Abstract
AIM: To assess the effect of human leukocyte antigen (HLA) mismatching on liver graft outcome and acute rejection from a meta-analysis of available cohort studies.
METHODS: Articles in PubMed/MEDLINE, EMBASE and the Cochrane database from January 1970 to June 2009, including non-English literature identified in these databases, were searched. Only studies comparing HLA or sub-phenotype matching with mismatching were extracted. The percentage of graft survival was extracted by “Engauge Digitizer” from survival curves if the raw data were not displayed. A meta-analysis was performed when at least 3 studies provided data.
RESULTS: Sixteen studies met the inclusion criteria. A lower number of HLA mismatches (0-2 vs 3-6) did reduce the incidence of acute rejection (relative risk: 0.77, P = 0.03). The degree of HLA mismatching (0-2 vs 3-6) had no significant effect on 1-year [hazard ratio (HR): 1.04, P = 0.68] and 5-year (HR: 1.09, P = 0.38) graft survival. In sub-phenotype analysis, the degree of HLA-A, B and DR mismatching (0 vs 1-2) had no significant effect on 1-year and 5-year graft survival, either. The HRs and P-values were 0.95, 0.71 (HLA-A, 1-year); 1.06, 0.60 (HLA-A, 5-year); 0.77, 0.16 (HLA-B, 1-year); 1.07, 0.56 (HLA-DR, 1-year); 1.18, 0.23 (HLA-DR, 5-year), respectively.
CONCLUSION: The results of this systematic review imply that good HLA compatibility can reduce the incidence of acute rejection in spite of having no influence on graft outcomes. To obtain a short recovery time and minimize rejection post transplantation, HLA matching studies should be considered before the operation.
Keywords: Human leukocyte antigen, Mismatching, Liver transplantation, Meta-analysis, Graft rejection
INTRODUCTION
In the past 2 decades, deaths and other complications of organ transplantation have decreased significantly as a result of improvements in anesthesiology and surgical techniques. In addition, development of immunosuppressive agents and new organ preservation solutions have been shown to play a role in the improved survival rate. However, acute or chronic rejection remains the most important reason of graft failure, especially for patients who suffer from mismatching of human leukocyte antigen (HLA).
The role of HLA matching between donor and recipient in organ transplant rejection and survival has been widely studied and proven to increase graft survival after kidney, heart, and other organ transplantation and to reduce the incidence of acute or chronic rejection[1-6]. In contrast, major histocompatibility complex analysis is not routinely performed in liver transplantation because its importance remains controversial, with different groups reporting disparate results. It was reported that some populations of patients gained benefit from high degrees of HLA matching[7-14]. Concern has been voiced about possible increased likelihood of recurrence of primary disease with good HLA compatibility[15-25].
We therefore performed a systematic review and meta-analysis on the efficacy of HLA mismatching in all published controlled clinical trials on the outcomes of liver transplantation.
MATERIALS AND METHODS
Search strategy
Relevant articles that were published between January 1970 and June 2009 in PubMed/MEDLINE, EMBASE and the Cochrane database, including the non-English literature were identified. The search strategy used the following single text words and combinations: living donor liver transplantation (LDLT), liver transplantation (LT), orthotopic liver transplantation (OLT), human leukocyte antigen (HLA), major histocompatibility complex (MHC), histocompatibility, matching and mismatching. Reference lists of relevant articles were cross checked for other potentially relevant articles.
Selection of trials and quality of the studies
Three separate authors (Lan X, Pu CL and Guo CB) independently reviewed and evaluated all articles for inclusion, which were classified as randomized control trial (RCT), controlled trial (CT), or descriptive study. After the initial article selection, the article dataset was reviewed and updated to capture any articles published between the final consensus review and the final data analysis (Zhang MM). Only cohort studies were indentified because of a lack of RCT.
The scoring system was adapted from Stahl, the Cochrane Collaboration and others[26-29]. This system suits not only RCT but CT or other studies well: (1) Was the trial design clearly stated? (2) Selection bias questions: Was the Patient selection process clearly stated? If the trial was an RCT, were patients randomly allocated to the therapeutic intervention? Were patients and clinicians blinded to the intervention? If the trial was not an RCT, were confounders controlled for? If the trial design was case control were matching procedures clearly described and implemented? Were patient recruitment procedures clearly described? Were the intervention and control groups selected similarly? (3) Performance bias questions: Was the intervention clearly described? Was intervention clearly measured? (4) Attrition bias questions: Were patients followed up? Were they followed up for 2 or more explicitly defined intervals? If patients were lost/dropped out other than because of death, were they accounted for? Were all outcome measures captured at the declared follow-up intervals? (5) Detection bias questions: Were the outcome measures clearly described? Was measurement of the outcome measures blinded? (6) Were appropriate statistical methods used? Were P-values clearly stated? Was life table analysis provided, etc.; and (7) Was the presentation of data adequate, for example, in the article were endpoints clearly defined i.e. graft survival, patient survival, duration of follow-up, re-transplantation rate, etc.? Were survival curves provided or were sufficient data to construct survival curves provided, were donor and recipient variables clearly defined and presented?
These questions were placed on a 3 point scale: unclear/inadequate (0), adequate (1), good (2). Articles were considered for inclusion if their summary score exceeded 30.
Data extraction
Graft loss was measured by hazard ratio (HR) and rejection was measured by relative risk (RR) at 1-year and 5-year in every study by 2 independent reviewers, reconciling any differences by consensus or when in doubt referring it to a third reviewer (Zhang MM) for arbitration. Graft survival rate was extracted for calculating corresponding HR using the formula recommended by Parmar et al[30]. Data was extracted by the software “Engauge 4.0” from survival curves if it was not shown in articles directly. Donor/recipient HLA compatibility for HLA class I (A and B), and HLA class II (DR) was measured as the number of mismatches, locus-specific (0 to 2 mismatches) and overall for the A, B, and DR loci (0 to 6 mismatches).
Meta-analysis
Both HR and RR were compared between 0 with 1-2 mismatches for each locus (mismatches of the HLA-A, B and C loci respectively) and 0-2 with 3-6 mismatches for overall HLA-A, B and DR loci. Comparability of the studies included in each pooled analysis was confirmed by examination of the χ2 Q (expressed as a P-value) and I2 statistics of heterogeneity. Statistical heterogeneity was defined as P < 0.10 or I2 > 50%. Lack of over-influence of one individual study to pooled estimates was confirmed by serial omission of each study and examination of the resulting estimate. To account for potential differences that were evident clinically but not identified by statistical tests, random effects models were used for each outcome measure. All statistical analyses were performed using Review Manager 5.0 which was a new program for determining HR.
RESULTS
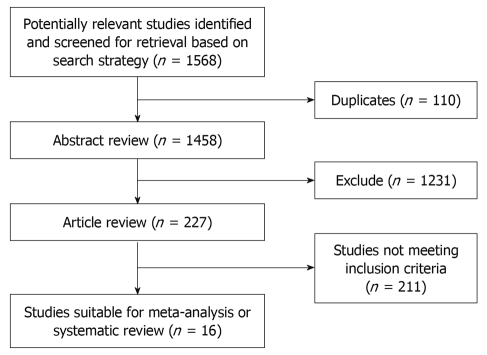
Results of the article selection are described in Figure 1. 1568 potentially relevant articles were identified in the search. The abstracts of these studies were reviewed by 2 independent investigators. One thousand four hundred and forty-two did not meet inclusion criteria as their summary score was less than 30. Publications eligible for analysis included 16 articles: 2 prospective studies[7,8] and 14 retrospective cohort studies[9-22]. Non RCTs were included in our studies. In 4 studies acute rejection rates were compared clearly between 0-2 mismatches and 3-6 mismatches of HLA[8,12-14]. That is too say, specific data could only be extracted in these 4 articles. In 10 and 8 studies 1-year and 5-year survival rates, respectively, were compared between 0-2 mismatches and 3-6 mismatches of HLA[7-10,12,13,15,17,19,21,22]. In 6 and 5 studies 1-year and 5-year survival rates, respectively, were compared or could be extracted from survival curves between 0 mismatches and 1-2 mismatches of the HLA-A epitope[7,8,10,17-22]. In 9 and 5 studies 1-year and 5-year survival rates, respectively, were compared or could be extracted from survival curves between 0 mismatches and 1-2 mismatches of the HLA-DR epitope[8,9,16-22]. In 6 studies 1-year survival rates were compared between 0 mismatches and 1-2 mismatches of the HLA-B epitope[8,17-19,21,22]. Although 0 mismatches of the HLA-B epitope were compared with 1-2 mismatches in 5-year survival rates in 3 articles, the statistical heterogeneity was P = 0.004 and I2 = 82% in the meta-analysis. Hence, the HR of the HLA-B epitope in 5-year survival rates was not included in our discussion. Details of these studies are described in Table 1. The methodological quality of the studies was assessed using a validated tool as described above (Table 2).
Figure 1.
Selection of articles.
Table 1.
Contents of included studies
| Author | Location | Immunosuppression | Number of patients | Contents |
| Meyer et al[9] | France | Cyclosporine, methylpred- nisolone and azathioprine | 162 | HLA-A, B and DR (5-yr graft survival); HLA-DR (1- and 5-yr graft survival) |
| Jakab et al[10] | American | NS | 631 | HLA-A, B and DR (1- and 5-yr graft survival); HLA-A and HLA-B (5-yr graft survival) |
| Neumanna et al[8] | Germany | Cyclosporine, azathioprine and prednisolone | 836 | HLA-A, B and DR (1- and 5-yr graft survival and rejection); HLA-A and HLA-DR (1- and 5-yr graft survival); HLA-B (1-yr graft survival) |
| Hashimoto et al[11] | Japan | Cyclosporine, methylpred- nisolone and azathioprine | 50 | HLA-A, B and DR (1- and 5-yr graft survival) |
| Langrehr et al[12] | Germany | Cyclosporine, azathioprine and prednisolone | 165 | HLA-A, B and DR (1- and 5-yr graft survival and rejection) |
| Suehiro et al[13] | Japan | Tacrolimus and Steroids | 104 | HLA-A, B and DR (1- and 5-yr graft survival and rejection) |
| Harihara et al[14] | Japan | Tacrolimus and Steroids | 85 | HLA-A, B and DR (rejection) |
| Balan et al[7] | American | Cyclosporine, prednisone, and azathioprine or tacrolimus | 799 | HLA-A, B and DR (1- and 5-yr graft survival); HLA-A (5-yr graft survival) |
| Sugawara et al[16] | Japan | Tacrolimus and methyl- prednisolone | 113 | HLA-DR (1-yr graft survival) |
| Doran et al[22] | Germany | NS | 446 | HLA-A, B and DR (1- yr graft survival); HLA-A and HLA-B (1-yr graft survival) |
| Poli et al[15] | Italy | Cyclosporine, azathioprine and tacrolimus | 814 | HLA-DR (5-yr graft survival) |
| Yagihashi et al[18] | American | Cyclosporine, azathioprine and tacrolimus | 347 | HLA-A, HLA-B and HLA-DR (1-yr graft survival) |
| Nikaein et al[21] | American | Cyclosporine and prednisone | 701 | HLA-A, B and DR (1-yr graft survival); HLA-A (1- and 5-yr graft survival); HLA-B and HLA-DR (1-yr graft survival) |
| Markus et al[20] | American | NS | 527 | HLA-A (5-yr graft survival); HLA-DR (1-yr graft survival) |
| Donaldson et al[19] | Britain | Cyclosporine, azathioprine | 466 | HLA-A, B and DR (1-yr graft survival and rejection); HLA-A and HLA-B (1-yr graft survival) |
| Knechtle et al[17] | American | NS | 324 | HLA-A, B and DR (1-yr graft survival); HLA-A, HLA-B and HLA-DR (1-yr graft survival) |
NS: Not specified; HLA: Human leukocyte antigen.
Table 2.
Methodological quality of the controlled trials
| Study | Selection criteria specified | Study design | Score | Other causing of death report | Dropouts explained | Funding |
| Meyer C | Yes | RCS | 30 | No | No | NS |
| Jakab SS | Yes | RCS | 32 | Yes | Yes | NS |
| Neumanna UP | Yes | PCS | 31 | No | No | NS |
| Morioka D | Yes | RCS | 30 | No | No | NS |
| Langrehr JM | Yes | RCS | 33 | Yes | Yes | NS |
| Suehiro T | Yes | RCS | 30 | Yes | No | NS |
| Harihara Y | Yes | RCS | 30 | No | No | NS |
| Vijayan B | Yes | PCS | 35 | Yes | Yes | NS |
| Sugawara Y | Yes | RCS | 30 | Yes | No | NS |
| Doran | Yes | RCS | 30 | No | No | NS |
| Poli F | Yes | RCS | 31 | No | No | NS |
| Yagihashi A | Yes | RCS | 30 | No | No | NS |
| Afzal N | Yes | RCS | 33 | No | No | NS |
| Markus BH | Yes | RCS | 32 | No | Yes | NS |
| Donaldson P | Yes | RCS | 32 | No | Yes | NS |
| Knechtle SJ | Yes | RCS | 31 | No | Yes | NS |
RCS: Retrospective cohort studies; NS: Not specified.
Meta-analysis of HLA epitope
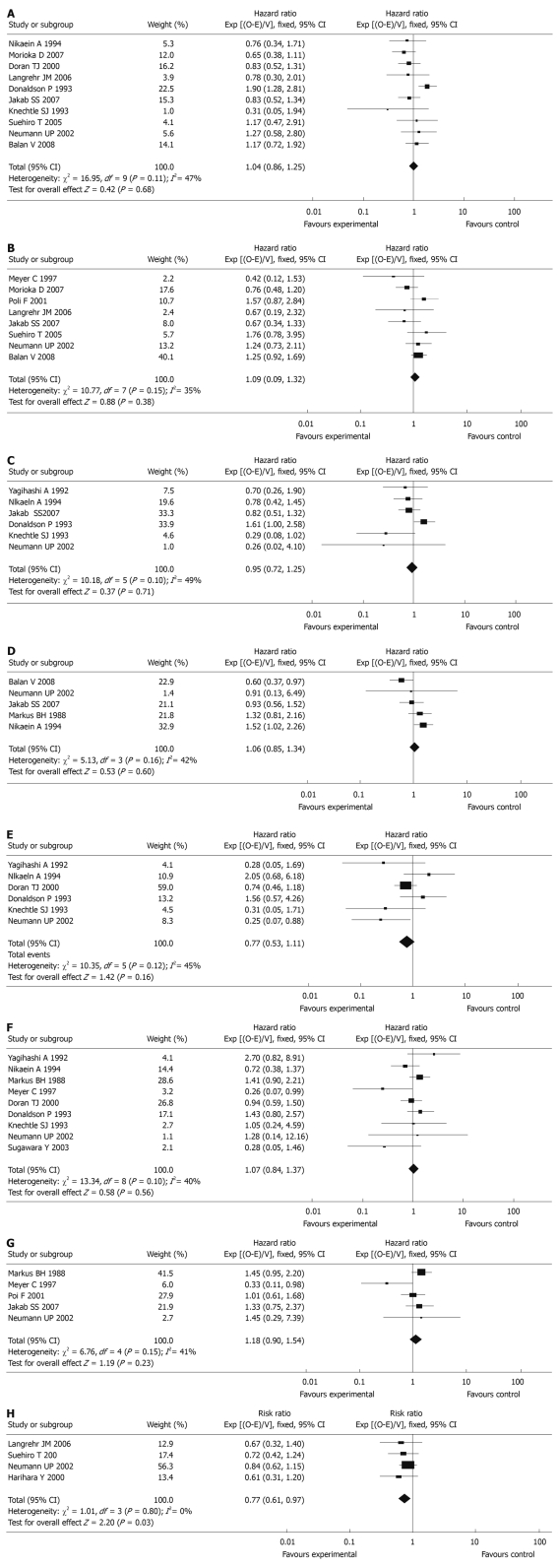
HLA-A, B and DR (0-2 mismatches vs 3-6 mismatches): In the studies included in the meta-analysis, a total of 4260 patients were included in 10 articles (1-year graft survival) and 3180 patients were included in 8 articles (5-year graft survival). No differences between 0-2 mismatches and 3-6 mismatches of HLA-A, B, and DR epitopes were seen in terms of 1-year graft survival [HR: 1.04, 95% confidence interval (CI): 0.86-1.25, P = 0.68] and 5-year graft survival (HR: 1.09, 95% CI: 0.90-1.32, P = 0.38, Figure 2).
Figure 2.
Meta-analysis of cohort trials comparing the effect of different mismatches of human leukocyte antigen epitopes on graft survival and acute rejection. A: 0-2 vs 3-6 mismatches of human leukocyte antigen (HLA)-A, B, DR epitopes on 1-year graft survival; B: 0-2 vs 3-6 mismatches of HLA-A, B, DR epitopes on 5-year graft survival; C: 0 vs 1-2 mismatches of HLA-A epitopes on 1-year graft survival; D: 0 vs 1-2 mismatches of HLA-A epitopes on 5-year graft survival; E: 0 vs 1-2 mismatches of HLA-B epitopes on 1-year graft survival; F: 0 vs 1-2 mismatches of HLA-DR epitopes on 1-year graft survival; G: 0 vs 1-2 mismatches of HLA-DR epitopes on 5-year graft survival; H: 0-2 vs 3-6 mismatches of HLA-A, B and DR epitopes on acute rejection.
HLA-A epitopes (0 mismatch vs 1-2 mismatches): Of the studies included in the meta-analysis, there were a total of 2049 patients in 6 articles (1-year graft survival) and 2138 patients in 5 articles (5-year graft survival). No differences between 0 mismatch and 1-2 mismatches of the HLA-A epitopes were seen in terms of 1-year graft survival (HR: 0.95, 95% CI: 0.72-1.25, P = 0.71) and 5-year graft survival (HR: 1.06, 95% CI: 0.85-1.34, P = 0.60, Figure 2).
HLA-B epitopes (0 mismatch vs 1-2 mismatches): A total of 1969 patients were included in 6 articles (1-year graft survival). No differences between 0 mismatch and 1-2 mismatches of the HLA-B epitopes were seen in terms of 1-year graft survival (HR: 0.77, 95% CI: 0.53-1.11, P = 0.16, Figure 2).
HLA-DR epitopes (0 mismatch vs 1-2 mismatches): A total of 2688 patients were included in 9 articles (1-year graft survival) and 2175 patients were included in 5 articles (5-year graft survival). No differences between 0 mismatch and 1-2 mismatches of the HLA-DR epitopes were seen in terms of 1-year graft survival (HR: 1.07, 95% CI: 0.84-1.37, P = 0.56) and 5-year graft survival (HR: 1.18, 95% CI: 0.90-1.54, P = 0.23, Figure 2).
HLA epitopes and acute rejection
A total of 1268 patients were included in 4 articles (acute rejection within 3 mo after transplantation). Significant differences between 0-2 mismatches and 3-6 mismatches of HLA-A, B and DR epitopes were seen in terms of acute rejection (RR: 0.77, 95% CI: 0.61-0.97, P = 0.03, Figure 2).
DISCUSSION
This is the first systematic review and meta-analysis on the effect of HLA mismatching in short and long term liver graft outcome and acute rejection. We identified and analyzed 16 unique cohort studies and all HLA locus-specific analyses were performed by standard lymphocytotoxicity tests with confirmation by polymerase chain reaction, with HLA-A, B and DR locus mismatches being compared. The results clearly showed that a lower number of HLA mismatches (0-2 vs 3-6) did reduce the incidence of acute rejection. The degree of HLA mismatching (0-2 vs 3-6) had no significant effect on 1-year and 5-year graft survival. Furthermore, we found no difference between 0 mismatches and 1-2 mismatches in 1-year and 5-year graft survival of HLA-A, HLA-B and HLA-DR on subgroup analysis.
The role of HLA matching between donor and recipient in liver transplant rejection and graft survival has been determined in some cohort studies and there still is no consensus view[7-15,31]. This systematic review analyzed the different data of various studies and has given our own results. However, the main objective in performing this analysis was to assess the necessity of donor-recipient HLA matching before liver transplantation.
As the role of HLA matching between donor and recipient in organ transplant rejection and survival had been proven to increase graft survival after kidney and heart transplantation, it has been debated whether these matches affected the outcomes of the liver graft similarly. In liver transplantation, organ allocation relies mostly on ABO blood group, recipients’ body weight, and clinical urgency, and the outcome of liver grafts relies mostly on complications after transplantation; HLA matching is usually not taken into account and the literature is inconsistent on the role of this parameter. In fact, any complications after transplantation are associated with graft outcome and rejection. Liver artery thrombosis, venous thromboembolic complications, seventh-day syndrome, primary graft non-function, and serious infection can decrease survival[32-36]. Compared to HLA mismatching, these complication are more important for long term graft survival.
Although the liver graft was considered to be a kind of immune-free organ, in our meta-analysis a lower number of HLA mismatches (0-2 vs 3-6) did reduce the incidence of acute rejection. It has become clear in recent years that mismatching of HLA in liver grafts led to endothelialitis induced by the recipient’s natural killer cells and so rejection was instigated. The association of acute rejection with 3 other risk factors (cold ischemia time greater than 15 h, pretransplantation elevation of aspartate transaminase, and older donor age) are less readily explained, but suggest that nonallogeneic and allogeneic immunological injury may be related. Both a long cold ischemia time and older donor age predispose an allograft liver to injury shortly after transplantation, which evokes immunological reactions that are not necessarily triggered by allogeneic differences.
Although a meta-analysis may provide a high level of scientific evidence, it is important to realize the limitations of interpreting results of meta-analyses. One major limitation to the meta-analysis is that inferences are based on aggregate analysis of relatively heterogeneous studies. We acknowledge the potential heterogeneity of combining studies from different centers in different geographic locations with different treatment protocols. In our systematic review, results obtained from each study were considered to be homogeneous (heterogeneity test was P > 0.10 and I2 < 50% in all available studies) in spite of there being no RCTs in this meta-analysis. Although we did not investigate through meta-regression any differences in the use of immunosuppressants or differences in study centers, the treatment protocols were nearly the same: cyclosporine or tacrolimus, azathioprine and prednisolone and no mycophenolate mofetil were used (Table 1).
Additionally, some studies did not report results with the measures that we chose for data extraction. It is the second limitation we must deal with. Survival rates under 1-year or 5-year were extracted by special software from survival curves if they was not shown in articles directly. We did not even obtain any data from some cohort studies, but including or excluding these articles also did not affect our conclusions.
The length of post transplantation follow-up was another limitation of many of the trials that we analyzed. Although most trials reported follow-up of some patients up to 5 years or even longer, some reported follow-up only to 1 year or 6 mo. Long-term graft survival, including HBV, HCV and hepatocellular carcinoma recurrence, may only become apparent or more pronounced after many years of post liver transplantation follow-up, and hence we may have underestimated the mortality in our study. In other words, we may have overestimated the role of HLA mismatching in liver graft loss.
Despite these limitations, our meta-analysis suggests that a lower number of HLA mismatching did reduce the incidence of acute rejection. The degree of HLA mismatching had no significant effect on 1-year and 5-year graft survival. Performing good donor-recipient HLA matching appears to be associated with a reduction in the incidence of acute rejection. Thus to obtain a shorter recovery time and avoid more rejection post transplantation, HLA matching examinations should be considered before surgery.
COMMENTS
Background
The role of human leukocyte antigen (HLA) matching between donor and recipient in organ transplant rejection and survival has been widely studied and proven to increase graft survival and to reduce the incidence of acute or chronic rejection. In contrast, major histocompatibility complex analysis is not routinely performed in liver transplantation because its importance remains controversial.
Research frontiers
Different groups have reported disparate results on the effect of HLA matching: some patients acquired benefit from high degrees of HLA matching but concern has been voiced about a greater likelihood of recurrence of primary disease with good HLA compatibility.
Innovations and breakthroughs
This is the first systematic review and meta-analysis on the effect of HLA mismatching in short and long term liver graft outcome and acute rejection. Importantly, the authors have some different conclusions compared to traditional views. Good donor-recipient HLA matching appears to be associated with a reduction in the incidence of acute rejection although there is no effect on 1-year and 5-year survival rates.
Applications
The percentage of graft survival was extracted by “Engauge Digitizer” from survival curves if the raw data was not presented. All statistical analyses were performed using Review Manager 5.0 which was a new program for determining HR.
Peer review
The authors aimed to assess the effect of HLA mismatching in liver graft outcome and acute rejection from available cohort studies by a systematic review and meta-analysis. The design of the study is rational and reliable, and the statistical methods used are appropriate. The article is also well organized. The conclusion may provide reliable and valuable information for clinical practice.
Footnotes
Peer reviewer: Tsung-Hui Hu, MD, PhD, Associate Professor, Director of Hepato-Gastroenterology, Department of Internal Medicine, Chang Gung Memorial Hospital, Kaohsiung Medical Center, 123, Ta-Pei Road, Niao-Sung Hsiang, Kaohsiung Hsien 833, Taiwan, China
S- Editor Wang YR L- Editor Cant MR E- Editor Ma WH
References
- 1.Opelz G. Success rate and impact of HLA matching on kidney graft survival in highly immunized recipients. Collaborative Transplant Study. Transpl Int. 1992;5 Suppl 1:S601–S603. doi: 10.1007/978-3-642-77423-2_176. [DOI] [PubMed] [Google Scholar]
- 2.Lee PC, Zhu L, Terasaki PI, Everly MJ. HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation. 2009;88:568–574. doi: 10.1097/TP.0b013e3181b11b72. [DOI] [PubMed] [Google Scholar]
- 3.Meng HL, Jin XB, Li XT, Wang HW, Lü JJ. Impact of human leukocyte antigen matching and recipients’ panel reactive antibodies on two-year outcome in presensitized renal allograft recipients. Chin Med J (Engl) 2009;122:420–426. [PubMed] [Google Scholar]
- 4.Kaczmarek I, Deutsch MA, Rohrer ME, Beiras-Fernandez A, Groetzner J, Daebritz S, Schmoeckel M, Spannagl M, Meiser B, Reichart B. HLA-DR matching improves survival after heart transplantation: is it time to change allocation policies? J Heart Lung Transplant. 2006;25:1057–1062. doi: 10.1016/j.healun.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Wong JY, Tait B, Levvey B, Griffiths A, Esmore DS, Snell GI, Williams TJ, Kotsimbos TC. Epstein-Barr virus primary mismatching and HLA matching: key risk factors for post lung transplant lymphoproliferative disease. Transplantation. 2004;78:205–210. doi: 10.1097/01.tp.0000128611.71038.0c. [DOI] [PubMed] [Google Scholar]
- 6.Taylor CJ, Smith SI, Sharples LD, Parameshwar J, Cary NR, Keogan M, Wallwork J, Large SR. Human leukocyte antigen compatibility in heart transplantation: evidence for a differential role of HLA matching on short- and medium-term patient survival. Transplantation. 1997;63:1346–1351. doi: 10.1097/00007890-199705150-00024. [DOI] [PubMed] [Google Scholar]
- 7.Balan V, Ruppert K, Demetris AJ, Ledneva T, Duquesnoy RJ, Detre KM, Wei YL, Rakela J, Schafer DF, Roberts JP, et al. Long-term outcome of human leukocyte antigen mismatching in liver transplantation: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Hepatology. 2008;48:878–888. doi: 10.1002/hep.22435. [DOI] [PubMed] [Google Scholar]
- 8.Neumann UP, Langrehr JM, Lang M, Schmitz V, Menzel S, Steinmueller T, Neuhaus P. Impact of HLA matching upon outcome after liver transplantation. Transplant Proc. 2002;34:1499–1500. doi: 10.1016/s0041-1345(02)02946-9. [DOI] [PubMed] [Google Scholar]
- 9.Meyer C, Parissiadis A, Compagnon P, Nisand G, Woehl-Jaegle ML, Ellero B, Herrera J, Boudjema K, Tongio MM, Jaeck D, et al. Effect of HLA compatibility on liver transplantation: is it a predictive factor of postoperative outcome? Transplant Proc. 1997;29:2332–2334. doi: 10.1016/s0041-1345(97)00389-8. [DOI] [PubMed] [Google Scholar]
- 10.Jakab SS, Navarro VJ, Colombe BW, Daskalakis C, Herrine SK, Rossi S. Human leukocyte antigen and adult living-donor liver transplantation outcomes: an analysis of the organ procurement and transplantation network database. Liver Transpl. 2007;13:1405–1413. doi: 10.1002/lt.21264. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Sugawara Y, Makuuchi M. Impact of human leukocyte antigen mismatching on outcomes of living donor liver transplantation for primary biliary cirrhosis. Liver Transpl. 2007;13:938–939. doi: 10.1002/lt.21118. [DOI] [PubMed] [Google Scholar]
- 12.Langrehr JM, Puhl G, Bahra M, Schmeding M, Spinelli A, Berg T, Schönemann C, Krenn V, Neuhaus P, Neumann UP. Influence of donor/recipient HLA-matching on outcome and recurrence of hepatitis C after liver transplantation. Liver Transpl. 2006;12:644–651. doi: 10.1002/lt.20648. [DOI] [PubMed] [Google Scholar]
- 13.Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, Hashimoto K, Mochida Y, Maehara Y, Kuwano H. Influence of HLA compatibility and lymphocyte cross-matching on acute cellular rejection following living donor adult liver transplantation. Liver Int. 2005;25:1182–1188. doi: 10.1111/j.1478-3231.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 14.Harihara Y, Makuuchi M, Kawasaki S, Hashikura Y, Kawarasaki H, Takayama T, Kubota K, Ito M, Mizuta K, Yoshino H, et al. Influence of HLA compatibility on living-related liver transplantation. Transplant Proc. 2000;32:2107. doi: 10.1016/s0041-1345(00)01590-6. [DOI] [PubMed] [Google Scholar]
- 15.Poli F, Frison S, Cardillo M, Scalamogna M, Longhi E, Crespiatico L, Porta E, Sirchia G. A retrospective analysis of HLA matching and other factors on liver graft outcome. Transplant Proc. 2001;33:1368–1369. doi: 10.1016/s0041-1345(00)02514-8. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara Y, Makuuchi M, Kaneko J, Saiura A, Imamura H, Kokudo N. Risk factors for acute rejection in living donor liver transplantation. Clin Transplant. 2003;17:347–352. doi: 10.1034/j.1399-0012.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 17.Knechtle SJ, Kalayolu M, D’Alessandro AM, Mason B, Pirsch JD, Sollinger HW, Steen DC, Belzer FO. Histocompatibility and liver transplantation. Surgery. 1993;114:667–671; discussion 671-672. [PubMed] [Google Scholar]
- 18.Yagihashi A, Kobayashi M, Noguchi K, Konno A, Yoshida Y, Terasawa K, Starzl TE, Iwaki Y. HLA matching effect in liver transplantation. Transplant Proc. 1992;24:2432–2433. [PMC free article] [PubMed] [Google Scholar]
- 19.Donaldson P, Underhill J, Doherty D, Hayllar K, Calne R, Tan KC, O’Grady J, Wight D, Portmann B, Williams R. Influence of human leukocyte antigen matching on liver allograft survival and rejection: “the dualistic effect”. Hepatology. 1993;17:1008–1015. [PubMed] [Google Scholar]
- 20.Markus BH, Duquesnoy RJ, Gordon RD, Fung JJ, Vanek M, Klintmalm G, Bryan C, Van Thiel D, Starzl TE. Histocompatibility and liver transplant outcome. Does HLA exert a dualistic effect? Transplantation. 1988;46:372–377. [PMC free article] [PubMed] [Google Scholar]
- 21.Nikaein A, Backman L, Jennings L, Levy MF, Goldstein R, Gonwa T, Stone MJ, Klintmalm G. HLA compatibility and liver transplant outcome. Improved patient survival by HLA and cross-matching. Transplantation. 1994;58:786–792. [PubMed] [Google Scholar]
- 22.Doran TJ, Geczy AF, Painter D, McCaughan G, Sheil AG, Süsal C, Opelz G. A large, single center investigation of the immunogenetic factors affecting liver transplantation. Transplantation. 2000;69:1491–1498. doi: 10.1097/00007890-200004150-00047. [DOI] [PubMed] [Google Scholar]
- 23.Bishara A, Brautbar C, Zamir G, Eid A, Safadi R. Impact of HLA-C and Bw epitopes disparity on liver transplantation outcome. Hum Immunol. 2005;66:1099–1105. doi: 10.1016/j.humimm.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Gubernatis G, Kemnitz J, Tusch G, Pichlmayr R. HLA compatibility and different features of liver allograft rejection. Transpl Int. 1988;1:155–160. doi: 10.1007/BF00348839. [DOI] [PubMed] [Google Scholar]
- 25.Ozawa M, Terasaki PI, Lee JH, Castro R, Alberu J, Alonso C, Alvarez I, Toledo R, Alvez H, Monterio M, et al. 14th International HLA and Immunogenetics Workshop: report on the Prospective Chronic Rejection Project. Tissue Antigens. 2007;69 Suppl 1:174–179. doi: 10.1111/j.1399-0039.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- 26.Chalmers TC, Smith H Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 27.Petitti DB. Meta-analysis, Decision Analysis, and Cost-effectiveness analysis. New York: Oxford University Press; 1994. [Google Scholar]
- 28.Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J, Lansing K, Bozorgzadeh A. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4:451–457. [PubMed] [Google Scholar]
- 29.Stahl JE, Kreke JE, Malek FA, Schaefer AJ, Vacanti J. Consequences of cold-ischemia time on primary nonfunction and patient and graft survival in liver transplantation: a meta-analysis. PLoS One. 2008;3:e2468. doi: 10.1371/journal.pone.0002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Moya-Quiles MR, Muro M, Torío A, Sánchez-Bueno F, Miras M, Marín L, García-Alonso AM, Parrilla P, Dausset J, Alvarez-López MR. Human leukocyte antigen-C in short- and long-term liver graft acceptance. Liver Transpl. 2003;9:218–227. doi: 10.1053/jlts.2003.50043. [DOI] [PubMed] [Google Scholar]
- 32.Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg. 2009;208:896–903; discussion 903-905. doi: 10.1016/j.jamcollsurg.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 33.Tao YF, Teng F, Wang ZX, Guo WY, Shi XM, Wang GH, Ding GS, Fu ZR. Liver transplant recipients with portal vein thrombosis: a single center retrospective study. Hepatobiliary Pancreat Dis Int. 2009;8:34–39. [PubMed] [Google Scholar]
- 34.Kyoden Y, Tamura S, Sugawara Y, Matsui Y, Togashi J, Kaneko J, Kokudo N, Makuuchi M. Portal vein complications after adult-to-adult living donor liver transplantation. Transpl Int. 2008;21:1136–1144. doi: 10.1111/j.1432-2277.2008.00752.x. [DOI] [PubMed] [Google Scholar]
- 35.Memon MA, Karademir S, Shen J, Koukoulis G, Fabrega F, Williams JW, Foster P. Seventh Day Syndrome--acute hepatocyte apoptosis associated with a unique syndrome of graft loss following liver transplantation. Liver. 2001;21:13–17. doi: 10.1034/j.1600-0676.2001.210102.x. [DOI] [PubMed] [Google Scholar]
- 36.Vertemati M, Sabatella G, Minola E, Gambacorta M, Goffredi M, Vizzotto L. Morphometric analysis of primary graft non-function in liver transplantation. Histopathology. 2005;46:451–459. doi: 10.1111/j.1365-2559.2005.02110.x. [DOI] [PubMed] [Google Scholar]