Summary
Osteoporosis and arthritis are highly prevalent diseases and a significant cause of morbidity and mortality worldwide. These diseases result from aberrant tissue remodeling leading to weak, fracture-prone bones or painful, dysfunctional joints. The nuclear factor of activated T cells (NFAT) transcription factor family controls diverse biologic processes in vertebrates. Here, we review the scientific evidence that links NFAT-regulated gene transcription to bone and joint pathology. A particular emphasis is placed on the role of NFATs in bone resorption and formation by osteoclasts and osteoblasts, respectively. In addition, emerging data that connect NFATs with cartilage biology, angiogenesis, nociception, and neurogenic inflammation are explored. The goal of this article is to highlight the importance of tissue remodeling in musculoskeletal disease and situate NFAT-driven cellular responses within this context to inspire future research endeavors.
Keywords: NFAT, osteoporosis, osteoarthritis, rheumatoid arthritis, osteoclast, osteoblast
Introduction
In 2000, the United Nations and World Health Organization endorsed the first 10 years of the 21st century as the Bone and Joint Decade (1). This action was taken in response to the growing worldwide burden of musculoskeletal conditions, such as osteoporosis (OP), osteoarthritis (OA), and rheumatoid arthritis (RA). These highly prevalent diseases lead to morbidity and mortality, reduce quality of life, and are a leading cause of work disability. As populations around the world age, these diseases are likely to further impact already heavily taxed health care systems (2). From a pathophysiologic standpoint, these disorders display a common theme of aberrant tissue remodeling that ultimately results in bone or joint failure.
A unique feature of the vertebrate body plan is a rigid endoskeleton of bone, a remarkable biomaterial composed of collagen fibrils and inorganic calcium phosphate crystals. The vertebrate skeleton serves numerous functions. These include protecting the viscera and central nervous system, providing a niche for hematopoietic cell development, acting as a mineral reservoir, and, most conspicuously, providing a structural frame for gross locomotion and fine motor skills. Bone is a dynamic tissue that continuously remodels to preserve structural integrity. Bone-forming osteoblasts and bone-resorbing osteoclasts control this remodeling process (3, 4). An imbalance between these two cell types, favoring resorption over formation, results in a negative bone balance and a weak, fracture-prone, osteoporotic skeleton (5, 6).
To facilitate movement, bones articulate at specialized structures termed diarthrodial joints composed of a cap of smooth, low friction, articular cartilage on the bone surface, and a lining that bridges the joint, called the synovium. Articular cartilage is rich in both type II collagen and proteoglycans. While collagen provides much of the tensile strength of cartilage, proteoglycans avidly retain water, giving articular cartilage the ability to deform and rebound in response to impact stress like a turgid gel (7). The synovium is composed of a lining layer of fibroblast-like and macrophage-like synoviocytes, connective tissue and a capillary and lymphatic network (8). Disease processes like OA and RA disturb the architecture of the articular cartilage, synovium, and periarticular bone leading to pain and disability and ultimately total joint failure (9–11). Here, we focus on the emerging, multifaceted role of the nuclear factor of activated T cells (NFAT) transcription factor (TF) family in common diseases of bone and joints.
The calcineurin/NFAT transcriptional axis
NFATs are a family of inducible transcriptional regulators identified in T cells over 20 years ago (12). This gene family consists of five members designated NFATc1 through NFATc4 and NFAT5. Calcium signaling pathways regulate NFATc1 through NFATc4, whereas NFAT5 is activated by osmotic stress. Adding to the complexity of this system, NFAT family members may have more than one isoform. For example, there are three protein isoforms of NFATc1: A, B, and C, which are driven from two different promoters and may have different biologic activities (13). NFATs contain an N-terminal regulatory domain as well as a DNA-binding motif with homology to the Rel domain of NF-κB (14). In unstimulated cells, NFAT proteins are localized to the cytoplasm by hyperphosphorylation of the N-terminal regulatory domain. Signaling pathways that promote a sustained influx of calcium activate the phosphatase calcineurin, which dephosphorylates the regulatory domain and exposes a nuclear localization sequence (reviewed in 13). Thus, calcineurin inhibitors, like cyclosporine A (CsA) and FK506, have been used for the last 2 decades to probe this pathway. In the nucleus, NFAT proteins cooperate with other TFs, like activator protein-1 (AP-1) family members, to regulate gene transcription (15). Shown in Fig. 1 are genes regulated by NFATs in various cell types discussed in this review. While both the regulatory and DNA-binding domains of NFAT are evolutionarily ancient, the genetic condensation of these domains did not occur until the emergence of vertebrates. Thus, it has been proposed that NFATs are poised to control developmental patterns specific to vertebrate physiology (proposed and reviewed in 16, 17). Indeed, studies have linked NFATs to an array of physiologic processes in vertebrate systems, including the central nervous system, vasculature, heart, skeletal muscle, immune system, integument, gut, lung, bone, and cartilage (17–19). The capacity to partner with other tissue or cell-specific TFs likely underlies the ability of NFATs to regulate these seemingly unrelated organ systems (17). Emerging evidence suggests that NFATs play a central role in pathologic processes in vertebrate systems as well. In this review, the role of NFATs is explored within the context of diseases affecting the quintessential components of the vertebrate body plan: the bone and joints.
Overview of pathologic bone and joint remodeling
The pathophysiology of OP, RA, and OA has been reviewed extensively elsewhere (6, 8, 10, 20). In this section, a brief overview of cellular and molecular physiology of each disease is provided to facilitate an understanding of the role of NFAT in these conditions.
OP pathogenesis
OP afflicts 10 million Americans over the age of 50 with fragility fractures occurring in approximately 1.5 million individuals per year (21). Age-related and post-menopausal bone loss are the most prevalent forms of primary OP and are influenced by genetic and acquired risk factors (6, 22). Chronic inflammatory conditions, endocrinologic abnormalities, or medications result in secondary OP (5, 23). Regardless of the initiating events, low bone mass ultimately results from an imbalance between bone-forming osteoblasts and bone-resorbing osteoclasts, leading to a relative increase in bone catabolism.
Osteoblasts are derived from multipotent mesenchymal progenitors that also give rise to myocytes, adipocytes, and chondrocytes. Direction of these progenitors toward the osteoblast lineage is largely guided by TFs such as Runx2 and Osterix. Mutations in mice or humans in the genes encoding these TFs lead to reduced numbers of osteoblasts and OP (24–26). At sites of new bone formation, osteoblasts secrete osteoid, an unmineralized precursor of bone composed primarily of type I collagen. Osteoblasts also release vesicles containing molecules such as annexin and alkaline phosphatase, which promote the eventual mineralization of osteoid to form mature bone (27). Osteoclasts are bone-resorbing multinucleated giant cells that differentiate from myeloid precursors in response to macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) (28). While M-CSF promotes growth and survival of pre-osteoclasts, RANKL initiates the signaling and transcriptional networks leading to osteoclast differentiation. After differentiation, osteoclasts adhere to the bone surface where they secrete acid and proteolytic enzymes to degrade the inorganic and organic constituents of bone, respectively (29). Loss-of-function mutations in mice and humans in the pathways that promote either osteoclast differentiation or their capability to resorb bone lead to a high bone mass state termed osteopetrosis (30). This can be caused by mutations in genes encoding (i) M-CSF, RANKL, or either of their receptors, (ii) components of RANK signaling pathway, (iii) TFs that guide osteoclast gene expression, or (iv) the bone degradation machinery (30–40). A soluble decoy receptor for RANKL, called osteoprotegerin (OPG), negatively regulates osteoclast differentiation in vivo (41). Interestingly, bone remodeling by osteoblasts and osteoclasts is an interdependent process. For example, osteoblasts are thought to be the physiologically relevant source of M-CSF, RANKL, and OPG in bone and thus regulate osteoclast differentiation. Likewise, soluble growth factors released from degraded bone as well as factors secreted by or presented on the cell surface of osteoclasts are thought to promote osteoblastogenesis (42). Thus, under physiologic circumstances, an osteoclast-mediated resorption cycle is almost always followed by new bone formation by osteoblasts (43). As discussed below, the calcineurin/NFAT axis plays a critical role in both osteoclasts and osteoblasts and genetic or pharmacologic disruption of this pathway leads to quantitative changes in bone mass.
RA pathogenesis
RA is a chronic, inflammatory, autoimmune condition that predominantly affects peripheral diarthrodial joints (reviewed in 44). RA is more common in women and thought to arise in genetically predisposed individuals exposed to inciting environmental stimuli. The inflammatory process in the synovium is driven by pro-inflammatory cytokines, like tumor necrosis factor α (TNFα). As the synovium becomes inflamed, it fills with T and B cells as well as innate immune cells like macrophages, dendritic cells, and mast cells. Moreover, the normally thin synovial lining proliferates and undergoes intense angiogenesis leading to hyperplasia of the synovium into a structure termed the pannus. Ultimately, unrestrained inflammation leads to destruction of the articular cartilage and erosion of periarticular bone. The pannus crawls over the articular cartilage and promotes its degradation by mechanisms that include direct effects of inflammatory cytokines on chondrocytes, as well as the production of degradative enzymes by synoviocytes (8). Osteoclasts are thought to mediate bone erosions in RA, since these bone-resorbing cells can be found at the sites where pannus tissue interfaces with periarticular bone (45). Moreover, while mice deficient in osteoclasts can develop inflamed joints, they are protected from bone erosions (46). Two features distinguish bone resorption at sites of inflammation from physiologic bone remodeling. First, the source of RANKL in the rheumatoid joint is not osteoblasts but rather T lymphocytes and fibroblast-like synoviocytes (FLS) within the inflamed synovium (47). Second, a cycle of periarticular bone resorption in RA patients is not followed by bone formation by osteoblasts (43). This `uncoupling' may be due to local production of mediators, like the Wnt inhibitor Dkk-1, which block osteoblastogenesis (48).
The importance of the calcineurin/NFAT pathway in RA is highlighted by the clinical efficacy of calcineurin inhibitors, like CsA and FK506, in these patients (49). The untoward side effect profile of these medications however impedes their widespread use, making a better understanding of this pathway in RA an important goal. Interestingly, one of their major side effects of calcineurin inhibitors, excessive hair growth, is a result of inhibition of NFATc1 activity in hair follicle stem cells, where this TF blocks cell proliferation (18). Evidence suggests that the calcineurin/NFAT pathway is crucial to a number of pathophysiologic processes in RA including inflammation, angiogenesis, osteoclast formation, cartilage catabolism, and pain sensation.
OA pathogenesis
OA is the most common joint disease with a majority of individuals over the age of 65 demonstrating radiographic or clinical manifestations (50). Patients with OA experience pain, stiffness and functional impairment of the affected joint, often culminating in the need for total arthroplasty. The cause of this disease is unresolved but likely involves genetic and biomechanical factors as well as age-related biochemical changes in joint tissues. The pathogenesis of OA has been reviewed in detail elsewhere (9, 10). Briefly, adult articular cartilage is composed of relatively small number of chondrocytes (2–5% per volume) interspersed within a unique matrix composed of type II and other collagens, a proteoglycan called aggregan, and hyaluronic acid. Articular cartilage is avascular and hypoxic, and thus articular chondrocytes display a low rate of turnover and synthetic function. During the OA process, activation of catabolic enzymes including matrix metalloproteinases (MMPs) and aggrecanases leads to softening of the articular cartilage. A low-grade inflammatory response ensues, characterized by the cytokines TNFα and interleukin-1 (IL-1), which likely further drives catabolic enzyme expression. Lastly, poorly characterized signals promote increased turnover of subchondral and periarticular bone leading to a net increase in bone formation, which can be radiographically appreciated as subchondral sclerosis and bony periarticular outgrowths called osteophytes. Although data on the role of the calcineurin/NFAT pathway in cartilage biology and osteoarthritis is limited, the literature suggests that this pathway may play a central role in chondrocyte proliferation and differentiation as well as in mitigating the production of catabolic enzymes.
Calcineurin/NFATs and inflammatory arthritis
The most direct evidence for the importance of the calcineurin/NFAT pathway in inflammatory arthritis comes from the utility of calcineurin inhibitors, like CsA, in treating patients with this disease (reviewed in 49, 51). The importance of NFATs in mediating T-cell biology, a key cellular element in the pathogenesis of inflammatory arthritis (52), has also been extensively reviewed elsewhere (53) and is not be addressed here. In this section, we cover those publications that have directly investigated the role of calcineurin and/or NFAT in experimental systems of RA.
Calcineurin is expressed at higher levels in synovial biopsies obtained from RA patients compared with OA patients (54). The expression pattern is broad, with synoviocytes, inflammatory cells, and endothelial cells all demonstrating immunoreactivity. Inhibition of calcineurin in RA FLS, with either CsA or a peptide inhibitor, blocks spontaneous production of TNFα (55) and attenuates the production of IL-6 or MMPs after stimulation with pro-inflammatory cytokines, like IL-1β (54). Treatment of FLS or synovial fluid mononuclear cells from RA patients with CsA also results in spontaneous synthesis of the anti-inflammatory cytokine IL-10 (54, 55). These data suggest that calcineurin, perhaps via NFAT, can augment pro-inflammatory and suppress anti-inflammatory pathways in the inflamed joint.
Direct evidence that NFAT proteins play an important role in the pathogenesis of RA or mouse models of arthritis is limited. NFAT5 as well as all four calcium-regulated NFATs (NFATc1–c4) are expressed in the synovium of patients with RA (51, 56). Which NFAT family members are expressed by which cell types in the inflamed joint has not been reported. Only one study has reported an arthritis model in NFAT-deficient mice (57). In wildtype mice, transfer of anti-glucose 6-phosphate isomerase antibodies in K/B×N serum induces a symmetric polyarthritis that is independent of adaptive immune cells but dependent on innate immune cells, such as mast cells and neutrophils (58–61). In contrast, when NFATc2 or NFATc2/c3 doubly deficient mice are injected with K/B×N arthritogenic serum, an asymmetric oligoarthritis ensues, affecting only one fore or hind limb per animal (57). Two additional observations were made in this study. First, the joints that developed arthritis in NFAT-deficient mice had as severe or even more severe inflammation and damage compared with those of wildtype mice. Second, injection of fivefold higher doses of arthritogenic serum into NFAT-deficient mice still resulted in an asymmetric oligoarthritis. These observations suggest that the pattern of arthritis in NFAT-deficient mice is not the result of an attenuated dose response to the K/B×N serum. Given that inflammation in this model is lymphocyte independent, these findings also support a relatively novel role for NFATs in innate immunity. Future work will better define (i) the upstream pathways that activate calcium signaling and NFAT activation during the initiation of joint inflammation, (ii) the specific innate immune cells in which NFAT activation is most important in arthritis, (iii) whether other NFAT family members (i.e. NFATc1 or NFATc4) regulate the symmetry and intensity of joint inflammation, and (iv) the transcriptional targets of NFAT in leukocytes and synoviocytes in the inflamed joint.
NFATc1: master regulator of osteoclast development and bone resorption
Osteoclasts are bone-resorbing multinucleated cells responsible for pathologic bone loss in conditions such as OP and RA (28). In this section, we review data generated in the last seven years that has revealed a pivotal role for NFATc1 in osteoclast differentiation in health and disease
Induction and activation of NFATc1 during osteoclastogenesis
In 2002, Takayanagi et al. (62) initially identified a role for NFATc1 in osteoclastogenesis by gene expression profiling of osteoclast precursors stimulated with the osteoclastogenic cytokine RANKL. Expression of NFATc1 is low in osteoclast precursors, but following RANKL stimulation, expression is dramatically induced. The induction of NFATc1 expression and its subsequent activation by calcineurin-mediated dephosphorylation require multiple signaling pathways (reviewed in 63). First, the NF-κB and c-Fos pathways downstream of RANK promote NFATc1 expression. Second, costimulatory receptors that engage immunoreceptor tyrosine-based activation motif (ITAM)-bearing adapter molecules, such as Fc receptor common γ subunit (FcRγ) and DNAX-activating protein 12 (DAP12), activate spleen tyrosine kinase (SYK). An interesting recent publication identified the tyrosine kinases TEC and Bruton's tyrosine kinase (BTK) as a nodal point in osteoclast differentiation, integrating signals downstream of RANK and the costimulatory receptors to activate phosholipase C and promote calcium influx (64). Calcium influx in turn activates the phosphatase calcineurin, which forms a complex with NFATc1 and dephosphorylates its regulatory domain to expose a nuclear localization sequence, resulting in the translocation of active NFATc1 into the nucleus (63). The activation of NFATc1 during osteoclastogenesis drives further expression of NFATc1 via an autoregulatory mechanism (65) and promotes the expression of genes needed for bone resorption (reviewed below). Loss of calcineurin activity by either pharmacologic inhibition or genetic ablation suppresses osteoclast formation in mouse as well as human osteoclast precursors (62, 66–68). Conversely, transduction of a constitutively active form of calcineurin promotes the expression of NFATc1 target genes in osteoclasts (67). Signaling mechanisms independent of calcium influx and calcineurin may also exist to activate NFATc1 during osteoclastogenesis. The inositol 1,4,5-trisphosphate receptors (IP3Rs) promote calcium release from intracellular stores in the endoplasmic reticulum. A study by Kuroda et al. (69) has shown that IP3R2 is expressed in osteoclasts and is critical for calcium influx and osteoclast differentiation induced by recombinant RANKL. In contrast, when osteoblasts are used as a source of RANKL, NFATc1 is activated in osteoclasts in manner independent of IP3R2, calcium influx, and calcineurin. Taken together, these data emphasize how multiple signaling pathways that drive osteoclastogenesis converge on NFATc1 to promote the expression and activation of this master transcriptional regulator of bone resorption.
NFATc1: master regulator of osteoclastogenesis in vivo
In the seminal description of the role of NFATc1 in osteoclastogenesis, Takayanagi et al. (62) found that embryonic stem cells lacking NFATc1 were incapable of differentiating into osteoclasts in vitro. Since NFATc1-deficient mice die at E13.5 because of cardiac valve defects (70), the function of NFATc1 in osteoclasts in vivo was not interrogated in this study. Three separate approaches have since been employed to tackle this important question. First, Asagiri et al. (65) showed that NFATc1-deficient stem cells could not rescue the severe osteopetrotic phenotype of c-Fos knockout mice in adoptive transfer and blastocyst complementation experiments. Second, Winslow et al. (71) rescued the lethal phenotype of NFATc1 knockout mice by intracardiac expression of NFATc1 and showed that this strain displays dramatic osteopetrosis at birth. However, these mice die in the perinatal period, making an analysis of the role of NFATc1 in the mature skeleton or in pathologic models of bone loss impossible. To circumvent this issue and examine the role of NFATc1 in the skeleton of growing and adult mice, our laboratory generated a conditional knockout of NFATc1 using a Cre-loxP strategy and deleted NFATc1 in mice at 10 days of age using Mx1-Cre (19). NFATc1-deleted mice develop severe osteoclast-poor osteopetrosis characterized by increased bone mass, short, club-shaped long bones and growth plate dysplasia due to failure to degrade primary spongiosa resulting in accumulation of calcified cartilage. Furthermore, NFATc1-deficient primary osteoclast precursors failed to form osteoclasts in vitro in response to RANKL or in a co-culture system with wildtype osteoblasts (19). Taken together, these three studies confirm the role of NFATc1 in osteoclastogenesis and bone resorption during growth and development.
The role of NFATc1 in inflammation induced bone loss: cherubism studies
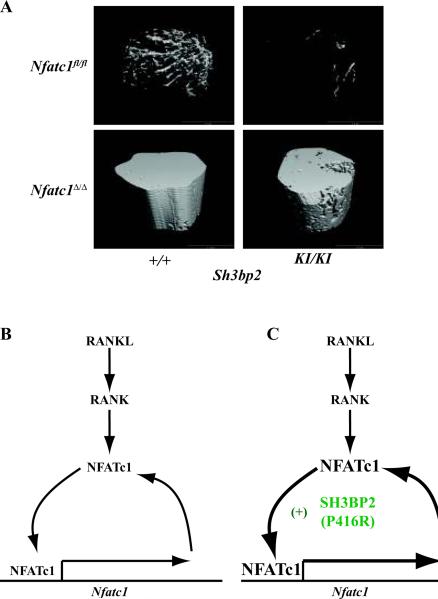
To investigate whether NFATc1-mediated osteoclastogenesis is important for inflammation-induced bone loss, we crossed our NFATc1 conditional knockout mice with a genetic model of cherubism (19, 72). Cherubism is a rare, autosomal dominant, pediatric disorder characterized by fibroinflammatory infiltrates in the face resulting in disfiguring swelling and osteolysis of the jaw. This disorder is caused by mutations in SH3BP2, which encodes a signaling adapter molecule (73). The most common genetic alteration in cherubism patients is a gain-of-function missense mutation in exon 9 of SH3BP2, which results in substitution of proline to arginine (P418R). Cherubism mice have been genetically engineered wherein this missense mutation (P416R in mice) was knocked-in (KI) to the Sh3bp2 locus (72). Homozygous Sh3bp2KI/KI mice exhibit multi-organ inflammation and severe systemic bone loss. Both of these phenotypes are dependent on the cytokine TNFα, which is overproduced by Sh3bp2KI/KI macrophages (72). TNFα has been implicated in bone loss associated with inflammatory conditions such as RA as well as postmenopausal OP (74). Furthermore, osteoclast precursors from Sh3bp2KI/KI mice show increased responsiveness to M-CSF and RANKL stimulation in vitro, forming exceptionally large osteoclasts with higher numbers of nuclei per cell (72). Since NFATc1 is a master regulator of osteoclastogenesis (75) and NFATs have been shown to promote TNFα production (76, 77), we deleted NFATc1 in young cherubism mice, prior to the onset of their dramatic phenotype (19). Ablation of NFATc1 in Sh3bp2KI/KI mice had no effect on inflammation but protected the mice from systemic bone loss (Fig. 2A). Thus, NFATc1 uncouples systemic inflammation from osteopenia in this unique model and may be a therapeutic target for TNFα-dependent bone loss. In addition, osteoclastogenesis was completely abolished in Sh3bp2KI/KI mice lacking NFATc1. In the absence of NFATc1 protein, mutant SH3BP2(P416R) was unable to promote autoregulation of NFATc1 expression (19). These data are consistent with a recent report that overexpression of SH3BP2 in a murine macrophage cell line promotes nuclear translocation of NFATc1 and the activity of an NFAT luciferase reporter construct (78). Taken together, these studies suggest that mutant SH3BP2(P416R) functions upstream of NFATc1 in the developing osteoclast and amplifies the NFATc1 autoregulatory loop to drive differentiation (Fig. 2B). Further experimentation will be needed to resolve whether other NFAT family members compensate for NFATc1 to promote pro-inflammatory cytokine production in this model or if mutant SH3BP2 functions independent of the calcineurin/NFAT axis to augment TNFα production in macrophages.
Regulation of osteoclast gene expression by NFATc1
Accumulating evidence suggests that NFATc1 directly regulates a number of genes important for bone resorption in the osteoclast. We have shown that NFATc1 regulates two sets of RANKL-induced genes in the developing osteoclast: (i) genes that are absolutely dependent on NFATc1 for expression such as calcitonin receptor (Calcr), osteoclast-associated receptor (Oscar), and integrin β3 (Itgb3) and (ii) those that are augmented by NFATc1 but can be partially induced by RANKL, even in the absence of NFATc1, such as matrix metalloproteinases 9 and 14 (Mmp9, Mmp14), tartrate-resistant acid phosphatase 5 (Acp5), cathepsin K (Ctsk), carbonic anhydrase-2 (Car2), Chloride channel-7 (Clcn7), and Nfatc1 itself (19). Chromatin immunoprecipitation (ChIP) experiments have shown that NFATc1 binds the promoters of many of these genes including Acp5, Ctsk, Oscar, Nfatc1, and Itgb3 during osteoclastogenesis (62, 79–82). Other NFATc1 regulated genes in osteoclasts are shown in Fig. 1. Taken together, these studies confirm that NFATc1 is a master regulator of the osteoclast `transcriptome' and suggest that a deeper resolution of NFATc1 target genes in the osteoclast may reveal novel regulators of bone resorption. In the next section, we describe the identification of a novel NFATc1-dependent transcript in the osteoclast, which encodes for an anion exchanger called SLC4A2 (AE2).
In the absence of NFATc1, RANKL dramatically stimulates expression of OPG in bone marrow osteoclast precursors (19). OPG is a decoy receptor for RANKL that negatively regulates osteoclast differentiation. Hence, overexpression of OPG leads to high bone mass (41), whereas lack of OPG results in osteoporosis (83). Osteoblasts are thought to be the primary source of OPG in bone (84). Our data indicate that a signal is generated downstream of RANK to promote OPG expression in osteoclast precursors. In wildtype cells, the induction of NFATc1 appears to directly repress this signal, as NFATc1 can both be identified by ChIP at the OPG promoter and attenuate the expression of a reporter construct containing the proximal OPG promoter (19). We hypothesize that perturbation of this inhibitory pathway could generate osteoclast precursors that synthesize an autocrine negative regulator of their own differentiation and represent a novel mechanism to inhibit bone destruction.
Identification of SLC4A2/AE2 as critical regulator of bone resorption
Bone degradation depends on the ability of the mature osteoclast to polarize its membrane when it attaches to the bone surface to form a resorption lacuna (Fig. 3A). The apical membrane of the osteoclast forms a highly invaginated structure, called a ruffled border, where acid is secreted to dissolve the alkaline salts of bone mineral. In the cytoplasm of the osteoclast, carbonic anhydrase generates the primary source of protons for the vacuolar type H+-ATPase located in the ruffled border membrane. To maintain electroneutrality, chloride ions are also transported into the lacuna. Mutations in mice or humans in the genes encoding either the proton pump or chloride channel results in dysfunctional osteoclasts leading to osteopetrosis (30). To prevent cytoplasmic alkalinization, electroneutral exchange of bicarbonate for chloride occurs through an anion exchanger in the osteoclast basolateral membrane (85). The genetic identity of this exchanger had eluded identification until recently.
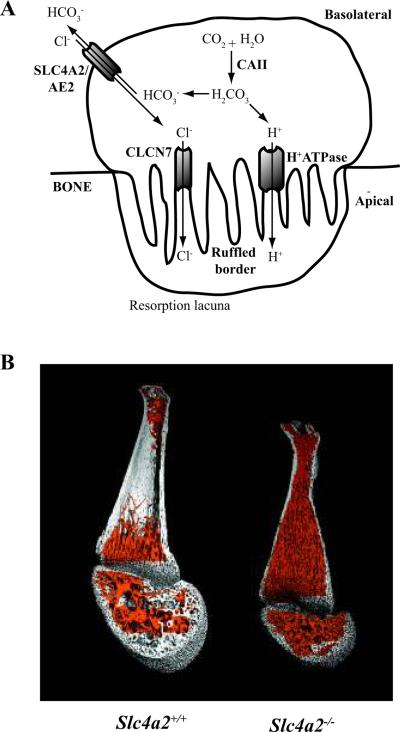
Among the genes that we identified by microarray analysis to be induced by RANKL, only in NFATc1-sufficient osteoclast precursors was SLC4A2 (AE2) (86). AE2 is a bicarbonate/chloride anion exchanger expressed in a variety of tissues including bone, kidney, intestine, stomach, liver, choroid plexus, testes, salivary glands, and the cochlea (87). The use of alternative promoters leads to multiple Slc4a2 mRNA isoforms (Slc4a2a, Slc4a2b1, Slc4a2b2, Slc4a2c1, and Slc4a2c2) (88), with osteoclasts preferentially expressing the Slc4a2a isoform (86). In 2004, Gawenis et al. (89) described the generation of mice lacking AE2. These mice are growth retarded, edentulous, and exhibit early lethality. Furthermore, they are achlorhydric. We recently analyzed the skeletal phenotype of AE2-deficient mice and showed that they fail to resorb the primarily cartilaginous skeletal anlagen and hence develop severe osteopetrosis (Fig. 3B). In contrast to NFATc1 knockout mice, which display an absence of osteoclasts in vivo (19), exceptionally large osteoclasts were identified in the bone marrow of AE2-deficient mice (86). Interestingly, many osteoclasts in the AE2 knockout mice were apoptotic, suggesting that AE2 may promote osteoclast survival. Moreover, we showed that AE2 deficient osteoclasts are unable to resorb calcified matrix in vitro and cannot form an acidified resorption lacuna. Recently, another group confirmed our finding of osteopetrosis in AE2 knockout mice and demonstrated that AE2 localizes to the basolateral membrane of the osteoclast and is required for the formation of a ruffled border (90). Many questions regarding the role of AE2 in osteoclast biology remain unresolved. First, we would like to understand whether AE2 is important for osteoclast function in pathologic conditions such as RA and OP. Second, we plan to explore how AE2 deficiency enhances osteoclast apoptosis. Third, we will resolve whether AE2 is a direct transcriptional target of NFATc1. Lastly, we would like to know whether AE2 functions in the other major cell type in skeleton, the bone-forming osteoblast.
Calcineurin/NFATs in osteoblast biology and bone formation
In addition to its well-described role in osteoclastogenesis, a function for the calcineurin/NFAT axis in osteoblasts is emerging, though a consensus among investigators has not been reached. A better understanding of this pathway in bone formation is important if its pharmacologic manipulation is considered as a therapeutic strategy for disorders of bone loss.
Osteoblasts express both the catalytic (CnA) and regulatory subunits (CnB) of calcineurin. Overexpression of CnA enhances osteoblast differentiation in vitro, as demonstrated by increased expression of Runx2, alkaline phosphatase, bone sialoprotein, and osteocalcin (91). This increase is associated with enhanced bone formation in calvarial osteoblast cultures. Consistent with these data, CnAα-deficient mice show diminished bone formation and osteoporosis, associated with reduced rates of bone formation (91). In contrast, disruption of the calcineurin regulatory subunit (Cnb1) in osteoblasts leads to a dramatic increase in bone mass, bone formation, and osteoblast numbers, as well as a decrease in bone resorption with decreased osteoclast numbers (92). The negative effect of Cnb1 deletion on osteoclastogenesis was due to higher levels of OPG and lower levels of RANKL produced by Cnb1-deficient osteoblasts. Accordingly, Cnb1-deficient osteoblasts were unable to support osteoclastogenesis in vitro (92). Further experimentation is needed to resolve the discrepancies between these two seemingly conflicting reports on the role of calcineurin in osteoblasts (91, 92).
Yeo et al. (93) demonstrated recently that pharmacologic inhibition of calcineurin also displays contrasting effects on bone formation and osteoblastogenesis. In vivo and in vitro treatment with low CsA concentrations led to an increase in bone mass and augmentation of osteoblast differentiation, whereas high concentrations had an inhibitory effect. The response to low dose CsA was associated with increased expression and activation of Fra-2, an AP-1 family member that plays an important positive role in osteoblasts (93). Furthermore, this group showed that osteoblast differentiation driven by low CsA concentrations could be inhibited by overexpression of constitutively active NFATc1, suggesting that the activation of osteoblastogenesis by low concentration of CsA is mediated by suppression of the NFAT axis. In contrast, another report found that a different calcineurin inhibitor, FK506, suppresses bone formation in vivo and inhibits mineral production by osteoblasts in vitro regardless of the dose (94). Although these studies suggest that calcineurin is important in osteoblasts, the observations that high and low doses of CsA have differential effects on bone formation (93) and that deletion of the catalytic and regulatory subunits of calcineurin result in apparently opposite phenotypes (91, 92) indicate that further experimentation will be needed to clarify this pathway in these cells.
Three studies have directly analyzed the role of NFATs in osteoblasts. First, Koga et al. (94) showed that NFATc2-deficient mice have low bone mass with reduced bone formation rates. In this study, NFAT was found to cooperate with another osteoblast-specific transcription factor, Osterix, to drive expression of the type I collagen gene (Col1a1), the predominant collagen in bone. Another study by Winslow et al. (71) supported a positive regulatory role for NFAT in bone formation. This group studied mice expressing a constitutively active variant of NFATc1 in osteoblasts and showed that this strain has elevated bone mass and osteoblast numbers. In addition, increased osteoclast numbers and bone resorption was observed (71). Augmented osteoclastogenesis in these mice appeared to be due to NFATc1-driven expression of the chemokine ligand CCL8 by osteoblasts. These data indicate that bone formation and resorption are integrated processes regulated by NFAT signaling in osteoblasts through the expression of chemokines that attract monocytic osteoclast precursors. Despite these two rather convincing studies, Choo et al. (95) recently proposed a negative role for NFAT in osteoblasts. This group showed that constitutively active NFATc1 inhibits alkaline phosphatase activity, mineralization, and the expression of osteoblast differentiation marker genes in an in vitro culture system. NFATc1 was found to repress the promoter for osteocalcin, a late marker of osteoblastogenesis, through the formation of a complex with histone deacetylase 3 (HDAC3) (95). Consistent with this observation, another study showed that HDACs negatively regulate osteoblast differentiation and osteocalcin expression (96). Finally, NFATc1 was recently identified as a negative regulator of estrogen receptor α (ERα) expression in a human osteosarcoma line (97). Since estrogen has a net anabolic effect on the skeleton (98), the repression of ERα expression by NFATc1 may indirectly attenuate bone formation.
More experimentation is needed to resolve the role of the calcineurin/NFAT axis in osteoblasts in vivo. Many of the studies presented above report dramatic phenotypes when this pathway is perturbed in mice or in vitro, but a consistent picture has not emerged. Future experiments should include the analysis of mouse mutants where NFAT family members have been specifically deleted in cells of the osteoblast lineage under physiologic conditions and in models of OP and arthritis.
Calcineurin/NFATs in cartilage homeostasis and pathobiology
The few studies that have examined the role of the calcineurin/NFAT axis in cartilage biology suggest that this pathway is a central regulator of chondrocyte physiology and pathology. In 2000, Ranger et al. (99) reported that NFATc2-deficient mice develop reduced range of motion and contractures of the shoulders, hips, knees, and ankles. These joint abnormalities were more prevalent in female mice, worsened with age, and arose as a consequence of hyperproliferation of articular chondrocytes, as well as ectopic chrondrogenesis in the periarticular soft tissues. These ectopic cartilage rests underwent endochondral ossification leading to radiodense calcifications encasing the affected joints. Furthermore, the authors demonstrated that NFATc2 was upregulated during chondrocyte differentiation and that NFATc2-deficient chondrocytes expressed higher levels of chondrocyte marker genes, such as type II and type X collagen, compared with wildtype cells. Moreover, NFATc2-deficient chondrocytes showed an intrinsic increase in proliferation ex vivo and some NFATc2 knockout mice even developed chondrosarcomas. Thus, the authors concluded that NFATc2 is an endogenous negative regulator of the cartilage phenotype and a putative tumor suppressor gene (99). Consistent with these observations, treatment of ATDC5 cells, a clonal mouse chondrogenic cell line, with the calcineurin inhibitor FK506 promotes chondrocyte differentiation (100). These data indicate that the Calcineurin/NFAT pathway represses chondrogenic differentiation and that agents targeting this pathway may promote cartilage regeneration. A recent publication (101) revisited the articular phenotype of NFATc2-deficient mice and found that at early time points (< 4 months) the joints of these animals display a reduction in proteoglycans, type II collagen, and aggrecan and increased levels of type X collagen, catabolic enzymes, and pro-inflammatory cytokines, like IL-1β. The authors suggested that the phenotype of these mice is more consistent with early onset osteoarthritis rather than a primary increase in chondroproliferation.
The Ranger study (99) also showed that murine chondrocytes express all four calcium-regulated NFATs (NFATc1-c4), suggesting that family members other than NFATc2 may play redundant or compensatory roles in this tissue. Accordingly, a few recent studies indicate that NFATs control the balance between anabolic and catabolic pathways in cartilage. First, NFATc1 and NFATc2 transactivate the ADAMTS-9 (a disintegrin and metalloprotease with thrombospondin motifs 9) and ADAMTS-4 (aggrecanase-1) promoters, respectively (102, 103). These aggecanases cleave aggrecan, the major proteoglycan in cartilage, and thereby promote cartilage destruction in RA and OA (10). Second, the pro-inflammatory cytokine IL-1β, which may play a pathogenic role in both RA and OA, induces NFATc1 expression in human articular chondrocytes (103). Pharmacologic inhibition of calcineurin prevents IL-1β-stimulated production of the degradative enzymes MMP-1, MMP-3, and ADAMTS-4 in chondrocytes isolated from OA knees (104). These data suggest that catabolic pathways activated by IL-1β in chondrocytes involve the calcineurin/NFAT pathway. Most importantly, Yoo et al. (104) recently showed that mice treated with CsA are protected from experimentally induced knee OA. Obviously these findings juxtapose those described by Wang et al. (101) of early osteoarthritis in the NFATc2-deficient mice. Taken together, the data in this section suggest that the calcineurin/NFAT pathway regulates both chondrocyte proliferation and the expression of anabolic and catabolic pathways in cartilage (99–104). Future studies should address the contribution of individual NFAT family members to chrondrocyte biology in vivo and evaluate novel NFAT inhibitors, with more favorable side effect profiles than CsA, for their protective and regenerative effects in models of arthritis and in human joint diseases.
Calcineurin/NFATs in angiogenesis
Joints affected by RA develop a characteristic synovial lesion termed the pannus. Pannus tissue contains hyperproliferative synoviocytes and inflammatory cells as well as a large number of new blood vessels. This robust angiogenesis provides oxygen and nourishment as well as a conduit for immune cells to the inflamed synovium (105, 106). The role of NFATs in synovial angiogenesis in RA has not been directly evaluated. Strong circumstantial evidence reviewed here, however, suggests that this pathway may be involved in this aspect of RA.
NFAT proteins are involved in a diverse array of cardiovascular processes (reviewed in 107). The most direct and dramatic evidence has come from genetic studies in knockout mice. For example, mice deficient in either NFATc1 or both NFATc3 and NFATc4 die during embryonic development. Whereas the former expire due to defective cardiac valve formation (70), the latter display excessive and disorganized blood vessel assembly (108). In contrast, the physiologic and pathologic role of NFATs in the vasculature during adult life, including within the context of arthritis, is not as well understood (107). One report found that CsA inhibits angiogenesis in synovial explants from RA but not OA patients (109). This experiment suggests that the production of or response to angiogenic factors in RA biopsies requires calcineurin and perhaps NFAT transcription factors.
Numerous proangiogenic factors have been implicated in the pathogenesis of RA (110). The vascular endothelial growth factor (VEGF) family appears to be particularly important. The VEGF family consists of five members (VEGFA, VEGFB, VEGFC, VEGFD, and placental growth factor). These ligands bind at least three receptors with different affinities and cellular distributions to promote responses including angiogenesis during development and tumor growth (111). The synovial fluid and serum of RA patients contain elevated levels of VEGF (112, 113), and both genetic and pharmacologic manipulation of the VEGF pathway inhibits arthritis in mouse models of RA. For example, mice deficient in VEGFB or those treated with blocking reagents to VEGF or VEGFR show reduced synovial pathology in multiple models of RA (106, 114). Conversely, overexpression of VEGF by gene transfer in mice promotes synovial angiogenesis and pathology (115).
The VEGF pathway appears to be regulated by NFAT at multiple levels. In 2001, Hernandez et al. (116) reported that CsA inhibits the induction of cyclooxygenase-2 (COX-2) by VEGF in human endothelial cells and that NFAT sites in a COX-2 promoter construct are required for VEGF-mediated transactivation. Importantly, CsA inhibited VEGF-mediated angiogenesis in vitro and in vivo. This inhibition could be reversed by treatment with prostaglandin E2 (PGE2), suggesting that the induction of COX-2 expression by NFAT, and hence prostaglandin production, is a necessary step in VEGF-induced angiogenesis (116).
Regulation of the VEGF pathway by NFAT may be more complex than NFAT simply mediating downstream responses to this growth factor. Jinnin et al. (117) recently reported that NFAT promotes expression of VEGFR1 (FLT1). VEGFR1 is a high affinity VEGF receptor, with limited signaling capacity in endothelial cells. It prevents VEGF from activating VEGFR2, the primary receptor coordinating VEGF responses (111). In human infantile hemangiomas, a benign endothelial tumor, downregulation of the NFAT pathway leads to reduced VEGFR1 expression and thus enhanced VEGFR2 signaling and precocious, disorganized angiogenesis (117). Interestingly, levels of VEGFR1 and its specific ligand, placental growth factor (PLGF), are increased in inflamed synovial tissue, and pharmacologic agents targeting this receptor inhibit arthritis in animal models (118–120). In addition to a possible pro-angiogenic role, PLGF promotes inflammatory cytokine production in mononuclear cells via a calcineurin-dependent pathway, suggesting that NFAT may also mediate downstream VEGFR1 responses (118). Lastly, CsA inhibits the production of VEGF from RA FLS (121). Taken together, these studies indicate that NFAT is positioned both upstream and downstream in the VEGF pathway, capable of regulating receptor (117) and ligand (121) levels as well as post-receptor transcriptional responses (116, 118). Further experimentation is needed to resolve which aspects of VEGF biology regulated by NFAT are most important to the pathophysiology of arthritis and joint remodeling.
Pro-angiogenic molecules beyond the VEGF family may also involve NFAT activation. For example, basic fibroblast growth factor (bFGF), which is elevated in the synovial fluid of patients with severe RA (122, 123), promotes NFATc2 activation in human endothelial cells (124). In addition, secreted frizzle-related protein 2, a Wnt pathway modulator, was recently shown to induce nuclear translocation of NFATc3 in murine endothelial cells and promote angiogenesis in vitro through a calcineurin-dependent pathway (125). In the future, investigators will resolve which upstream growth factors and cytokines regulate the NFAT pathway to promote synovial angiogenesis in inflammatory arthritis.
Neuropeptides, nociception, and NFAT
The synovial membrane, joint capsule, and periarticular bone are richly innervated with afferent sensory and post-ganglionic sympathetic neurons that relay pain signals to the central nervous system and regulate vascular tone and permeability, respectively (126). These neurons also release neuropeptides, such as substance P (SP), bombesin/gastrin releasing peptide (BN/GRP), and vasoactive intestinal peptide (VIP), which are synthesized in proximal ganglia and transported to the peripheral nerve endings (reviewed in 126, 127). In addition to effects on vasculature, neuropeptides directly regulate synovial and inflammatory cells in arthritis. For example, SP induces synoviocyte proliferation and the secretion of pro-inflammatory mediators and degradative enzymes, such as PGE2 and collagenases (128). Moreover, human peripheral blood mononuclear cells produce proinflammatory cytokines, like IL-1β, TNFα, and IL-6 when stimulated with SP (127). Both SP and BN/GRP are elevated in the synovial fluid of patients with RA, and their levels correlate with the presence of pro-inflammatory cytokines (129). In contrast to SP, VIP has anti-inflammatory properties. For example, VIP inhibits experimental collagen induced arthritis in mice through its ability to (i) preferentially promote non-pathogenic Th2 differentiation, (ii) inhibit the production of pro-inflammatory cytokines by synovial membrane cells, and (iii) induce regulatory T-cell differentiation through tolerogenic dendritic cells (130, 131). Direct effects of neuropeptides on endothelial cells, mast cells, macrophages, and osteoclasts have also been demonstrated (126). Together these data highlight the ability of the nervous system to promote or mitigate joint remodeling through neuropeptides.
A review of limited literature suggests that neuropeptides induce transcriptional responses in target cells via the NFAT pathway. SP mediates signaling in target cells through G protein-coupled neurokinin receptors (NK-R) that promote calcium influx (132). In endothelial cells, SP induces the expression of the adhesion molecule ICAM-1, important for the recruitment of inflammatory cells, through a calcineurin-dependent pathway. NFAT-binding sites were identified in the ICAM-1 promoter and shown to bind NFATc2 by electrophoretic mobility shift assays (132). In primary rat spinal neuron cultures, SP promotes nuclear translocation of NFATc4 and the activation of an NFAT luciferase reporter construct (133). In addition, BN/GRP promotes COX-2 expression through NFAT activation in colon cancer cells (134). In contrast to SP and BN/GRP, the anti-inflammatory neuropeptide VIP inhibits the activation of NFAT. Delgado et al. (135) showed that VIP blocks nuclear accumulation of NFATc2 in T lymphocytes after antigen receptor cross-linking through the activation of an inhibitory protein kinase A pathway. These data indicate that the regulation of NFAT is an important transcriptional pathway downstream of multiple neuropeptides. Further investigation will be needed to resolve the role of NFAT in the neuropeptide pathways that are most relevant to pain perception, inflammation, and joint remodeling in arthritis.
Pain is a nearly ubiquitous symptom in RA. A better understanding of pain in this disease is important, as almost 70% of patients indicate that pain control is the area of their healthcare they would like to see improved (136). Not only do RA patients experience pain at sites of joint inflammation, but many also develop a widespread pain amplification syndrome called fibromyalgia (137). Pain amplification may involve transcriptional changes in sensory neurons that lead to heightened responses to noxious or innocuous stimuli (peripheral sensitization). Bradykinin (BK) is a nine amino acid peptide generated at the sites of tissue damage. In rat dorsal root ganglion neurons, BK promotes nuclear translocation of NFAT and the expression of COX-2 via a calcineurin-dependent pathway (138). Similarly, neurotrophins, whose expression in synovial fluid cells may be elevated in patients with inflammatory arthritis (139), also promote COX-2 expression through calcineurin (140). The production of prostaglandins by COX-2 is thought to play an important role in peripheral sensitization (141). Thus, the ability of NFAT to induce COX-2 expression in neurons may represent a final common pathway to amplify nociceptive responses in inflammatory states.
Concluding remarks
Pathologic tissue remodeling in OP, RA, and OA is a complex process that involves the coordinated activity of cells native and foreign to bone and joints. In RA for example, an influx of inflammatory cells into the synovium, accompanied by robust angiogenesis, leads to the signs and symptoms of inflammation and the activation of catabolic pathways in cartilage and periarticular bone, culminating in irreversible joint damage. Depending on the cellular context, members of the vertebrate-specific NFAT transcription factor family influence an array of biologic processes. In this review, the role of NFATc1 as a master regulator of osteoclast differentiation and bone resorption during growth, development, and inflammatory processes has been explored in detail. In addition, emerging evidence reviewed here links NFATs to bone formation, chondrocyte proliferation, cartilage catabolism, angiogenesis, nociception, and the response to neuropeptides. Thus, a multifaceted role for NFAT in the remodeling of bone and joints, the quintessential components of the vertebrate body plan, is beginning to crystallize.
Given the plethora of biologic processes linked to NFATs (17), it is unlikely that new pharmacologic agents directly targeting this pathway will have more success or be more specific than currently available therapeutics like CsA and FK506. We propose that a better understanding of the receptors and their ligands that engage the NFAT pathway to cause tissue-specific pathology could lead to the identification of new therapeutic targets. Indeed, blocking antibodies to RANKL, which induces NFATc1 in osteoclast precursors to drive bone resorption, is currently in late stage clinical development for the treatment of OP and RA (142, 143). Likewise, elucidation of the cell-specific transcriptional targets of NFAT that participate in the tissue remodeling process also will yield novel molecules for drug discovery. Lastly, knowledge of transcriptional regulators that cooperate with NFAT to impart tissue-specific gene regulation, such as Osterix in osteoblasts (94) or Pu.1 in osteoclasts (81), may reveal approaches to mitigate NFAT-driven pathology in bone and joints while minimizing effects on physiologic processes controlled by NFAT in other organ systems.
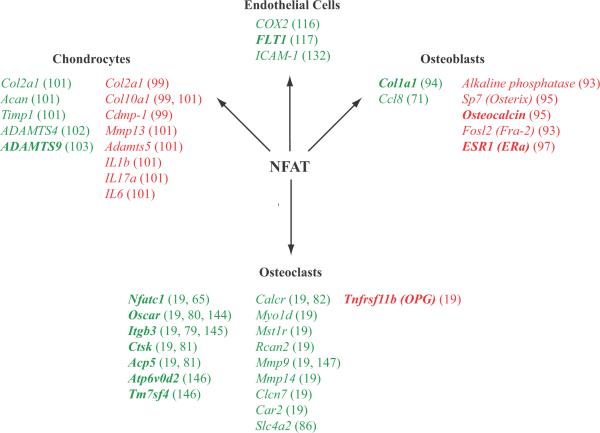
Fig. 1. Regulation of gene expression by NFAT in endothelial cells, chondrocytes, osteoblasts, and osteoclasts.
Genes included on this list were shown to be NFAT regulated by promoter reporter assays, ChIP experiments, overexpression studies, or differential regulation in wildtype versus NFAT-deficient cells. Genes whose expression is augmented by NFAT are shown in green. Genes whose expression is repressed by NFAT are shown in red. The promoters of the genes in bold have been shown by ChIP to be direct NFAT targets.
Fig. 2. Mutant SH3BP2 promotes osteoclastogenesis via NFATc1.
(A) Micro-computed tomography (μ-CT) images of the distal femoral metaphysis of wildtype (Sh3bp2+/+) and cherubism mice (Sh3bp2KI/KI) either sufficient (Nfatc1+/+) or deficient (NFATc1Δ/Δ) in NFATc1. Note the dramatic osteoporosis in Sh3bp2KI/KI mice compared with wildtype mice (upper right and upper left panels). In the absence of NFATc1 (lower right panel), osteoporosis is reversed in Sh3bp2KI/KI mice and these mice display osteopetrosis similar to Sh3bp2+/+ lacking NFATc1 (lower left panel). (B) Signals emanating from RANK during osteoclast differentiation induce the expression and activation of NFATc1, which promotes additional NFATc1 expression by autoregulation of the Nfatc1 promoter (65). (C) Mutant SH3BP2 (P416R) augments the osteoclast differentiation program by driving NFATc1 autoamplification (19). Image in (A) courtesy of Dr. Ted Gross from the University of Washington.
Fig. 3. SLC4A2/AE2 is necessary for bone resorption by osteoclasts.
(A) Diagram of the osteoclast acidification pathway. Carbonic anhydrase II (CAII) generates carbonic acid (H2CO3), which dissociates into a bicarbonate ion (HCO3−) and a proton (H+). The proton is secreted into the resorption lacuna via a vacuolar-type H+ATPase located in the ruffled border of the apical membrane. A chloride (Cl−) equivalent is also released via CLCN7 into the resorption lacuna to maintain electroneutrality. Excess cytoplasmic HCO3− is exchanged for Cl− via SLC4A2/AE2 located in the basolateral membrane, which prevents intracellular alkalinization (85, 86). (B) μCT reconstruction of femurs from a wild-type mouse (left) and a mouse deficient in the HCO3−/Cl− exchanger, SLC4A2/AE2 (right). Absence of AE2 perturbs the bone resorbing function of osteoclasts, leading to a massive accumulation of trabecular bone (pseudocolored in orange). Image in (B) courtesy of Nicholas James Brady from the μCT facility at the Harvard School of Dental Medicine.
Acknowledgements
Dr. Antonios O. Aliprantis holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and is supported by Award Number K08AR054859 from the National Institute Of Arthritis And Musculoskeletal And Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Arthritis And Musculoskeletal And Skin Diseases or the National Institutes of Health.
References
- 1.Harris ED., Jr The bone and joint decade: a catalyst for progress. Arthritis Rheum. 2001;44:1969–1970. doi: 10.1002/1529-0131(200109)44:9<1969::AID-ART342>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 2.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 3.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 4.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips K, Aliprantis A, Coblyn J. Strategies for the prevention and treatment of osteoporosis in patients with rheumatoid arthritis. Drugs Aging. 2006;23:773–779. doi: 10.2165/00002512-200623100-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ralston SH, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20:2492–2506. doi: 10.1101/gad.1449506. [DOI] [PubMed] [Google Scholar]
- 7.Simkin PA, Gardner GC. The musculoskeletal system and joint physiology. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 4th edn. Elsevier; Philadelphia: 2008. pp. 33–43. [Google Scholar]
- 8.Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–270. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 11.Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev. 2005;208:228–251. doi: 10.1111/j.0105-2896.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 12.Shaw J, Utz P, Durand D, Toole J, Emmel E, Crabtree G. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. [PubMed] [Google Scholar]
- 13.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 14.Graef IA, Gastier JM, Francke U, Crabtree GR. Evolutionary relationships among Rel domains indicate functional diversification by recombination. Proc Natl Acad Sci USA. 2001;98:5740–5745. doi: 10.1073/pnas.101602398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18:1–9. [PubMed] [Google Scholar]
- 16.Graef IA, Chen F, Crabtree GR. NFAT signaling in vertebrate development. Curr Opin Genet Dev. 2001;11:505–512. doi: 10.1016/s0959-437x(00)00225-2. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol. 2007;17:251–260. doi: 10.1016/j.tcb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aliprantis AO, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118:3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 21.Services UDoHaH . Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Office of the Surgeon General; Rockville, MD: 2004. [PubMed] [Google Scholar]
- 22.Carlsten H. Immune responses and bone loss: the estrogen connection. Immunol Rev. 2005;208:194–206. doi: 10.1111/j.0105-2896.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 23.Canalis E, Bilezikian JP, Angeli A, Giustina A. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593–598. doi: 10.1016/j.bone.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Lian JB, et al. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- 25.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 26.Otto F, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 27.Balcerzak M, et al. The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim Pol. 2003;50:1019–1038. [PubMed] [Google Scholar]
- 28.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 29.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 30.Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004;351:2839–2849. doi: 10.1056/NEJMra040952. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 32.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 34.Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takayanagi H, Sato K, Takaoka A, Taniguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005;208:181–193. doi: 10.1111/j.0105-2896.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 36.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005;208:126–140. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 37.Rho J, Takami M, Choi Y. Osteoimmunology: interactions of the immune and skeletal systems. Mol Cells. 2004;17:1–9. [PubMed] [Google Scholar]
- 38.Jimi E, Ghosh S. Role of nuclear factor-kappaB in the immune system and bone. Immunol Rev. 2005;208:80–87. doi: 10.1111/j.0105-2896.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzo J, Choi Y. Osteoimmunology. Immunol Rev. 2005;208:5–6. doi: 10.1111/j.0105-2896.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 40.Xing L, Schwarz EM, Boyce BF. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005;208:19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 41.Simonet WS, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 42.Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Goldring SR. Bone and joint destruction in rheumatoid arthritis: what is really happening? J Rheumatol Suppl. 2002;65:44–48. [PubMed] [Google Scholar]
- 44.Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36:372–378. doi: 10.1016/s1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 45.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl):ii84–86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettit AR, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gravallese EM, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 48.Diarra D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 49.Kitahara K, Kawai S. Cyclosporine and tacrolimus for the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:238–245. doi: 10.1097/BOR.0b013e328099af80. [DOI] [PubMed] [Google Scholar]
- 50.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 51.Pessler F, Dai L, Cron RQ, Schumacher HR. NFAT transcription factors--new players in the pathogenesis of inflammatory arthropathies? Autoimmun Rev. 2006;5:106–110. doi: 10.1016/j.autrev.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Buch MH, Vital EM, Emery P. Abatacept in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2008;10(Suppl):S5. doi: 10.1186/ar2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 54.Yoo SA, et al. Calcineurin is expressed and plays a critical role in inflammatory arthritis. J Immunol. 2006;177:2681–2690. doi: 10.4049/jimmunol.177.4.2681. [DOI] [PubMed] [Google Scholar]
- 55.Cho ML, et al. Cyclosporine differentially regulates interleukin-10, interleukin-15, and tumor necrosis factor a production by rheumatoid synoviocytes. Arthritis Rheum. 2002;46:42–51. doi: 10.1002/1529-0131(200201)46:1<42::AID-ART10026>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 56.Masuda K, et al. Molecular profile of synovial fibroblasts in rheumatoid arthritis depends on the stage of proliferation. Arthritis Res. 2002;4:R8. doi: 10.1186/ar427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerth AJ, Pham CT, Peng SL. Regulation of the symmetry and intensity of immune complex-mediated synovitis by nuclear factor of activated T cells. Arthritis Rheum. 2004;50:3392–3395. doi: 10.1002/art.20579. [DOI] [PubMed] [Google Scholar]
- 58.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 59.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 60.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen M, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 63.Takayanagi H. The role of NFAT in osteoclast formation. Ann NY Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 64.Shinohara M, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 65.Asagiri M, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishida N, et al. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem. 2002;277:41147–41156. doi: 10.1074/jbc.M205063200. [DOI] [PubMed] [Google Scholar]
- 67.Sun L, et al. Evidence that calcineurin is required for the genesis of bone-resorbing osteoclasts. Am J Physiol Renal Physiol. 2007;292:F285–291. doi: 10.1152/ajprenal.00415.2005. [DOI] [PubMed] [Google Scholar]
- 68.Day CJ, et al. Gene array identification of osteoclast genes: differential inhibition of osteoclastogenesis by cyclosporin A and granulocyte macrophage colony stimulating factor. J Cell Biochem. 2004;91:303–315. doi: 10.1002/jcb.10780. [DOI] [PubMed] [Google Scholar]
- 69.Kuroda Y, Hisatsune C, Nakamura T, Matsuo K, Mikoshiba K. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc Natl Acad Sci U S A. 2008;105:8643–8648. doi: 10.1073/pnas.0800642105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ranger AM, et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392:186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- 71.Winslow MM, et al. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell. 2006;10:771–782. doi: 10.1016/j.devcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 72.Ueki Y, et al. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 “cherubism” mice. Cell. 2007;128:71–83. doi: 10.1016/j.cell.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 73.Ueki Y, et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28:125–126. doi: 10.1038/88832. [DOI] [PubMed] [Google Scholar]
- 74.Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–168. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 75.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 76.Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14:13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 77.Kaminuma O, et al. Differential contribution of NFATc2 and NFATc1 to TNF-alpha gene expression in T cells. J Immunol. 2008;180:319–326. doi: 10.4049/jimmunol.180.1.319. [DOI] [PubMed] [Google Scholar]
- 78.Lietman SA, Yin L, Levine MA. SH3BP2 is an activator of NFAT activity and osteoclastogenesis. Biochem Biophys Res Commun. 2008;371:644–648. doi: 10.1016/j.bbrc.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP. NFATc1 regulation of the human beta3 integrin promoter in osteoclast differentiation. Gene. 2006;372:92–102. doi: 10.1016/j.gene.2005.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim K, et al. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem. 2005;280:35209–35216. doi: 10.1074/jbc.M505815200. [DOI] [PubMed] [Google Scholar]
- 81.Sharma SM, et al. MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. J Biol Chem. 2007;282:15921–15929. doi: 10.1074/jbc.M609723200. [DOI] [PubMed] [Google Scholar]
- 82.Shen Z, Crotti TN, Flannery MR, Matsuzaki K, Goldring SR, McHugh KP. A novel promoter regulates calcitonin receptor gene expression in human osteoclasts. Biochim Biophys Acta. 2007;1769:659–667. doi: 10.1016/j.bbaexp.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 83.Mizuno A, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 84.Glass DA, 2nd, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 85.Rousselle AV, Heymann D. Osteoclastic acidification pathways during bone resorption. Bone. 2002;30:533–540. doi: 10.1016/s8756-3282(02)00672-5. [DOI] [PubMed] [Google Scholar]
- 86.Wu J, Glimcher LH, Aliprantis AO. HCO3-/Cl- anion exchanger SLC4A2 is required for proper osteoclast differentiation and function. Proc Natl Acad Sci USA. 2008;105:16934–16939. doi: 10.1073/pnas.0808763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol. 2006;91:153–161. doi: 10.1113/expphysiol.2005.031765. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z, Schultheis PJ, Shull GE. Three N-terminal variants of the AE2 Cl-/HCO3-exchanger are encoded by mRNAs transcribed from alternative promoters. J Biol Chem. 1996;271:7835–7843. doi: 10.1074/jbc.271.13.7835. [DOI] [PubMed] [Google Scholar]
- 89.Gawenis LR, et al. Mice with a targeted disruption of the AE2 Cl-/HCO3- exchanger are achlorhydric. J Biol Chem. 2004;279:30531–30539. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- 90.Josephsen K, et al. Targeted disruption of the Cl-/HCO3- exchanger Ae2 results in osteopetrosis in mice. Proc Natl Acad Sci USA. 2009;106:1638–1641. doi: 10.1073/pnas.0811682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun L, et al. Calcineurin regulates bone formation by the osteoblast. Proc Natl Acad Sci USA. 2005;102:17130–17135. doi: 10.1073/pnas.0508480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeo H, et al. Conditional disruption of calcineurin B1 in osteoblasts increases bone formation and reduces bone resorption. J Biol Chem. 2007;282:35318–35327. doi: 10.1074/jbc.M702435200. [DOI] [PubMed] [Google Scholar]
- 93.Yeo H, Beck LH, McDonald JM, Zayzafoon M. Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone. 2007;40:1502–1516. doi: 10.1016/j.bone.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koga T, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11:880–885. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 95.Choo MK, Yeo H, Zayzafoon M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone. 2009 doi: 10.1016/j.bone.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schroeder TM, Nair AK, Staggs R, Lamblin AF, Westendorf JJ. Gene profile analysis of osteoblast genes differentially regulated by histone deacetylase inhibitors. BMC Genomics. 2007;8:362. doi: 10.1186/1471-2164-8-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Penolazzi L, et al. Induction of estrogen receptor alpha expression with decoy oligonucleotide targeted to NFATc1 binding sites in osteoblasts. Mol Pharmacol. 2007;71:1457–1462. doi: 10.1124/mol.107.034561. [DOI] [PubMed] [Google Scholar]
- 98.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 99.Ranger AM, et al. The transcription factor NFATp is a repressor of chondrogenesis. J Exp Med. 2000;191:9–21. doi: 10.1084/jem.191.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nishigaki F, Sakuma S, Ogawa T, Miyata S, Ohkubo T, Goto T. FK506 induces chondrogenic differentiation of clonal mouse embryonic carcinoma cells, ATDC5. Eur J Pharmacol. 2002;437:123–128. doi: 10.1016/s0014-2999(02)01269-4. [DOI] [PubMed] [Google Scholar]
- 101.Wang J, et al. Transcription factor Nfat1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. J Pathol. 2009 doi: 10.1002/path.2578. DOI 10.1002/path.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thirunavukkarasu K, et al. Regulation of the human ADAMTS-4 promoter by transcription factors and cytokines. Biochem Biophys Res Commun. 2006;345:197–204. doi: 10.1016/j.bbrc.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 103.Yaykasli KO, et al. ADAMTS9 activation by interleukin 1 beta via NFATc1 in OUMS-27 chondrosarcoma cells and in human chondrocytes. Mol Cell Biochem. 2009;323:69–79. doi: 10.1007/s11010-008-9965-4. [DOI] [PubMed] [Google Scholar]
- 104.Yoo SA, et al. Calcineurin modulates the catabolic and anabolic activity of chondrocytes and participates in the progression of experimental osteoarthritis. Arthritis Rheum. 2007;56:2299–2311. doi: 10.1002/art.22731. [DOI] [PubMed] [Google Scholar]
- 105.Koch AE. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998;41:951–962. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 106.Yoo SA, Kwok SK, Kim WU. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic intervention. Mediators Inflamm. 2008;2008:129873. doi: 10.1155/2008/129873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nilsson LM, Nilsson-Ohman J, Zetterqvist AV, Gomez MF. Nuclear factor of activated T-cells transcription factors in the vasculature: the good guys or the bad guys? Curr Opin Lipidol. 2008;19:483–490. doi: 10.1097/MOL.0b013e32830dd545. [DOI] [PubMed] [Google Scholar]
- 108.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 109.Ribatti D, et al. An experimental study in the chick embryo chorioallantoic membrane of the anti-angiogenic activity of cyclosporine in rheumatoid arthritis versus osteoarthritis. Inflamm Res. 2000;49:418–423. doi: 10.1007/s000110050610. [DOI] [PubMed] [Google Scholar]
- 110.Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. Angiogenesis in rheumatoid arthritis. Histol Histopathol. 2006;21:557–566. doi: 10.14670/HH-21.557. [DOI] [PubMed] [Google Scholar]
- 111.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 112.Koch AE, et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- 113.Lee SS, et al. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19:321–324. [PubMed] [Google Scholar]
- 114.Choi ST, Kim JH, Seok JY, Park YB, Lee SK. Therapeutic effect of anti-vascular endothelial growth factor receptor I antibody in the established collagen-induced arthritis mouse model. Clin Rheumatol. 2009;28:333–337. doi: 10.1007/s10067-008-1075-x. [DOI] [PubMed] [Google Scholar]
- 115.Clavel G, et al. Relationship between angiogenesis and inflammation in experimental arthritis. Eur Cytokine Netw. 2006;17:202–210. [PubMed] [Google Scholar]
- 116.Hernandez GL, et al. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jinnin M, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoo SA, et al. Role of placenta growth factor and its receptor flt-1 in rheumatoid inflammation: a link between angiogenesis and inflammation. Arthritis Rheum. 2009;60:345–354. doi: 10.1002/art.24289. [DOI] [PubMed] [Google Scholar]
- 119.Ikeda M, Hosoda Y, Hirose S, Okada Y, Ikeda E. Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J Pathol. 2000;191:426–433. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH649>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 120.Luttun A, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 121.Cho ML, et al. Cyclosporine inhibition of vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Arthritis Rheum. 2002;46:1202–1209. doi: 10.1002/art.10215. [DOI] [PubMed] [Google Scholar]
- 122.Manabe N, Oda H, Nakamura K, Kuga Y, Uchida S, Kawaguchi H. Involvement of fibroblast growth factor-2 in joint destruction of rheumatoid arthritis patients. Rheumatology (Oxford) 1999;38:714–720. doi: 10.1093/rheumatology/38.8.714. [DOI] [PubMed] [Google Scholar]
- 123.Sharaki OA, El-Guiziry DA, Abou-Zeid AA, El-Noueam KI, Helal AE, Gaballah AE. Clinical usefulness of basic fibroblast growth factor and E-selectin in patients with rheumatoid arthritis. Egypt J Immunol. 2004;11:91–100. [PubMed] [Google Scholar]
- 124.Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med. 2004;199:1513–1522. doi: 10.1084/jem.20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Courtwright A, et al. Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling pathway. Cancer Res. 2009;69:4621–4628. doi: 10.1158/0008-5472.CAN-08-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Niissalo S, Hukkanen M, Imai S, Tornwall J, Konttinen YT. Neuropeptides in experimental and degenerative arthritis. Ann NY Acad Sci. 2002;966:384–399. doi: 10.1111/j.1749-6632.2002.tb04239.x. [DOI] [PubMed] [Google Scholar]
- 127.Green PG. Gastrin-releasing peptide, substance P and cytokines in rheumatoid arthritis. Arthritis Res Ther. 2005;7:111–113. doi: 10.1186/ar1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lotz M, Carson DA, Vaughan JH. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987;235:893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- 129.Grimsholm O, Rantapaa-Dahlqvist S, Forsgren S. Levels of gastrin-releasing peptide and substance P in synovial fluid and serum correlate with levels of cytokines in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R416–426. doi: 10.1186/ar1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 131.Ganea D, Gonzalez-Rey E, Delgado M. A novel mechanism for immunosuppression: from neuropeptides to regulatory T cells. J Neuroimmune Pharmacol. 2006;1:400–409. doi: 10.1007/s11481-006-9044-0. [DOI] [PubMed] [Google Scholar]
- 132.Quinlan KL, et al. Substance P activates coincident NF-AT- and NF-kappa B-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J Immunol. 1999;163:5656–5665. [PubMed] [Google Scholar]
- 133.Seybold VS, Coicou LG, Groth RD, Mermelstein PG. Substance P initiates NFAT-dependent gene expression in spinal neurons. J Neurochem. 2006;97:397–407. doi: 10.1111/j.1471-4159.2006.03744.x. [DOI] [PubMed] [Google Scholar]
- 134.Corral RS, Iniguez MA, Duque J, Lopez-Perez R, Fresno M. Bombesin induces cyclooxygenase-2 expression through the activation of the nuclear factor of activated T cells and enhances cell migration in Caco-2 colon carcinoma cells. Oncogene. 2007;26:958–969. doi: 10.1038/sj.onc.1209856. [DOI] [PubMed] [Google Scholar]
- 135.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit expression of Fas ligand in activated T lymphocytes by regulating c-Myc, NF-kappa B, NF-AT, and early growth factors 2/3. J Immunol. 2001;166:1028–1040. doi: 10.4049/jimmunol.166.2.1028. [DOI] [PubMed] [Google Scholar]
- 136.Heiberg T, Kvien TK. Preferences for improved health examined in 1,024 patients with rheumatoid arthritis: pain has highest priority. Arthritis Rheum. 2002;47:391–397. doi: 10.1002/art.10515. [DOI] [PubMed] [Google Scholar]
- 137.Ranzolin A, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:794–800. doi: 10.1002/art.24430. [DOI] [PubMed] [Google Scholar]
- 138.Jackson JG, Usachev YM, Thayer SA. Bradykinin-induced nuclear factor of activated T-cells-dependent transcription in rat dorsal root ganglion neurons. Mol Pharmacol. 2007;72:303–310. doi: 10.1124/mol.107.035048. [DOI] [PubMed] [Google Scholar]
- 139.Barthel C, et al. Nerve growth factor and receptor expression in rheumatoid arthritis and spondyloarthritis. Arthritis Res Ther. 2009;11:R82. doi: 10.1186/ar2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Groth RD, Coicou LG, Mermelstein PG, Seybold VS. Neurotrophin activation of NFAT-dependent transcription contributes to the regulation of pro-nociceptive genes. J Neurochem. 2007;102:1162–1174. doi: 10.1111/j.1471-4159.2007.04632.x. [DOI] [PubMed] [Google Scholar]
- 141.Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 142.Miller PD. Denosumab: anti-RANKL antibody. Curr Osteoporos Rep. 2009;7:18–22. doi: 10.1007/s11914-009-0004-5. [DOI] [PubMed] [Google Scholar]
- 143.Cohen SB, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 144.Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem. 2005;280:32905–32913. doi: 10.1074/jbc.M505820200. [DOI] [PubMed] [Google Scholar]
- 145.Crotti TN, et al. PU.1 and NFATc1 mediate osteoclastic induction of the mouse beta3 integrin promoter. J Cell Physiol. 2008;215:636–644. doi: 10.1002/jcp.21344. [DOI] [PubMed] [Google Scholar]
- 146.Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP) Mol Endocrinol. 2008;22:176–185. doi: 10.1210/me.2007-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sundaram K, Nishimura R, Senn J, Youssef RF, London SD, Reddy SV. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp Cell Res. 2007;313:168–178. doi: 10.1016/j.yexcr.2006.10.001. [DOI] [PubMed] [Google Scholar]