Abstract
Background
A 58-year-old white man who was being followed by his hepatologist for nonalcoholic steatohepatitis-related liver cirrhosis and portal hypertension and who had been found to have a biopsy-proven hepatocellular carcinoma (HCC) on routine screening, self-referred to our center for a second opinion on the management of his HCC.
Investigations
Laboratory investigations, CT scan of the abdomen and chest, bone scan and technetium macroaggregated albumin scan.
Diagnosis
The patient had unresectable HCC.
Management
The patient underwent two treatments with Yttrium-90 glass microspheres, which were performed as outpatient procedures 1 month and 3 months after diagnosis. He underwent orthotopic liver transplantation (OLT) 1 year after the initial diagnosis of HCC. The post-OLT immunoregimen included OKT3 plus rituximab and high-dose steroids. On discharge from hospital he was on immunosuppressive treatment with tacrolimus. He had de novo autoimmune hepatitis 6 months post-OLT, which was treated with a short course of low-dose steroids and addition of mycophenolate mofetil.
The case
A 58-year-old white man with a 10-year history of type II diabetes (without microvascular or macro vascular complica tions), obesity, hypertension with evidence of left ventricular hypertrophy, hypothyroidism and non-alcoholic steatohepatitis-related liver cirrhosis (Child– Pugh class B, model for end-stage liver disease [MELD] score 10) with biopsy-proven hepatocellular carcinoma (HCC; Barcelona clinic liver cancer [BCLC] stage a, cancer of the liver Italian program [CLIP] score 1) presented for a second opinion on the management of his HCC. Previous routine ultrasound liver screening had found the mass to be about 8 cm in diameter. A CT scan repeated on the day of presentation for evaluation of the size of the tumor showed an 8 cm × 10 cm × 11 cm heterogeneously enhancing lesion without satellite nodules or portal vein involvement (Figure 1). During evaluation he had an episode of bleeding from gastric fundic varices that reduced his hemoglobin levels to 64 g/l (6.4 g/dl; normal range 140–175 g/l or 14.0–17.5 g/dl), consistent with clinically significant portal hypertension. Due to the large size of the tumor and underlying liver disease with significant portal hypertension, he was deemed not to be a candidate for surgical resection, radiofrequency ablation (RFA) or chemoembolization.
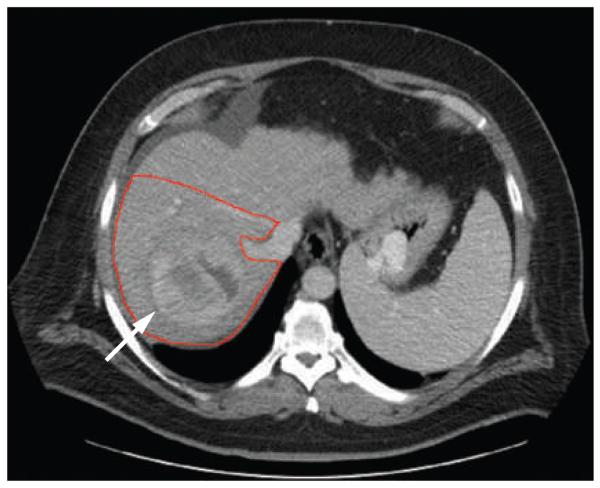
Figure 1.
A baseline CT scan showing the tumor (arrow) before treatment with Yttrium-90 glass microspheres with a calculated treatment volume of 1,518 ml.
The patient's baseline α-fetoprotein concentration was 5.3 μg/l (normal <20 μg/l). Hepatitis C antibody, hepatitis B surface antigen, hepatitis B core antibody, anti-nuclear antibody and anti-smooth muscle antibody were not detected. The patient's other laboratory values are shown in Table 1.
Table 1.
The patient's laboratory values
| Laboratory variable | Before radioembolization |
Before OLT | Normal range |
|---|---|---|---|
| Serum sodium (mmol/l) | 143 | 136 | 136–142 |
| Potassium (mmol/l) | 3.4 | 4.9 | 3.5–5.0 |
| Chloride (mmol/l) | 105 | 102 | 96–106 |
| Bicarbonate (mmol/l) | 29 | 25 | 21–28 |
| Blood urea nitrogen (mmol/l) | 12.5 | 10.7 | 2.9–8.2 |
| Serum creatine (μmol/l) | 91.5 | 129.6 | 8–31 |
| Serum albumin (g/l) | 36 | 36 | 35–50 |
| Alanine aminotransferase (μkat/l) |
0.58 | 0.47 | 0.17–0.68 |
| Aspartate aminotransferase (μkat/l) |
0.73 | 0.95 | 0.17–0.51 |
| Total bilirubin (μmol/l) | 13.7 | 15.4 | 5.0–21.0 |
| Alkaline phosphatase (μkat/l) | 1.27 | 1.3 | 0.5–2.0 |
| International normalized ratio | 1.2 | 1.1 | NA |
Abbreviations: NA, not applicable; OLT, orthotopic liver transplantation.
The patient was enrolled in the FDA-approved humanitarian device exemption protocol of transarterial radio-embolization with Yttrium-90 (90Y) glass microspheres (Therasphere®, MDS nordion, ottawa, Canada). At the initial planning angiogram (Figure 2), the technetium macroaggregated albumin scan showed a lung-shunt fraction of 6%. A CT scan showed a target volume of 1,518 ml (Figure 1). the patient underwent two treatments with 90Y glass microspheres without any adverse effects, which were performed as outpatient procedures by percutaneous access to the hepatic artery 1 month and 3 months after diagnosis. the doses of 90Y delivered were 110 Gy (3.65 GBq) for a target volume of 1,518 cm3 at 1 month, and 97 Gy (2.82 GBq) for a target volume of 1,327 cm3 at 3 months. a Bremsstrahlung scan performed after the first treatment confirmed localization of the microspheres in the tumor (Figure 3). The cumulative dose delivered to the lungs was estimated to be <30 Gy. A CT scan performed 7 months after the initial treatment showed a substantial decrease in the size of the mass to 5 cm × 4 cm with absence of arterial enhancement, which was suggestive of complete tumor necrosis (Figure 4). The patient had no tumor or tumor necrosis-related symptoms. The portal and hepatic veins remained patent. Given the excellent response to 90Y treatment, the seemingly nonaggressive biology of the tumor, as shown by lack of metastases (assessed by bone scan, head CT and chest CT scans) and by stable disease, the patient was referred for liver transplantation and was listed with a MELD score of 15.
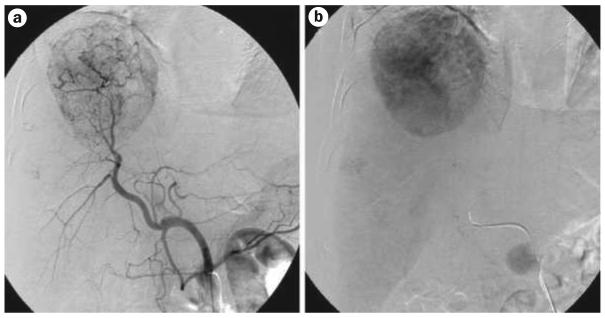
Figure 2.
The contrast angiogram with a | arterial phase and b | venous phase images.
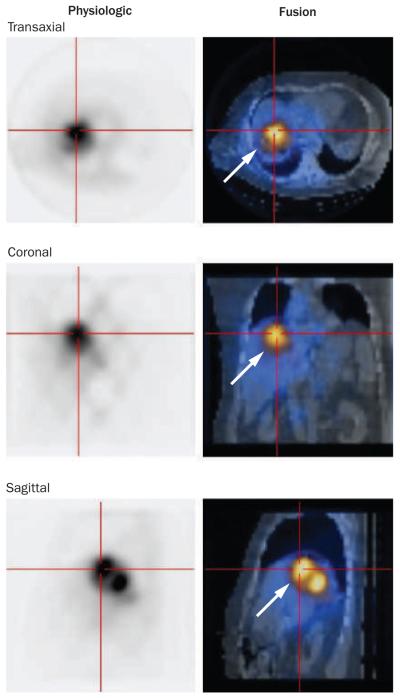
Figure 3.
Bremsstrahlung scan confirming the localization of the infused Yttrium-90 glass microspheres in the tumor (arrows).
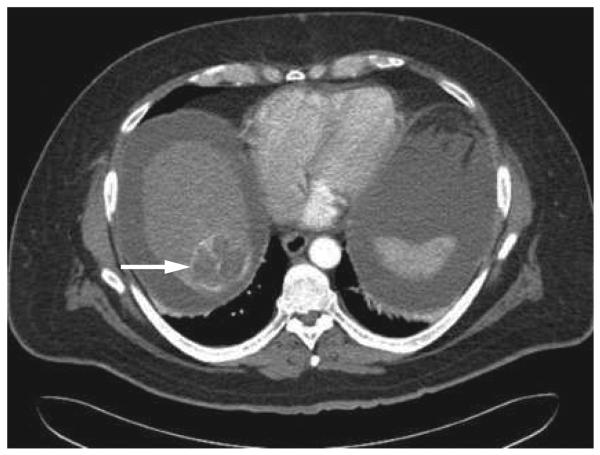
Figure 4.
CT scan showing the decrease in tumor size (arrow) and loss of vascular enhancement after treatment with Yttrium-90 glass microspheres.
Orthotopic liver transplantation (OLT) was performed 1 year after the initial diagnosis of HCC. The explant showed signs of cirrhosis and necrosis with a xanthogranulomatous inflammatory reaction, which was consistent with the tumor necrosis (measuring 4 cm in diameter) seen in the dome of the right lobe of the liver. There was no lymph node or diaphragmatic involvement. The immediate postoperative course was complicated by intraperitoneal bleeding. On exploratory laparotomy; this required packing for hemostasis.
The immediate post-OLT conditioning immunoregimen included intravenous antithymocyte globulin (OKT3) 150 mg every 48 h for three doses, and monoclonal antibody, rituximab 150 mg/m2 (a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of B cells) and high-dose steroids. on postoperative day 8 the patient was discharged on maintenance immunosuppressive treatment with tacrolimus with a target trough level in the range of 7–9 ng/ml to be taken indefinitely.
The patient developed de novo autoimmune hepatitis 6 months post-OLT, which was treated with a short course of low-dose steroids and the addition of the immunosuppressant mycophenolate mofetil at a dose of 1,000 mg orally twice daily. The most recent MRI, which was performed approximately 4.5 years after the initial HCC diagnosis and 3.5 years post-OLT, was negative for recurrence of HCC and the patient continues to do well.
Discussion of diagnosis
The incidence of HCC in the US has tripled over the past 30 years. It is now estimated that there are 20,000 new cases of HCC diagnosed each year and an equal number of deaths.1-4 HCC is also the gastro intestinal malignancy associated with the highest mortality rate and there are limited treatment options for those patients who have advanced disease.5 Liver cirrhosis of any etiology markedly increases the risk of HCC and the American Association for The Study of Liver Diseases (AASLD) guidelines6 advise that regular screening for HCC needs to be performed using liver ultrasonography every 6 months for patients with cirrhosis of any cause as well as for individuals with chronic HBV infection without cirrhosis that has been acquired at birth or in early childhood, after the age of 40 for Asian born males, after the age of 50 for Asian born females, and after the age of 20 for African born individuals of either gender.
HCC is often asymptomatic at the early stage of the disease, but acute decompensation of liver disease, spontaneous bacterial peritonitis, or right upper quadrant abdominal pain in patients with cirrhosis should alert the clinician to the possible presence of HCC.
The case patient has been followed up by his hepatologist for nonalcoholic steatohepatitis-related cirrhosis since 2002. He was found to have a mass in his liver during screening for HCC with serial ultrasound. Initially the mass was about 8 cm in diameter, but a repeat CT scan at our center showed that the mass had increased in size to 11 cm in diameter. The encapsulated nature of the mass and lack of satellite nodules in spite of the large size of the tumor suggested a less invasive and nonmetastatic phenotype of the tumor and a better long-term outcome. Options for management of HCC were limited due to marked portal hypertension as evidenced by a gastric variceal bleed episode that occurred during the initial consultation phase.
Treatment and management
HCC was initially deemed a contraindication for OLT by the US Department of Health and Human Services because of the poor overall post-OLT 5-year survival rate of 30–40%.7 In 1996, however, Mazzaferro et al. established the Milan criteria for the selection of patients with HCC for OLT, and reported a recurrence rate of 8% and recurrence-free survival rate of 83%.3,8 This work established OLT as the standard therapy for patients with a solitary HCC ≤5 cm or with two or three tumors each ≤3 cm. In 2002, Yao et al. found no difference in survival rates post OLT in 46 patients who did not meet the Milan criteria but who met the proposed University of California San Francisco (UCSF) criteria (a single lesion >6.5 cm; or two to three lesions, none >4.5 cm, with total tumor diameter ≤8 cm), and found that modest expansion to meet to UCSF criteria allowed transplantation of 23% more patients without affecting the survival rate.9,10 In the past 5 years, the United Network for Organ Sharing (UNOS) has instituted exception scores (up to 22) to accommodate patients with HCC who have MELD scores lower than 22, but only if they meet the Milan or UCSF criteria. Advanced HCC is still a contraindication for liver transplantation. The case patient did not meet the milan or UCSF criteria because his single lesion was too large.
External beam radiotherapy has limited application for the treatment of HCC because of the risk of radiation-induced liver disease.11,12 The other treatment options for advanced HCC are systemic chemotherapy, chemoembolization, radiofrequency ablation, percutaneous ethanol injection and radioembolization.
Transarterial radioembolization with 90Y glass microspheres allows selective lobar, segmental or subsegmental treatment of HCC. 90Y microspheres consist of non-biodegradable glass microspheres with a 25 μm mean diameter, in which 90Y is an integral constituent of the glass. 90Y is a pure β-emitter with a physical half-life of 64.1 h. The average energy emission of 90Y is 0.9,367 MeV, with a mean tissue penetration of 2.5 mm and a maximum tissue penetration of 10 mm.12-15 Alternative transarterial radionuclide therapies include radioactive iodine-131 (131I), rhenium-188 (188Re), and 90Y resin microspheres.16 90Y resin microspheres are approved for use in the US by the FDA under a humanitarian device exemption protocol, and were available for use at our center. as HCCs are highly vascular, the glass microspheres preferentially distribute to the tumors, sparing the adjacent non-neoplastic tissue. we chose this option due to large size of tumor and relatively decompensated liver disease. Another advantage of 90Y glass microsphere therapy compared with chemoembolization is that there is reduced incidence of post embolization syndrome, so it can therefore be performed on an outpatient basis. Postembolization syndrome is due to ischemic injury to the vascular system and is characterized by systemic symptoms such as abdominal pain, nausea, fatigue, and low to high-grade fevers with laboratory abnormalities. It occurs in approximately 60–80% of patients undergoing chemoembolization. It is usually self-limiting but the patients often require hospitaliza tion for the management of symptoms. Hence, the outpatient procedure of transarterial radioembolization is less expensive than repeated chemoembolization procedures.13-17 In addition, because 90Y glass microspheres used for radio embolization do not occlude the tumor vasculature, patients can subsequently receive further therapy with chemoembolization. Radioembolization with 90Y glass microspheres is particularly useful in patients with advanced HCC who are not candidates for resection. Previous publications have reported the use of 90Y glass microspheres as a bridge to liver transplantation, and as an alternative to RFA and resection in patients who initially were not candidates for liver transplantation or surgery.12,17
As the case patient had a large tumor and significant portal hypertension, which made surgical resection a high-risk procedure, he was an ideal candidate for treatment with 90Y glass microspheres. Treatment with radioembolization was uneventful, with no local-regional complications such as hematomas, no vascular complications and no systemic complications. A substantial decrease in the tumor mass was seen. As reported in earlier studies, the response to 90Y glass microspheretherapy is variable. Many tumor masses show loss of viability without a major decrease in tumor size, while a smaller proportion of tumors show substantial size decrease or even complete resolution of the tumor.12,17
Favorable characteristics for success of OLT in this case included HCC scored as BCLC class A and CLIP 1 and a good response to 90Y glass microsphere therapy.18,19 In addition, before listing for OLT a bone scan, head CT scan and chest CT scan ruled out metastasis. Post-OLT, we closely monitored the patient for recurrence of HCC and development of metastases. We monitored him with an abdominal MRI every 4 months for 1 year, and then he had an annual MRI. We also obtained an annual chest CT scan. We chose MRI because he had some baseline renal insufficiency due to his longstanding diabetic nephropathy and this worsened post OLT with the use of tacrolimus for maintenance of immunosuppression. When the patient had an episode of de novo autoimmune hepatitis, we added the immunosupressant mycophenolate mofetil, because it has fewer nephrotoxic effects.
HCC that extends beyond milan and UCSF criteria has a high incidence of tumor recurrence. However, in this case, the solitary, encapsulated nature of the tumor, the good response to radioembolization, no metastasis at the time of OLT and a low CLIP score indicated that a favorable outcome was possible. the patient's tumor-free survival more than 40 months post-OLT underscores the importance of careful risk stratification and decision making for each individual patient with HCC.
Conclusions
We report the case of a patient who remains tumor free more than 40 months post-OLT after radioembolization of a large HCC that was not eligible for the expanded UCSF criteria for liver transplantation.9,10
We underscore the role of 90Y glass microspheres as an alternative to chemoembolization for patients with unresectable HCC. This case is unique in that it describes successful downstaging of a large HCC with radioembolization that provided a bridge to OLT after which the patient is alive and cancer-free over 40 months post-transplant. We know of two HCC patients who had tumors of comparable size and a good response to 90Y glass microsphere therapy in 2006, but neither underwent OLT (personal communication, R. Salem).
Additional larger studies will be required to determine post-OLT outcomes after downstaging tumors before OLT with various modalities, including 90Y glass microspheres. The successful downstaging with radio-embolization and successful OLT of this patient with a large but solitary HCC, which had not metastasized and so was probably of nonaggressive biology, gives impetus to efforts to characterize the biological behavior of large HCCs using molecular methods, as this may allow selection of patients with a low risk of tumor dissemination for transplantation.
Acknowledgments
Lewis R. Roberts is supported by Mayo Clinic and Mayo Cancer Center, NIH Grants CA82862 and CA100882, an Industry Research Scholar Award from the Foundation for Digestive Health and Nutrition, a Harold Amos Medical Faculty Development Award from The Robert Wood Johnson Foundation, a generous gift from The Richard M. Schulze Family Foundation and the Miles and Shirley Fiterman Center for Digestive Diseases at the Mayo Clinic, Rochester, MN. The authors thank Vicki Campion for secretarial assistance.
Footnotes
Competing interests
L. R. Roberts declares associations with Isis Pharmaceuticals, Rosetta Genomics, Wako Diagnostics, MDS Nordion, and Bristol-Myers Squibb. See the article online for full details of the relationships. The other authors declare no competing interests.
References
- 1.Howe HL, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J. Natl Cancer Inst. 2001;93:824–842. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N. Engl. J. Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann. Intern. Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, et al. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberts SR, et al. Treatment options for hepatobiliary and pancreatic cancer. Mayo Clin. Proc. 2007;82:628–637. doi: 10.4065/82.5.628. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 7.Fung J, Marsh W. The quandary over liver transplantation for hepatocellular carcinoma: the greater sin? Liver Transpl. 2002;8:775–777. doi: 10.1053/jlts.2002.35336. [DOI] [PubMed] [Google Scholar]
- 8.Mazzaferro V, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 9.Yao FY, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765–774. doi: 10.1053/jlts.2002.34892. [DOI] [PubMed] [Google Scholar]
- 10.Yao FY, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 11.Goin JE, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J. Vasc. Interv. Radiol. 2005;16:205–213. doi: 10.1097/01.rvi.00001142592.89564.f9. [DOI] [PubMed] [Google Scholar]
- 12.Kulik LM, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J. Surg. Oncol. 2006;94:572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 13.Salem R, Hunter RD. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma: a review. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:S83–S88. doi: 10.1016/j.ijrobp.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 14.Geschwind JF, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Salem R, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J. Vasc. Interv. Radiol. 2005;16:1627–1639. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-Luna LE, et al. The clinical value of radioembolization in the treatment of inoperable liver cancer. US Gastroenterology & Hepatology Review. 2009;4:32–36. [Google Scholar]
- 17.Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107–S110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 19.The Cancer of the Liver Italian Program (CLIP) Investigators A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]