante; Inmaculada Lopez-Font has a Juan de la Cierva contract supported by the Spanish Ministry of Science and Innovation
Peer reviewers: Julia B Greer, MD, MPH, Department of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh Medical Center, M2, Presbyterian University Hospital, 200 Lothrop Street, Pittsburgh, PA 15213, United States; Yvette Mándi, Professor, MD, Department of Medical Microbiology and Immunobiology, University Szeged, Dom ter 10, Szeged, H6720, Hungary
S- Editor Wang YR L- Editor Webster JR E- Editor Ma WH
References
- 1.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 2.Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744. doi: 10.1002/bjs.5290. [DOI] [PubMed] [Google Scholar]
- 3.Compañy L, Sáez J, Martínez J, Aparicio JR, Laveda R, Griñó P, Pérez-Mateo M. Factors predicting mortality in severe acute pancreatitis. Pancreatology. 2003;3:144–148. doi: 10.1159/000070083. [DOI] [PubMed] [Google Scholar]
- 4.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340–1344. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de-Madaria E, Martínez J, Sempere L, Lozano B, Sánchez-Payá J, Uceda F, Pérez-Mateo M. Cytokine genotypes in acute pancreatitis: association with etiology, severity, and cytokine levels in blood. Pancreas. 2008;37:295–301. doi: 10.1097/MPA.0b013e31816726d5. [DOI] [PubMed] [Google Scholar]
- 6.Folch E, Gelpí E, Roselló-Catafau J, Closa D. Free radicals generated by xanthine oxidase mediate pancreatitis-associated organ failure. Dig Dis Sci. 1998;43:2405–2410. doi: 10.1023/a:1026617812283. [DOI] [PubMed] [Google Scholar]
- 7.Lindström O, Jarva H, Meri S, Mentula P, Puolakkainen P, Kemppainen E, Haapiainen R, Repo H, Kylänpää L. Elevated levels of the complement regulator protein CD59 in severe acute pancreatitis. Scand J Gastroenterol. 2008;43:350–355. doi: 10.1080/00365520701763209. [DOI] [PubMed] [Google Scholar]
- 8.Braganza JM. Mast cell: pivotal player in lethal acute pancreatitis. QJM. 2000;93:469–476. doi: 10.1093/qjmed/93.7.469. [DOI] [PubMed] [Google Scholar]
- 9.Yönetçi N, Oruç N, Ozütemiz AO, Celik HA, Yüce G. Effects of mast-cell stabilization in cerulein-induced acute pancreatitis in rats. Int J Pancreatol. 2001;29:163–171. doi: 10.1385/IJGC:29:3:163. [DOI] [PubMed] [Google Scholar]
- 10.Dib M, Zhao X, Wang XD, Andersson R. Role of mast cells in the development of pancreatitis-induced multiple organ dysfunction. Br J Surg. 2002;89:172–178. doi: 10.1046/j.0007-1323.2001.01991.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Dib M, Wang X, Widegren B, Andersson R. Influence of mast cells on the expression of adhesion molecules on circulating and migrating leukocytes in acute pancreatitis-associated lung injury. Lung. 2005;183:253–264. doi: 10.1007/s00408-004-2538-8. [DOI] [PubMed] [Google Scholar]
- 12.Purcell WM, Cohen DL, Hanahoe TH. Comparison of histamine and 5-hydroxytryptamine content and secretion in rat mast cells isolated from different anatomical locations. Int Arch Allergy Appl Immunol. 1989;90:382–386. doi: 10.1159/000235058. [DOI] [PubMed] [Google Scholar]
- 13.Aho HJ, Suonpää K, Ahola RA, Nevalainen TJ. Experimental pancreatitis in the rat. Ductal factors in sodium taurocholate-induced acute pancreatitis. Exp Pathol. 1984;25:73–79. doi: 10.1016/s0232-1513(84)80010-9. [DOI] [PubMed] [Google Scholar]
- 14.Folch E, Salas A, Panés J, Gelpí E, Roselló-Catafau J, Anderson DC, Navarro S, Piqué JM, Fernández-Cruz L, Closa D. Role of P-selectin and ICAM-1 in pancreatitis-induced lung inflammation in rats: significance of oxidative stress. Ann Surg. 1999;230:792–798; discussion 798-799. doi: 10.1097/00000658-199912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson CD. Platelet-activating factor and platelet-activating factor antagonists in acute pancreatitis. Dig Surg. 1999;16:93–101. doi: 10.1159/000018699. [DOI] [PubMed] [Google Scholar]
- 16.Closa D, Roselló-Catafau J, Fernández-Cruz L, Gelpí E. Prostaglandin D2, F2 alpha, E2, and E1 in early phase of experimental acute necrohemorrhagic pancreatitis in rats. Pancreas. 1994;9:73–77. doi: 10.1097/00006676-199401000-00011. [DOI] [PubMed] [Google Scholar]