Abstract
Pluripotent stem cells have great clinical potential for tissue regeneration/repair in humans. The use of embryonic stem (ES) cells is ethically controversial, leading to searches for other sources of pluripotent stem cells. Testicular spermatogonial stem cells (SSCs) produce the spermatogenic lineage. Under in vitro conditions, SSCs have the ability to give rise to pluripotent ES-like cells. We hypothesized that stem/progenitor spermatogonia could directly transdifferentiate into different tissue types if they were recombined with inductive mesenchymes from fetal/neonatal organs using a tissue separation/recombination methodology and grown in vivo. Green fluorescent protein transgenic mice were used to track cell lineages. Our results indicate that stem/progenitor spermatogonia recombined with the appropriate mesenchyme can directly transdifferentiate in vivo into tissues of all germ layers, including prostatic, uterine, and skin epithelium. In addition, transdifferentiated tissue expressed molecular, histological, and functional markers of the appropriate epithelium. The ability of stem/progenitor spermatogonia to directly generate various epithelia emphasizes their clinical potential, and if adult human SSCs have similar properties, this may have applications in human regenerative medicine.
Keywords: Stem/progenitor spermatogonia, Pluripotency, Prostate, Skin, Uterus
Introduction
The potential of embryonic stem (ES) cells to be used clinically in humans for tissue repair/replacement has generated intense interest. Despite their scientific promise, the extensive ethical, moral, and legal problems associated with these cells are significant impediments to their clinical utility.
To avoid problems associated with ES cells, recent work has shown that normal somatic cells from mice [1, 2] or human fibroblasts [3, 4] can be reprogrammed by the use of viral vectors to induce ectopic expression of four transcription factors associated with pluripotency. These induced pluripotent stem (iPS) cells can differentiate into a wide variety of tissues in vivo and in vitro. However, there was a high incidence of neoplasia in mice receiving iPS cells when one of the transforming factors was c-Myc [2, 5]. Although this problem has been circumvented by the use of other transcription factors for transformation [5], the low efficiency of transformation and safety concerns associated with the use of virally transformed cells in vivo complicate their clinical applicability.
Stem cells derived from other organs could have therapeutic potential for human regenerative medicine and would avoid ethical and safety constraints associated with ES and iPS cells. Testicular spermatogonial stem cells (SSCs) are promising in this regard. SSCs are a self-renewing population of germ cells that are located along the basement membrane of seminiferous tubules in the testis. SSCs from neonatal mice cultured for approximately 2 months gave rise to some colonies with an ES-like appearance [6]. After isolation and expansion, these cells were capable of forming hematopoietic, glial, and vascular cells, neurons, and myocytes in vivo and in vitro [6, 7]. Subsequent work [8–10] reported that ES-like cells could be obtained following long-term cultures of juvenile and adult SSCs. These ES-like cells gave rise to endodermal, mesodermal, and ectodermal derivatives, including cardiomyocytes. Recently Conrad et al. [11] and Kossack et al. [12] demonstrated that ES-like cells can be obtained from adult human SSCs, indicating that human SSCs can give rise to ES-like cells that are pluripotent and comparable with mouse ES-like cells derived from SSCs in terms of developmental potential.
Despite the potential of SSCs to give rise to ES-like cells, present methodologies for obtaining these cells limit their clinical applicability. For example, extended culture periods prior to ES-like cell appearance are problematical, especially considering the additional time required to expand these ES-like cells sufficiently for clinical use. Another concern is the low frequency of occurrence of these cells, with only a few isolated ES-like cells appearing among thousands of SSCs in vitro. This makes obtaining ES-like cells inefficient and unpredictable, and exacerbates problems associated with the long latency for generating these cells.
Zwaka and Thomson [13] suggested that early germ cells are the closest in vivo equivalent to ES cells, and this may account for the pluripotency of cells derived from SSCs. However, so far there is no evidence that SSCs can directly transdifferentiate into other tissues, because all published reports have involved the derivation of ES-like cells from SSCs and the subsequent differentiation of these ES-like cells into a variety of tissues.
We and others have demonstrated the importance of the SSC niche, which is the local microenvironment that establishes the balance between self-renewal and differentiation of SSCs [14]. Extensive studies have also shown that the connective tissue is a major regulator of epithelial differentiation. Furthermore, there are numerous reports that epithelium from one tissue experimentally recombined with the mesenchyme of another tissue (or its adult derivative, stroma) is instructively induced to acquire specific morphological and functional characteristics by the host mesenchyme. For example, mesenchyme from the urogenital sinus (UGS), the fetal precursor of the prostate in males, can instructively induce adult bladder epithelium to differentiate into prostatic epithelium [15]. Similarly, neonatal uterine mesenchyme (UtM) can instructively induce neonatal vaginal epithelium to form uterine epithelium (UtE) [16]. Based on the importance of the stem cell niche for SSC differentiation, previous work showing the pluripotential nature of ES-like cells derived from SSCs, and the known ability of mesenchyme to instructively induce responsive epithelium, we hypothesized that stem/progenitor spermatogonia could directly transdifferentiate into a variety of other tissue types after being removed from their niche and recombined with inductive mesenchyme from various fetal/neonatal organs. To test this hypothesis, we chose a tissue separation/recombination methodology that has been extensively used to elucidate stromal–epithelial interactions in reproductive and nonreproductive organs [17]. We have used transgenic mice expressing green fluorescent protein (GFP) ubiquitously to track cell lineages in tissue recombinants and directly verify that stem/progenitor spermatogonia were undergoing transdifferentiation.
Our results demonstrate that stem/progenitor spermatogonia recombined with the appropriate fetal or neonatal mesenchyme can directly transdifferentiate into tissues derived from all germ layers, including prostatic, uterine, and skin epithelium. In addition, these epithelia expressed appropriate molecular, histological, and functional markers, indicating complete transdifferentiation. This methodology provides an approach to directly and specifically obtain a wide variety of organized tissues from stem/progenitor spermatogonia distinct from teratomas, and these results emphasize the potential clinical applications of these cells. If similar results can be obtained with adult human SSCs, this approach may lead to therapeutic methodologies with distinct advantages over both ES and iPS cells.
Materials and Methods
Stem/Progenitor Spermatogonia and Gonocyte Isolation
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Illinois. Stem/progenitor spermatogonia were isolated from 5- to 6-day-old neonates and gonocytes were isolated from day 16.5 fetal C57BL/6 mice by mechanical isolation, enzymatic digestion, and differential plating [18, 19]. Briefly, testes from 6-10 pups or 15-20 fetuses were decapsulated (postnatal testis only) and enzymatically digested initially with 1% collagenase followed by extensive washing with Dulbecco's modified Eagle's medium (DMEM)/F-12 using gravity sedimentation to obtain a fraction of interstitial and endothelial cells. Subsequently, the cells were digested with 1% collagenase and 1.5% hyalurodinase to obtain a suspension of Sertoli and germ cells. The cells were then trypsinized to obtain a single-cell suspension and plated on lectin-coated plates for 2 hours for neonatal or 30-45 minutes for fetal cells (differential plating) [20] to remove contaminating Sertoli or peritubular cells. The enriched fraction of stem/progenitor spermatogonia was counted and resuspended in DMEM/F-12 supplemented with 10% synthetic Nu-Serum (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com), 50 U/ml penicillin, and 50 μg/ml streptomycin. All reagents were purchased from Sigma (St. Louis, MO, http://www.sigmaaldrich.com) or from HyClone/Fisher Scientific, (Pittsburgh, PA, http://www.hyclone.com). To track cell lineages derived from stem/progenitor spermatogonia and gonocytes in tissue recombinations and directly verify that these cells were undergoing transdifferentiation, transgenic C57BL/6 mice (C57BL/6-Tg(ACTB-EGFP)1Osb/J) expressing an enhanced GFP under the control of a chicken β-actin promoter and cytomegalovirus enhancer (Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) were used. The purity of enriched gonocytes and stem/progenitor spermatogonia was assessed by staining for germ cell nuclear antigen one (GCNA1). The enriched germ cell population from 5- to 6-day-old mice constituted Asingle, Apaired, and a few Aaligned spermatogonia. Thus the germ cell isolations contained SSCs and also stem progenitors that were capable of reverting to SSCs [21]. The interstitial, endothelial, and Sertoli cell fractions obtained with this separation procedure from neonatal testis were used for tissue recombination as negative controls.
Tissue Recombination
Timed pregnant C57BL/6 mice were sacrificed to obtain fetuses that were used as a source of UGS at day 16.5 and skin at day 14.5 of gestation. Neonatal uteri were obtained from 5-day-old females. As with the germ cells, both wild-type (wt) and GFP donors were used to facilitate tracing of cell lineages. The UGS was incubated with a 1% trypsin solution [22] to digest the basement membrane between the epithelium and mesenchyme, and the UGS was then separated into its connective tissue component (UGM) and epithelial component (UGE). Mesenchyme and epithelium from skin and uterus were also separated using trypsinization. Mesenchyme from all organs was recombined with homologous epithelium as a control, whereas tissue recombinants of various mesenchymes and stem/progenitor spermatogonia were prepared by placing the mesenchyme in a 1.5-ml Eppendorf tube, adding stem/progenitor spermatogonia, and then centrifuging at 500g for 1 minute. Approximately 3 × 104 stem/progenitor spermatogonia were used for each tissue recombinant. For tissue recombinations of UGM and stem/progenitor spermatogonia, one tissue recombinant represented 1 UGM from a 16.5-day fetus recombined with approximately 3 × 104 stem/progenitor spermatogonia/gonocytes. For uterine and skin tissue recombinations, uterine and skin mesenchymes of approximately 5 mm in size were recombined with 3 × 104 stem/progenitor spermatogonia. The resultant tissue recombinants, along with control homologous tissue recombinations, were placed on nutrient 1% agar plates and incubated overnight at 37° C and 5% CO2 [23]. Tissue recombinants prepared with GFP+ germ cells were examined under fluorescent light prior to grafting to ensure that gonocytes or stem/progenitor spermatogonia were present.
To establish whether cells giving rise to prostatic epithelium in tissue recombinations were stem/progenitor spermatogonia or other cell contaminants (interstitial, endothelial, peritubular cells, etc.) present in the enriched stem/progenitor spermatogonia preparations, tissue recombinants were prepared with UGM and Sertoli cells or UGM and a combination of endothelial and peritubular cell fractions from neonatal testes. UGM alone was grafted as a negative control. Previous literature has indicated that UGM grafted alone forms a fibromuscular stroma with no formation of prostatic epithelium [24, 25].
All tissue recombinations were grafted and grown for 4 weeks under the renal capsule of adult syngeneic hosts (male hosts for tissue recombinants with UGM, female hosts for those with UtM, male and female hosts for those containing skin mesenchyme) [23, 26]. The GFP+ stem/progenitor spermatogonia plus wt mesenchyme tissue recombinants were always grafted into wt hosts, and wt stem/progenitor spermatogonia plus GFP+ mesenchyme tissue recombinants were always grafted into GFP hosts to ensure that there would be no cells from the host that could be mistakenly identified as transdifferentiating spermatogonia. Some recovered grafts were frozen in liquid N2 for subsequent quantitative polymerase chain reaction (qPCR) analysis, whereas others were fixed in neutral-buffered formalin for immunohistochemistry or immunofluorescence. GFP expression was always confirmed in the recovered grafts to ensure that the transdifferentiated epithelium was of stem/progenitor spermatogonia or gonocyte origin before the grafts were subjected to subsequent analyses.
The mRNA expression levels of prostate secretory proteins, probasin, spermine-binding protein (SBP), and transglutaminase were determined in the tissue recombinants in response to testosterone. For this, hosts with tissue recombinants of UGM plus stem/progenitor spermatogonia or UGM alone were castrated after 4 weeks of the grafting period. After 3 weeks of castration, they were treated with testosterone (120 ng/0.1 ml corn oil) or vehicle (0.1 ml corn oil) for 4 days. The recovered grafts were frozen in liquid N2 for subsequent RNA extraction and real-time qPCR analysis.
To determine the potential of transdifferentiated UtE to respond to its main mitogenic hormone, 17β-estradiol (E2), some female hosts bearing tissue recombinants of UtM plus UtE or UtM plus stem/progenitor spermatogonia were ovariectomized and 2 weeks later were given 125 ng of E2 in 0.1 ml of oil or vehicle alone. Epithelial proliferation was then assessed 16 hours later, as described elsewhere [23]. Expression of lactoferrin and progesterone receptor (PR) was assessed in tissue recombinants in which the hosts were treated for 3 days with 125 ng of E2 in 0.1 ml of oil or vehicle alone and harvested 24 hours later, as described elsewhere [27, 28]. Host uterus was also used as a positive control.
Immunohistochemistry, Immunofluorescence, and Photomicrographs
Grafts were fixed in 10% neutral-buffered formalin, dehydrated through graded alcohols, and embedded in paraffin. Grafts were sectioned at 4 μm, deparaffinized, and rehydrated. To facilitate antigen detection, slides were placed in boiling 10 mM sodium citrate buffer (pH 6.0) for 10 minutes and then cooled to ambient temperature. Endogenous peroxidase activity was quenched by incubation with 0.3% H2O2 for 20 minutes. The primary antibodies used for immunohistochemistry were anti-mouse Ki67 (BD Biosciences) and anti-rabbit PR (Dako, Carpinteria, CA, http://www.dako.com), estrogen receptor (ER)α (Novocastra Ltd., Newcastle upon Tyne, U.K., http://www.novocastra.co.uk), and lactoferrin (Sigma). A biotinylated secondary antibody was used for Ki67 and horseradish peroxidase-conjugated antibodies were used for PR, ERα, and lactoferrin.
For immunofluorescence, all the procedures followed for immunohistochemistry, except peroxidase quenching, were performed. The primary antibodies used were anti-rabbit GFP (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), androgen receptor (AR) (gift from Gail Prins, University of Illinois–Chicago), pancytokeratin (Dako), NKX3.1 (Chemicon, Temecula, CA, http://www.chemicon.com), anti-rat GCNA1 (gift from George Enders, University of Kansas Medical Center), and cytokeratin 8 (Developmental Studies Hybridoma Bank, University of Iowa, http://dshb.biology.uiowa.edu/). The secondary antibodies used were fluorescein isothiocyanate (FITC)- and tetramethylr-hodamine isothiocyanate-conjugated anti-rat and anti-rabbit antibodies. Appropriate negative controls were run in parallel. Slides were mounted with Vectashield that contained 4′,6-diamidino-2-phenylindole (H-1200; Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) and photomicrographs were taken with an Olympus BX51 fluorescence microscope (Olympus Corp., Melville, NY, http://www.olympus-global.com) using bright-field illumination or appropriate fluorescence excitation and emission filters. For GCNA1 immunostaining in Figure 2G, a FITC-tagged secondary antibody was used and the positive cells were pseudocolored.
Figure 2.
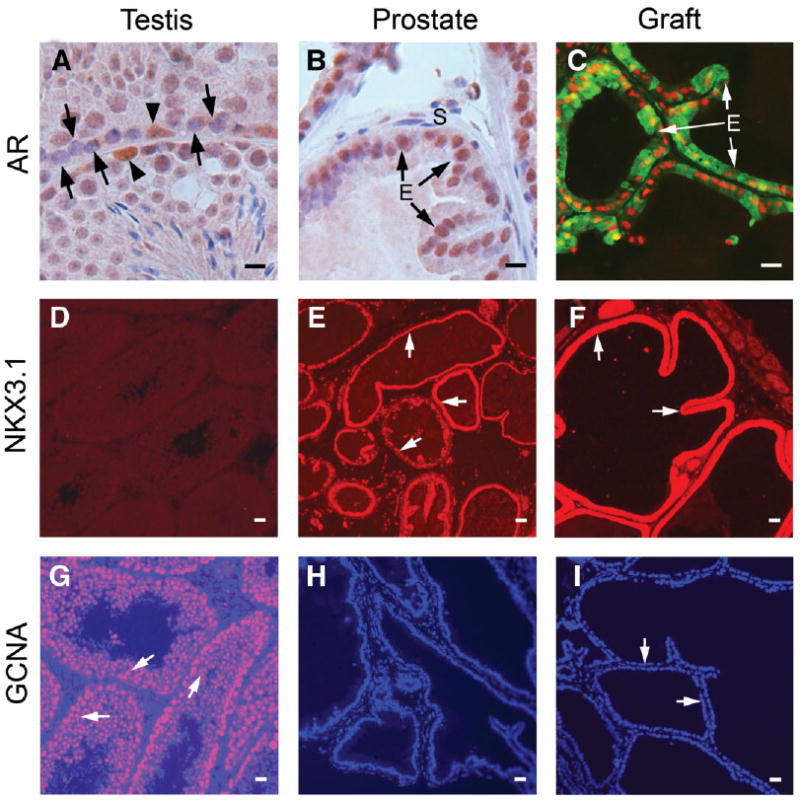
Transdifferentiated epithelium expresses molecular and functional markers of prostate tissue. Shown are immunohistochemistry of markers for prostatic epithelium—including AR and the prostatic transcription factor NKX3.1—and germ cells, GCNA1. (A): Sertoli cells (arrowheads) of the testis stain for AR, but spermatogonia (arrows) are negative. (B): Nuclei of normal prostatic epithelial cells are AR+. (C): Nuclei of the transdifferentiated epithelia in tissue recombinants at 4 weeks strongly express AR. The epithelia also express GFP, confirming transdifferentiation of GFP+ stem/progenitor spermatogonia. (D): Testis is negative for NKX3.1. (E): Normal prostatic epithelium shows strong staining for NKX3.1 (arrows). (F): Transdifferentiated epithelium (arrows) at 4 weeks from wt UGM plus GFP+ stem/progenitor spermatogonia tissue recombination shows intense immunofluorescence for NKX3.1. (G): Germ cells of the testis stain intensely for GCNA1 (arrows). (H): Prostatic epithelial cells lack GCNA1 expression. (I): Transdifferentiated epithelial cells (arrows) at 4 weeks from wt UGM plus GFP+ stem/progenitor spermatogonia tissue recombination also do not express GCNA1, indicating that transdifferentiation of stem/progenitor spermatogonia is accompanied by a total loss of germ cell phenotype. Scale bars = 10 μm. Abbreviations: AR, androgen receptor; E, epithelial cells; GCNA1, germ cell nuclear antigen 1; GFP, green fluorescent protein; UGM, urogenital sinus mesenchyme; wt, wild-type.
Real-Time PCR
RNA was extracted from the grafts using the RNeasy mini kit according to the manufacturer's instructions (Qiagen, Valencia, CA, http://www1.qiagen.com). Samples were treated with DNase to prevent DNA contamination. First-strand cDNA was synthesized from total RNA (0.5 μg) using Superscript reverse transcriptase III and random primers (Invitrogen). The expression value of each gene was normalized to the amount of an internal control gene (18S rRNA) cDNA to calculate a relative amount of RNA in each sample. The expression value of each gene in vehicle-treated controls of UGM plus stem/progenitor spermatogonia was arbitrarily defined as 1 unit. Real-time PCR assays were carried out in triplicate and the normalized expression values for all samples were averaged. A relative quantitative fold change was determined using the δδ Ct method (ABI Chemistry Guide #4330019; www.genomics.bham.ac.uk/Documents/4348358%20Real-time%20PCR%20Chemistry%20Guide%20RevA.pdf). ABI Taqman gene expression assays used for specific transcripts were: Mm00444381_m1 (probasin), Mm004488101_m1 (SBP), Mm00626039_m1 (transglutaminase 4), and Hs99999901_s1 (18S).
Statistical Analysis
Six or more replicates were obtained for all the tissue recombinations. Each replicate represented at least two tissue recombinants transplanted under the renal capsule of a host.
For mRNA expression analysis, three replicates of each of the tissue recombinants were obtained from both the testosterone-treated and vehicle-treated groups. Data were analyzed by oneway analysis of variance followed by Tukey–Kramer's multiple comparison tests. Statistical analyses were performed using Graph Pad Prism 4.0 (Graph Pad Software, Inc., San Diego, CA, http://www.graphpad.com). Data are expressed as the mean ± standard error of the mean, and differences between groups were considered significant at p < .05.
Results
Gonocyte and stem/progenitor spermatogonia populations from fetal and neonatal testes were highly enriched in germ cells. Approximately 70% of the cells stained positive for GCNA1, a marker specific for the germ cell lineage. An average of 5 × 105 stem/progenitor spermatogonia or gonocytes was obtained from each isolation. In tissue recombinants prepared with GFP+ stem/progenitor spermatogonia, there were substantial numbers of stem/progenitor spermatogonia in a homogeneous distribution across the tissue recombinants (Fig. 1A, 1B).
Figure 1.
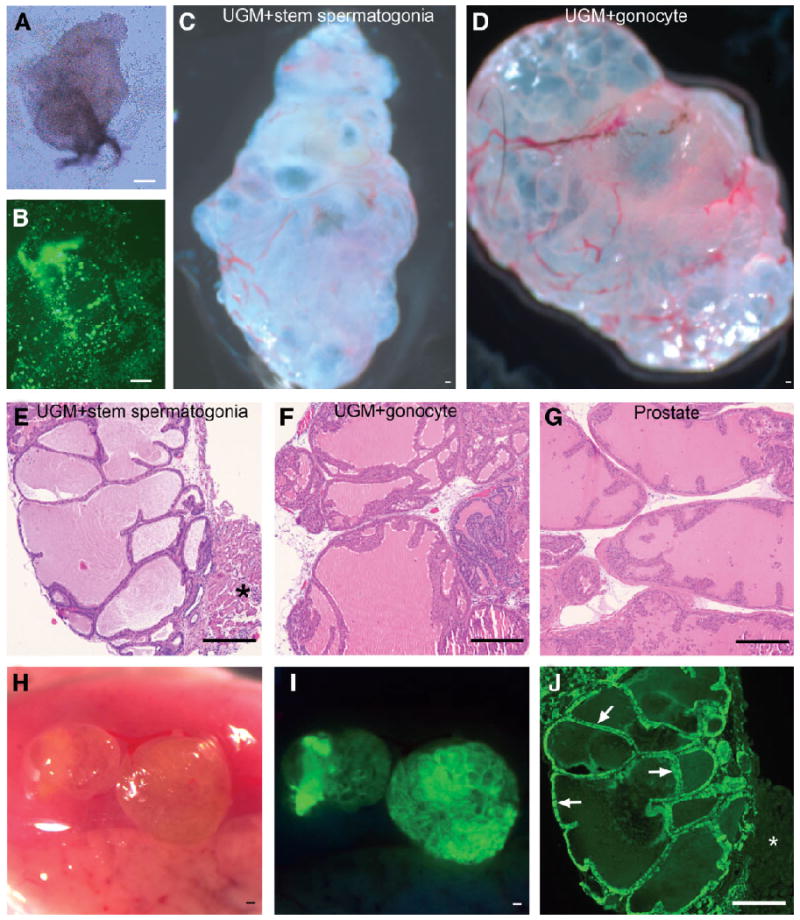
Transdifferentiation of stem/progenitor spermatogonia and gonocytes into prostatic epithelium. UGM from day 16.5 fetuses was recombined with stem/progenitor spermatogonia or gonocytes and grafted under the renal capsule of syngeneic male hosts. After 4 weeks of growth, the tissue recombinants formed prostatic epithelium that was of stem/progenitor spermatogonia or gonocyte origin. (A): Tissue recombinant of wt UGM plus GFP+ stem/progenitor spermatogonia after overnight incubation in agar plates. Approximately 3 × 104 stem/progenitor spermatogonia were placed on each UGM. (B): UV illumination of the tissue recombinant in (A) showing substantial numbers of GFP+ stem/progenitor spermatogonia and a homogeneous distribution over the wt UGM. (C): wt UGM plus stem/progenitor spermatogonia, whole-mount tissue recombinant at 4 weeks. (D): wt UGM plus gonocyte, whole-mount tissue recombinant at 4 weeks. Extensive vascularization was observed in recovered grafts, as well as ductal structures and secretions in both stem/progenitor spermatogonia and gonocyte recombinations (C, D). (E): wt UGM plus GFP+ stem/progenitor spermatogonia tissue recombinant histology at 4 weeks showing branched acini, lined with columnar epithelium that have characteristic folds, and containing luminal secretions, similar to normal prostate (G). *Renal tissue of host. (F): wt UGM plus gonocyte; tissue recombinant structure at 4 weeks is similar to that in (E) and (G). (G): Normal juvenile prostate. (H): wt UGM plus GFP+ stem/progenitor spermatogonia whole-mount tissue recombinant at 4 weeks, viewed with visible light under the renal capsule. (I): UV illumination of the tissue recombinant in (H) showing that the green fluorescence of the newly formed prostate tissue originated from GFP+ stem/progenitor spermatogonia. (J): Histological section of the wt UGM plus GFP+ stem/progenitor spermatogonia tissue recombinant shown in (I). Immunofluorescence showed the epithelium to be strongly GFP+ (arrows), confirming that GFP+ stem/progenitor spermatogonia transdifferentiated into prostatic epithelium. *Renal tissue of the host is GFP−. Scale bars in (A–J) = 100 μm. Abbreviations: GFP, green fluorescent protein; UGM, urogenital sinus mesenchyme; wt, wild-type.
Transdifferentiation of Stem/Progenitor Spermatogonia and Gonocytes into an Endodermal Derivative, Prostatic Epithelium
The UGM plus stem/progenitor spermatogonia or UGM plus gonocyte tissue recombinations showed striking growth over the 4-week in vivo grafting period. At recovery, extensive vascularization and an internal ductal structure were visible in whole mounts of both types of tissue recombinations (Fig. 1C, 1D).
UGM plus stem/progenitor spermatogonia or UGM plus gonocyte tissue recombinations formed prostatic tissue that was histologically identical to that in control UGM plus UGE tissue recombinants or prostate from juvenile (day 30) mice (Fig. 1E–1G). Grafts recovered from wt UGM plus GFP+ stem/progenitor spermatogonia tissue recombinants fluoresced green under UV illumination (Fig. 1H, 1I).
The wt UGM plus GFP+ stem/progenitor spermatogonia tissue recombinants had an epithelium that stained intensely for GFP (Fig. 1J), indicating that it was of stem/progenitor spermatogonia origin, whereas stromal cells lacked GFP staining. Similar results were obtained with wt UGM plus GFP+ gonocyte tissue recombinations (not shown).
Spermatogonia in normal testis lacked AR or the prostatic transcription factor NKX3.1, but epithelium of wt UGM plus GFP+ stem/progenitor spermatogonia tissue recombinants stained intensely for both of these, similar to normal prostate (Fig. 2A–2F). Thus, transdifferentiation of stem/progenitor spermatogonia into prostatic epithelium is accompanied by expression of characteristic prostatic epithelial proteins. Conversely, epithelium of wt UGM plus GFP+ stem/progenitor spermatogonia or normal prostate did not stain for GCNA1, whereas testicular germ cells stained intensely for GCNA1 (Fig. 2G–2I), indicating that transdifferentiation of stem/progenitor spermatogonia into prostatic epithelium was accompanied by loss of germ cell characteristics as well as acquisition of characteristics of prostatic epithelium. UGM plus UGE tissue recombinants serving as controls expressed prostate-specific proteins and lacked GCNA1 expression, similar to UGM plus stem/progenitor spermatogonia tissue recombinants. Tissue recombinants of UGM plus gonocytes also expressed AR and NKX3.1 and did not express GCNA1 (not shown).
Testosterone treatment induced 20- to 35-fold higher levels of mRNA for prostatic secretory proteins such as probasin, SBP, and transglutaminase in UGM plus stem/progenitor spermatogonia tissue recombinants, compared with levels in those from vehicle-treated hosts (Fig. 3); conversely, the expression level of mRNA for all these proteins was very low (tenfold lower) in both testosterone- and vehicle-treated tissue recombinants with UGM alone. mRNA levels in the testosterone- and vehicle-treated tissue recombinants with UGM alone were expressed relative to the vehicle-treated controls of UGM plus stem/progenitor spermatogonia tissue recombinants.
Figure 3.
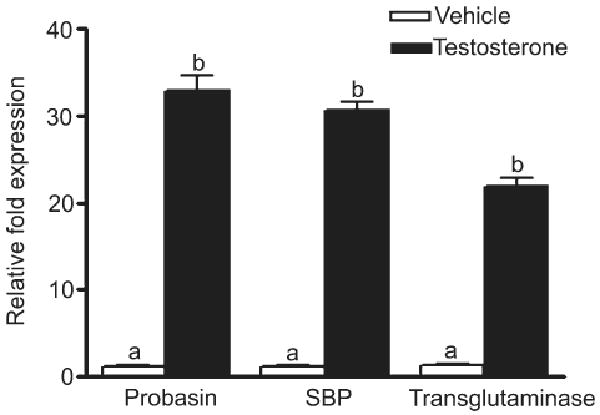
mRNA levels of prostate secretory proteins in tissue recombinants. After 4 weeks, hosts with UGM plus stem/progenitor spermatogonia or UGM alone tissue recombinants were castrated and treated with testosterone or vehicle for 4 days. The relative mRNA expression levels from the graft tissue of UGM plus stem/progenitor spermatogonia show significant increases for probasin, SBP, and transglutaminase in response to testosterone. Tissue recombinants with UGM alone had very low levels (tenfold lower) of all three secretory proteins both in the testosterone- and vehicle-treated groups. Results are expressed as the mean ± standard error of the mean. Values with different superscripts are significantly different (p < .001). Abbreviations: SBP, spermine-binding protein; UGM, urogenital sinus mesenchyme.
Approximately 70% of the UGM plus stem/progenitor spermatogonia tissue recombinants that were retrieved after the 4-week growth period formed histologically identifiable prostatic epithelium (Table 1). In contrast, none of the tissue recombinants consisting of UGM recombined with a mixture of peritubular and interstitial cells formed histologically identifiable prostatic epithelium, and only one tissue recombinant of UGM plus an enriched Sertoli cell fraction (n = 12) formed prostate. All grafts that contained UGM alone formed fibromuscular stroma devoid of epithelium (not shown).
Table 1.
Efficiency of stem/progenitor spermatogonia to transdifferentiate into prostatic epithelium
| Tissue recombinants | n of tissue recombinant grafts retrieved | n of grafts differentiated into prostatic epithelium | Percentage of tissue recombinants differentiated |
|---|---|---|---|
| UGM plus stem/progenitor spermatogonia | 74 | 53 | 71 |
| UGM plus interstitial, peritubular cells | 12 | 0 | 0 |
| UGM plus Sertoli cells | 12 | 1 | 8 |
Abbreviation: UGM, urogenital sinus mesenchyme.
Transdifferentiation of Stem/Progenitor Spermatogonia into a Mesodermal Derivative of Female Origin, UtE
Tissue recombinants of GFP+ UtM plus wt stem/progenitor spermatogonia contained simple columnar epithelium similar to normal murine UtE in vivo (Fig. 4A, 4B). Neither uterus nor tissue recombinants of UtE plus stem/progenitor spermatogonia expressed the germ cell marker GCNA1 (Fig. 4C, 4D). Tissue recombinants of GFP+ UtM plus wt stem/progenitor spermatogonia expressed cytokeratin 8, a marker of uterine epithelial cells, similar to murine UtE (Fig. 4E, 4F). The stromal cells strongly expressed GFP, whereas epithelium did not, indicating that the epithelium was of wt stem/progenitor spermatogonia origin (Fig. 4G, 4H). UtM plus stem/progenitor spermatogonia tissue recombinants had strong epithelial and stromal ERα staining when treated with E2, compared with vehicle-treated controls (Fig. 4I, 4J). E2 induced robust epithelial proliferation in UtM plus stem/progenitor spermatogonia tissue recombinants, whereas proliferative activity was minimal in these tissues in hosts given vehicle (Fig. 4K, 4L). E2 reduced PR expression in epithelium but induced it in stroma of UtM plus stem/progenitor spermatogonia tissue recombinants, which is a unique feature of mouse UtE. PR expression was low in the epithelium and stroma of vehicle-treated controls (Fig. 4M, 4N). UtM plus stem/progenitor spermatogonia tissue recombinants expressed high levels of epithelial lactoferrin in response to E2, whereas lactoferrin production was minimal in all tissue recombinants given only vehicle (Fig. 4O, 4P); host uteri showed epithelial proliferation and lactoferrin production that was essentially identical to that seen in the tissue recombinants.
Figure 4.
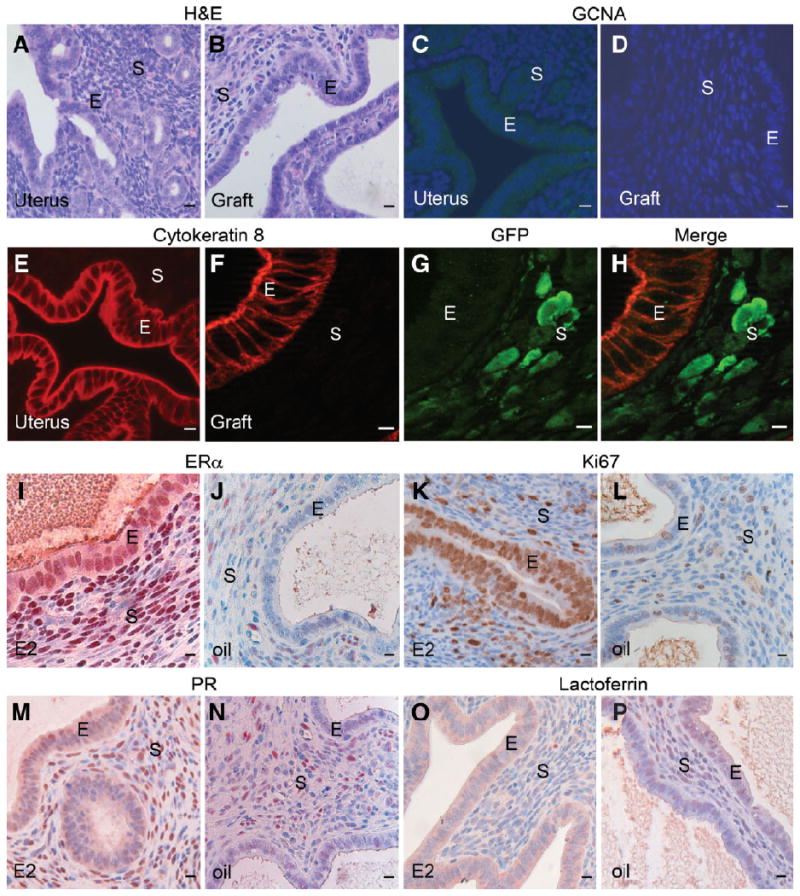
Transdifferentiation of stem/progenitor spermatogonia into UtE. Neonatal UtM was recombined with stem/progenitor spermatogonia and grown in vivo. After 4 weeks of growth, the tissue recombinants formed UtE of stem/progenitor spermatogonia origin. (A): Normal uterus showing epithelial and stromal histology. (B): GFP+ UtM plus wt stem/progenitor spermatogonia recombinant tissue showing uterine columnar epithelium and stroma, similar to (A). (C): Uterine cells do not express GCNA1; nuclei stained for DAPI. (D): Tissue recombinants with UtM plus stem/progenitor spermatogonia do not express GCNA1; nuclei stained for DAPI. (E): Normal murine uterus showing expression of cytokeratin 8 in the cytoplasm of epithelial cells. (F): GFP+ UtM plus wt stem/progenitor spermatogonia tissue recombinant showing immunofluorescent staining for cytokeratin 8 in the epithelium. (G): The same section as in (F) stained for GFP expresses the protein only in the stroma. (H): Merged image of (F) and (G) showing expression of GFP in the stroma and cytokeratin 8 in the epithelium, indicating that UtE in the tissue recombinants originated from stem/progenitor spermatogonia. (I): E2- treated GFP+ UtM plus wt stem/progenitor spermatogonia tissue recombinant expressed nuclear ERα in both the epithelium and stroma. (J): Vehicle-treated tissue recombinant has very low levels of ERα. (K): E2-treated GFP+ UtM plus wt stem/progenitor spermatogonia tissue recombinant showing immunohistochemical staining for Ki67, a proliferation marker that reveals an E2-induced epithelial proliferative response. (L): There is minimal proliferation in vehicle-treated controls. (M): E2-treated GFP+ UtM plus wt stem/progenitor spermatogonia tissue recombinant showing immunohistochemical staining for PR. E2 treatment resulted in lower expression of PR in the epithelium and greater expression in the stroma. (N): There was minimal expression of PR in vehicle-treated controls. (O): E2-treated GFP+ UtM plus wt stem/progenitor spermatogonia tissue recombinant showing immunohistochemical staining for lactoferrin (arrows), a uterine secretory protein that is typically induced by E2 treatment. (P): Vehicle-treated GFP+ UtM plus wt stem/progenitor spermatogonia tissue recombinant showing lack of immunohistochemical staining for lactoferrin. Scale bars = 10 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; E, epithelium; E2, estrogen; ERα, estrogen receptor α; GCNA1, germ cell nuclear antigen 1; GFP, green fluorescent protein; PR, progesterone receptor; S, stroma; UtE, uterine epithelium; UtM, uterine mesenchyme; wt, wild-type.
Transdifferentiation of Stem/Progenitor Spermatogonia into Nonreproductive Tissue of the Ectodermal Germ Layer
Tissue recombinants of dermis plus stem/progenitor spermatogonia produced a stratified squamous epithelium typical of epidermis and comparable with that seen in the positive control of skin tissue recombinants. Hair follicles and sebaceous glands were seen in dermis plus stem/progenitor spermatogonia tissue recombinations, similar to the positive controls (Fig. 5A, 5B). The GFP+ dermis plus wt stem/progenitor spermatogonia tissue recombinants expressed GFP in the dermis but not in the newly formed epidermis (Fig. 5C). Normal skin or dermis plus stem/progenitor spermatogonia did not express GCNA1 (Fig. 5D, 5E). The epidermis and epithelium surrounding hair follicles did not express GFP, indicating that the transdifferentiated epithelium was of wt stem/progenitor spermatogonia origin and expressed pancytokeratin, an epidermal marker (Fig. 5F–5I).
Figure 5.
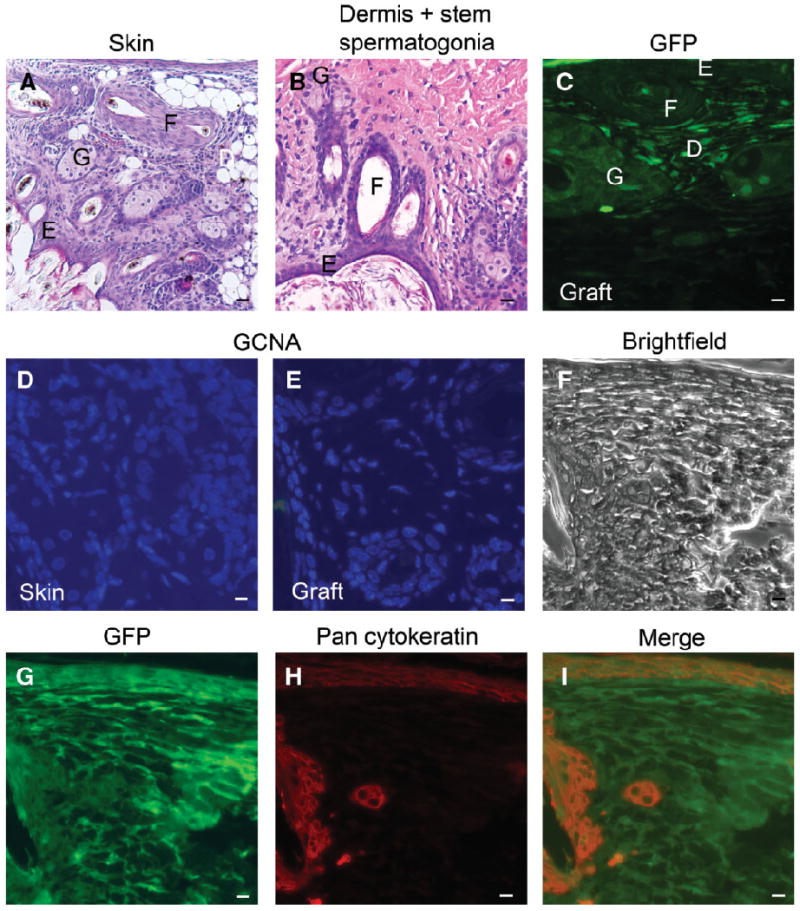
Transdifferentiation of stem/progenitor spermatogonia into skin, an ectodermal derivative. The dermis of skin from GFP+ day 14.5 fetuses was recombined with wt stem/progenitor spermatogonia and grown in vivo for 4 weeks. The tissue recombinants formed epidermis of stem/progenitor spermatogonia origin. (A): Histology of dermis plus epidermis tissue recombinants showing stratified squamous epithelium with hair follicles, and sebaceous glands. (B): Tissue recombinant of dermis plus stem/progenitor spermatogonia contained epithelium typical of normal skin, with hair follicles and sebaceous glands. (C): Tissue recombinant stained for GFP by immunofluorescence showed that dermal tissue expressed GFP whereas epithelium, hair follicles, and sebaceous glands are negative. (D): Normal skin does not express GCNA1; nuclei stained for DAPI. (E): Tissue recombinant of dermis plus stem/progenitor spermatogonia did not express GCNA1, similar to normal skin; nuclei stained for DAPI. (F): Bright-field image of tissue recombinant of GFP+ dermis plus wt stem/progenitor spermatogonia showing epithelium, hair follicles, sebaceous glands, and dermal tissue. (G): GFP immunostaining of the section in (F) showing expression in the dermis. (H): Pancytokeratin immunostaining of the tissue recombinant shows expression in epithelium, hair follicles, and sebaceous glands. (I): Merged image of (G) and (H) indicating the origin of epidermal cells from wt stem/progenitor spermatogonia. Scale bars = 10 μm. Abbreviations: D, dermal tissue; DAPI, 4′,6-diamidino-2-phenylindole; E, epithelium; F, hair follicles; G, sebaceous glands; GCNA1, germ cell nuclear antigen 1; GFP, green fluorescent protein; wt, wild-type.
Discussion
Our results indicate that stem/progenitor spermatogonia can directly transdifferentiate into other reproductive and nonreproductive epithelia when associated with various inductive mesenchymes in vivo. This is the first demonstration that freshly isolated stem/progenitor spermatogonia can transdifferentiate into tissues of all three germ layers when placed in an appropriate inductive environment. In addition, stem/progenitor spermatogonia can form organs and tissues that are morphologically normal and express appropriate functional markers.
Previous work demonstrating pluripotency of cells derived from neonatal and adult SSCs required specific in vitro culture conditions for up to several months to obtain ES-like cells and additional subculturing for expansion [6, 8, 10]. Those cells, which differed dramatically from the original SSCs, were induced to differentiate into various tissues in vivo and in vitro. The extended culture period needed to obtain and expand the ES-like cells from SSCs, along with the attendant costs and labor, will hinder clinical use of these cells. The present data indicate that extended culture of SSCs in order to produce, identify, and expand an ES-like population is unnecessary, and that freshly isolated stem/progenitor spermatogonia are themselves able to transdifferentiate when removed from their niche and placed in contact with an inductive mesenchyme.
Our results indicate that stem/progenitor spermatogonia and gonocytes are capable of transdifferentiating into an endodermal derivative, prostatic epithelium. In addition, freshly isolated stem/progenitor spermatogonia can give rise to an ectodermal derivative, epidermis, as well as a mesodermal derivative, UtE. Thus, freshly isolated stem/progenitor spermatogonia, like ES and iPS cells, can directly give rise to cell lineages characteristic of all the three germ layers.
The stem/progenitor spermatogonia and gonocytes that transdifferentiated into prostatic epithelium showed histological, molecular, and functional markers corresponding to normal prostatic epithelium, along with an apparent loss of unique spermatogonial characteristics, indicating that transdifferentiation of stem/progenitor spermatogonia into a new tissue under the influence of an inductive mesenchyme was complete. Similarly, stem/progenitor spermatogonia recombined with UtM produced transdifferentiation, as shown by the typical simple columnar uterine epithelial phenotype, expression of cytokeratin 8, ERα, and uterine epithelial secretory proteins, proliferative response to E2, the major mitogen for UtE, and expression of PR in uterine stroma, but not epithelium, in response to E2. The ability of stem/progenitor spermatogonia to transdifferentiate into keratinocytes and form epidermis with hair follicles further demonstrates the ability of stem/progenitor spermatogonia to fully transdifferentiate and manifest all unique characteristics of the induced tissue. In the case of skin, there is extensive scientific and clinical interest in inducing ES cells to produce epithelium of the skin [29]. Our present results show that stem/progenitor spermatogonia may be of equal utility for clinical applications related to using stem cells to regenerate skin epithelium in vivo and in vitro.
Recent work [30] has shown that when cells isolated from adult mouse seminiferous tubules were mixed with mammary epithelial cells and transplanted into cleared mammary fat pads, the mammary ductal structures that resulted after several weeks of growth contained some epithelial cells derived from the seminiferous tubules. However, the testis cells alone, without the presence of added mammary epithelial cells, were unable to differentiate into mammary ductal epithelial cells. Thus, although these earlier results also emphasize the plasticity of SSCs, these studies did not demonstrate full transdifferentiation or the ability of SSCs to generate an epithelium de novo, as we observed in the present study.
Approximately 70% of the UGM plus stem/progenitor spermatogonia tissue recombinants produced prostate, whereas tissue recombinants of UGM plus peritubular and interstitial cells never produced prostate, and prostate was obtained in only one of the 12 UGM plus Sertoli cell grafts. These findings suggest that stem/progenitor spermatogonia alone may be capable of undergoing prostatic epithelial transdifferentiation, because tissue recombinants prepared with peritubular cells and an interstitial fraction containing endothelial and vascular smooth muscle cells, various immune cells, as well as potentially bloodborne stem cells did not form prostate. Although one UGM plus Sertoli cell graft formed prostate, it is most likely a result of stem/progenitor spermatogonia contamination in the Sertoli cell fraction used for tissue recombination, because Sertoli cells are separated from stem/progenitor spermatogonia by differential plating and manual isolation. In any case, the formation of prostate in a single UGM plus Sertoli tissue recombination contrasts starkly with the high rate of prostatic transdifferentiation seen when stem/progenitor spermatogonia were used.
The mechanism of mesenchyme-induced transdifferentiation of stem/progenitor spermatogonia remains largely unknown. Future experiments using this system will allow us to dissect the molecular mechanisms that are involved in transdifferentiation of spermatogonia as well as the signals from the inductive mesenchyme that induce transdifferentiation. The signals involved could be complex and involve paracrine and circulating growth factors and extracellular matrix components.
All results presented here were obtained with neonatal stem/progenitor spermatogonia and gonocytes. Thus, a crucial question is whether adult SSCs are as responsive to transdifferentiation stimuli as the neonatal stem/progenitor spermatogonia and fetal gonocytes used here. Adult mouse [8, 10] and human [11, 12] SSCs have been shown to give rise to ES-like cells following extended culture in vitro. In addition, these multipotent adult germline stem cells express the microRNA clusters 290 and 302 that are ES-cell specific, suggesting the pluripotent nature of adult SSCs [31]. This suggests that adult mouse or human SSCs, like the neonatal mouse SSCs or gonocytes used here, may have the ability to directly differentiate into various tissues when placed in vivo in an appropriate inductive environment, but this remains to be definitively established.
An important question that remains unresolved by the present results is how the stem/progenitor spermatogonia transdifferentiate into other epithelia. Either they can arise from normal SSCs that can dedifferentiate to pluripotency and subsequently differentiate into other tissues or there could be a subpopulation of pluripotent SSCs, distinct from the SSCs committed to the germline, that transdifferentiate when exposed to the inductive influence of a novel microenvironment. Results showing dedifferentiation of a single SSC into ES-like cells and subsequent differentiation into cells of different germ layers [32] argue for the former. The latter concept is supported by recent work of Izadyar et al. [33] demonstrating that only a subpopulation of SSCs that are POU5F1+/c-Kit+ are pluripotent, whereas SSCs that are POU5F1+/c-Kit− give rise to only the spermatogenic lineage. Further work is necessary to resolve the origin and identity of the SSC population that can undergo transdifferentiation.
Acknowledgments
The authors thank Dr. Gerald R. Cunha for constructive suggestions on the manuscript, the Veterinary Diagnostic Histopathology Laboratory, University of Illinois for pancytokeratin staining, and Jon Ekman, Beckman Institute, University of Illinois, for cytokeratin 8, AR, and GFP microphotographs.
This work was supported by the Billie A. Field Endowment, University of Illinois (P.S.C.) and NIH grant R01-HD044543 (M.C.H.). Work at the University of Illinois was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR16515 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Disclosure of potential conflicts of interest is found at the end of this article.
Disclosure of Potential Conflicts of Interest: The authors indicate no potential conflicts of interest.
Author contributions: L.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; G.E.: administrative support, collection and/or assembly of data; N.K.: collection and/or assembly of data; Z.Z.: collection and/or assembly of data; R.A.H.: conception and design, manuscript writing; M.-C.H.: conception and design, financial support, manuscript writing, final approval of manuscript; P.S.C.: conception and design, financial support, administrative support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- 1.Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 6.Kanatsu-Shinohara M, Inoue K, Lee J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Baba S, Heike T, Umeda K, et al. Generation of cardiac and endothelial cells from neonatal mouse testis-derived multipotent germline stem cells. Stem Cells. 2007;25:1375–1383. doi: 10.1634/stemcells.2006-0574. [DOI] [PubMed] [Google Scholar]
- 8.Guan K, Nayernia K, Maier LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 9.Guan K, Wagner S, Unsöld B, et al. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–1625. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]
- 10.Seandel M, James D, Shmelkov SV, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrad S, Renninger M, Hennenlotter J, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 12.Kossack N, Meneses J, Shefi S, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Ouyang W, Grigura V, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha GR, Lung B, Reese B. Glandular epithelial induction by embryonic mesenchyme in adult bladder epithelium of BALB/c mice. Invest Urol. 1980;17:302–304. [PubMed] [Google Scholar]
- 16.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Dev Biol. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- 17.Cunha GR, Bigsby RM, Cooke PS, et al. Stromal-epithelial interactions in adult organs. Cell Differ. 1985;17:137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- 18.Dym M, Jia MC, Dirami G, et al. Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biol Reprod. 1995;52:8–19. doi: 10.1095/biolreprod52.1.8. [DOI] [PubMed] [Google Scholar]
- 19.He Z, Jiang J, Kokkinaki M, et al. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpino S, Morena AR, Petersen C, et al. A rapid method of Sertoli cell isolation by DSA lectin, allowing mitotic analyses. Mol Cell Endocrinol. 1998;146:121–127. doi: 10.1016/s0303-7207(98)00190-7. [DOI] [PubMed] [Google Scholar]
- 21.Barroca V, Lassalle B, Coureuil M, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 22.Bigsby RM, Cooke PS, Cunha GR. A simple efficient method for separating murine uterine epithelial and mesenchymal cells. Am J Physiol. 1986;251:E630–E636. doi: 10.1152/ajpendo.1986.251.5.E630. [DOI] [PubMed] [Google Scholar]
- 23.Cooke PS, Buchanan DL, Young P, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boutin EL, Battle E, Cunha GR. The response of female urogenital tract epithelia to mesenchymal inductors is restricted by the germ layer origin of the epithelium: Prostatic inductions. Differentiation. 1991;48:99–105. doi: 10.1111/j.1432-0436.1991.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi N, Cunha GR, Wong YC. Influence of male genital tract mesenchymes on differentiation of Dunning prostatic adenocarcinoma. Cancer Res. 1990;50:4747–4754. [PubMed] [Google Scholar]
- 26.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 27.Kurita T, Lee KJ, Cooke PS, et al. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod. 2000;62:831–838. doi: 10.1095/biolreprod62.4.831. [DOI] [PubMed] [Google Scholar]
- 28.Kurita T, Lee KJ, Cooke PS, et al. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- 29.Aberdam D. Derivation of keratinocyte progenitor cells and skin formation from embryonic stem cells. Int J Dev Biol. 2004;48:203–206. doi: 10.1387/ijdb.15272386. [DOI] [PubMed] [Google Scholar]
- 30.Boulanger CA, Mack DL, Booth BW, et al. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zovoilis A, Nolte J, Drusenheimer N, et al. Multipotent adult germline stem cells and embryonic stem cells have similar microRNA profiles. Mol Hum Reprod. 2008;14:521–529. doi: 10.1093/molehr/gan044. [DOI] [PubMed] [Google Scholar]
- 32.Kanatsu-Shinohara M, Lee J, Inoue K, et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78:681–687. doi: 10.1095/biolreprod.107.066068. [DOI] [PubMed] [Google Scholar]
- 33.Izadyar F, Pau F, Marh J, et al. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. 2008;135:771–784. doi: 10.1530/REP-07-0479. [DOI] [PubMed] [Google Scholar]


