Contents
Spermatogonial stem cells (SSCs) reside within specialized microenvironments called ‘niches’, which are essential for their maintenance and self-renewal. In the mammalian testis, the main components of the niche include the Sertoli cell, the growth factors that this nursing cell produces, the basement membrane, and stimuli from the vascular network between the seminiferous tubules. This review focuses on signalling pathways maintaining SSCs self-renewal and differentiation and describes potential mechanisms of regulation of the spermatogonial stem cell niche.
Stem Cells and their Niche
Stem cells are required for the growth, maintenance and repair of many tissues. They are characterized by their abilities to self-renew and to produce numerous differentiated daughter cells, enabling them to play a central role in tissue homeostasis. In order for their maintenance and self-renewal to be ensured, they receive essential signals from their microenvironment, which is called the stem cell ‘niche’. The concept of stem cell niche was first suggested by Schofield in 1978, to describe a microenvironment that supports stem cells in the mammalian hematopoietic system (Schofield 1978). Other stem cell niches have now been identified in most tissues of model organisms, including the intestine, skin, brain and testis. The niche regulates specific properties of the stem cell, including self-renewal, pluripotency, quiescence and the ability to differentiate into single or multiple lineages (Adams and Scadden 2006). To this effect, the niche can be defined as a complex interplay of short- and long-range stimuli between the stem cells, their differentiating daughters, neighbouring cells, and the extracellular matrix, collectively making up a microenvironment that controls stem cell behaviour. Ultimately, this behaviour will depend on cellular intrinsic factors that are modulated by these signals (Watt and Hogan 2000).
The Spermatogonial Stem Cell Niche
In the mammalian testis, the stem cells that are at the origin of spermatogenesis are called spermatogonial stem cells (SSCs). Spermatogonial stem cells reside in the basal part of the seminiferous epithelium. These cells are the only stem cells in the body that undergo self-renewal throughout life and transmit genetic information to the offspring (De Rooij and Russell 2000). Spermatogonial stem cells are morphologically undifferentiated single cells that are not connected by intercellular bridges like the more advanced germ cells (Dym and Fawcett 1971; Huckins 1971; Oakberg 1971). They reside on the basement membrane and are also in intimate contact with the Sertoli cells, the only somatic cells present within the seminiferous epithelium. Spermatogonial stem cells self-renew in order to maintain spermatogenesis throughout the life of adult male animals. These cells also differentiate to produce Apaired, Aaligned, A1–A4 spermatogonia and type B spermatogonia, through a series of steps that will amplify the number of germ cells. Finally, type B spermatogonia will differentiate into spermatocytes that will translocate to the inner layer of the seminiferous epithelium, undergo meiosis and give rise to haploid spermatids that will differentiate into spermatozoa. Although some advances have recently been made, the molecular mechanisms underlying the maintenance and self-renewal of SSCs only begin to be elucidated. In addition, the signal that mediates the decision of SSCs to differentiate rather than self-renew is still unknown. This slow progress is due to the fact that these cells exist in low numbers (0.03% of the total number of germ cells in an adult testis; Meistrich and Van Beek 1993) and that no specific membrane marker is available to isolate them unequivocally. However, enrichment techniques are now available, that allow significant advances in our understanding of the behaviour of SSCs in vitro (Kubota et al. 2003; Buageaw et al. 2005; Hofmann et al. 2005b; Braydich-Stolle et al. 2007). In addition, the transplantation technique established by the group of R. Brinster a decade ago provided researchers with an in vivo functional assay to assess self-renewal and germline transmission after genetic manipulations of these cells (Nagano et al. 2001; Brinster 2002).
In the mammalian testis, the somatic Sertoli cell, the basement membrane and the cellular components of the interstitial space between the seminiferous tubules (Shetty and Meistrich 2007) are crucial components of the niche. The Sertoli cell provides growth factors necessary for self-renewal such as glial cell line-derived neurotrophic factor (GDNF) and basic fibroblast growth factor (bFGF) (Meng et al. 2000; Kubota et al. 2004; Hofmann et al. 2005b), the basement membrane and integrins provide for anchorage (Shinohara et al. 1999), and stimuli from the vascular network and interstitial cells are crucial for the localization of undifferentiated spermatogonia along specific portions of the basement membrane (Yoshida et al. 2007). The integration of these signals provides the cues necessary for self-renewal and retention of the SSCs in their undifferentiated state. These extrinsic signals will modulate SSC intrinsic signals such as kinases, second messengers and transcription factors to ensure homeostasis.
Cues for Self-Renewal
One factor that is essential for SSC self-renewal is GDNF. It is a protein secreted by Sertoli cells after birth, and is specifically responsible for the maintenance and proliferation of SSCs in vivo and in vitro (Meng et al. 2000; Kubota et al. 2004; Hofmann et al. 2005b). Glial cell line-derived neurotrophic factor belongs to the transforming growth factor-beta superfamily, and signals through a multi-component receptor formed by the Ret tyrosine kinase transmembrane receptor and its co-receptor glial cell line-derived neurotrophic factor family receptor alpha-1 (GFRα-1). The binding of GDNF triggers the activation of multiple signalling pathways in responsive cells (Airaksinen and Saarma 2002).
Mice lacking GDNF or its receptors die within the first day of birth with renal and neuronal abnormalities (Schuchardt et al. 1994; Moore et al. 1996; Pichel et al. 1996; Sanchez et al. 1996). The problem of neonatal mortality can be overcome by grafting GDNF, GFRα-1 or Ret deficient neonatal testes under the back skin of immunodeficient mice, and subsequently monitoring the development of the grafted testes (Naughton et al. 2006). This strategy revealed that any disruption of GDNF-mediated Ret signalling results in a lack of SSC self-renewal and induces the progressive loss of spermatogenesis by germ cell depletion. In comparison, normal spermatogenesis and maintenance of SSC populations was observed in the grafted wild type (WT) testes. Thus, GDNF, Ret and GFRa-1 are all crucial for SSC maintenance, emphasizing the essential role of the GDNF/Ret/GFRa1 signalling pathway in SSCs.
Spermatogonial stem cells have been difficult to isolate because of their low numbers and the lack of surface markers specific enough for immunosorting. Some laboratories, including ours, have isolated SSCs using antibodies against the GFRα-1 receptor (Buageaw et al. 2005; Hofmann et al. 2005b). Although expressed by both the stem cell and its direct progeny, the Apaired spermatogonia, GFRα-1 is an adequate marker for purifying SSCs from testes using antibody selection. Using gravity sedimentation on a 2–4% bovine serum albumin (BSA) gradient followed by magnetic beads isolation with a GFRα-1 antibody, we obtained a cell purity of up to 98%. However, while the purity is high, the number of cells recovered is low. Other investigators have been successful at enriching SSCs using antibodies to thymus cell antigen 1 (Thy-1) and fluorescence-activated cell sorting, producing an enrichment that was sufficient for most of their studies (Kubota et al. 2003). Glial cell line-derived neurotrophic factor, together with other growth factors, has recently been used to expand the number of SSCs in long-term cultures provided that they are grown onto feeder layers (Kanatsu-Shinohara et al. 2003; Ogawa et al. 2003; Kubota et al. 2004).
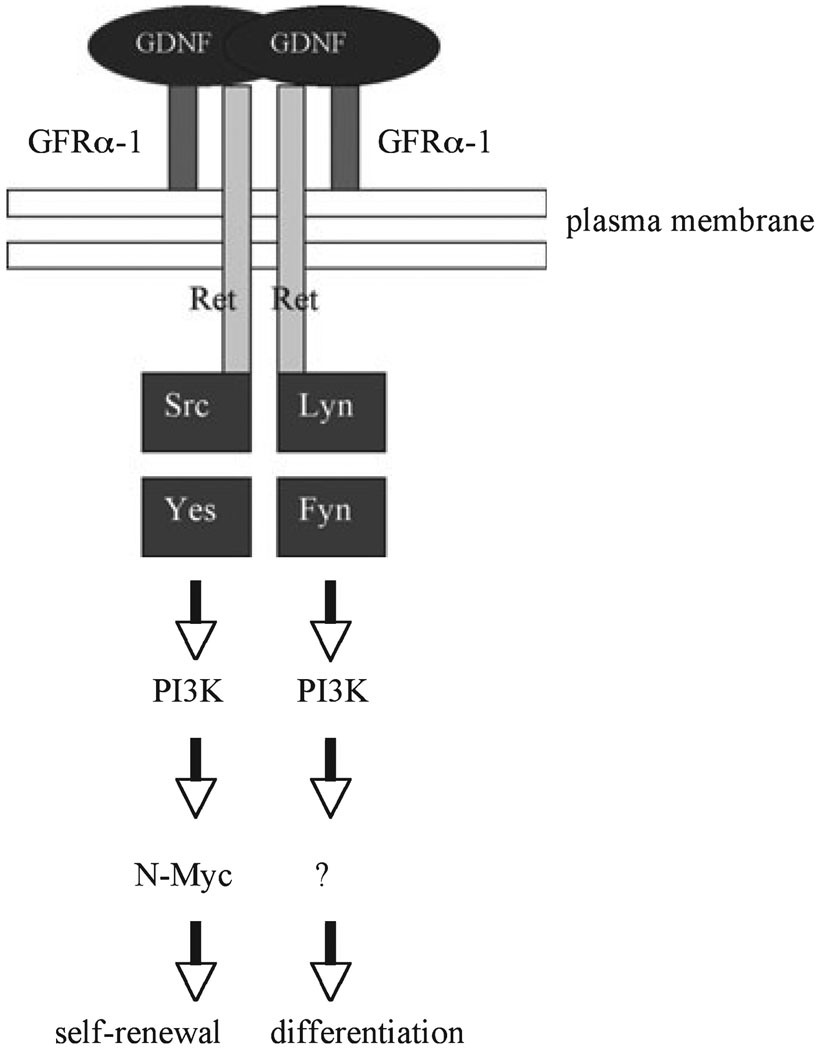
We recently elucidated some of the pathways induced by GDNF in SSCs by using serum-free short-term cultures of SSCs and a spermatogonial stem cell line that we established (Hofmann et al. 2005a). After stimulation by GDNF, we observed that several kinases from the Src family co-precipitate with the Ret receptor in SSCs (Braydich-Stolle et al. 2007). Further, we demonstrated that Src activates a PI3K/Akt signalling pathway, which ultimately leads to N-Myc expression and promotes SSC proliferation (Fig. 1). Subsequently, the groups of T. Shinohara and R. Brinster established the in vivo relevance of this signalling axis for SSC self-renewal by using germ cell transplantations after downregulating Akt and Src expression by RNA interference or pharmacological inhibitors (Lee et al. 2007; Oatley et al. 2007).
Fig. 1.
Glial cell line-derived neurotrophic factor can signal through activation of Src kinases in SSCs. The GDNF can promote cell cycle progression via activation of Src family kinases (SFKs) such as Src, Yes, Fyn and Lyn. The SFKs further activate a PI3K/Akt pathway, which ultimately leads to an increase in N-myc gene expression (from Braydich-Stolle et al. 2007)
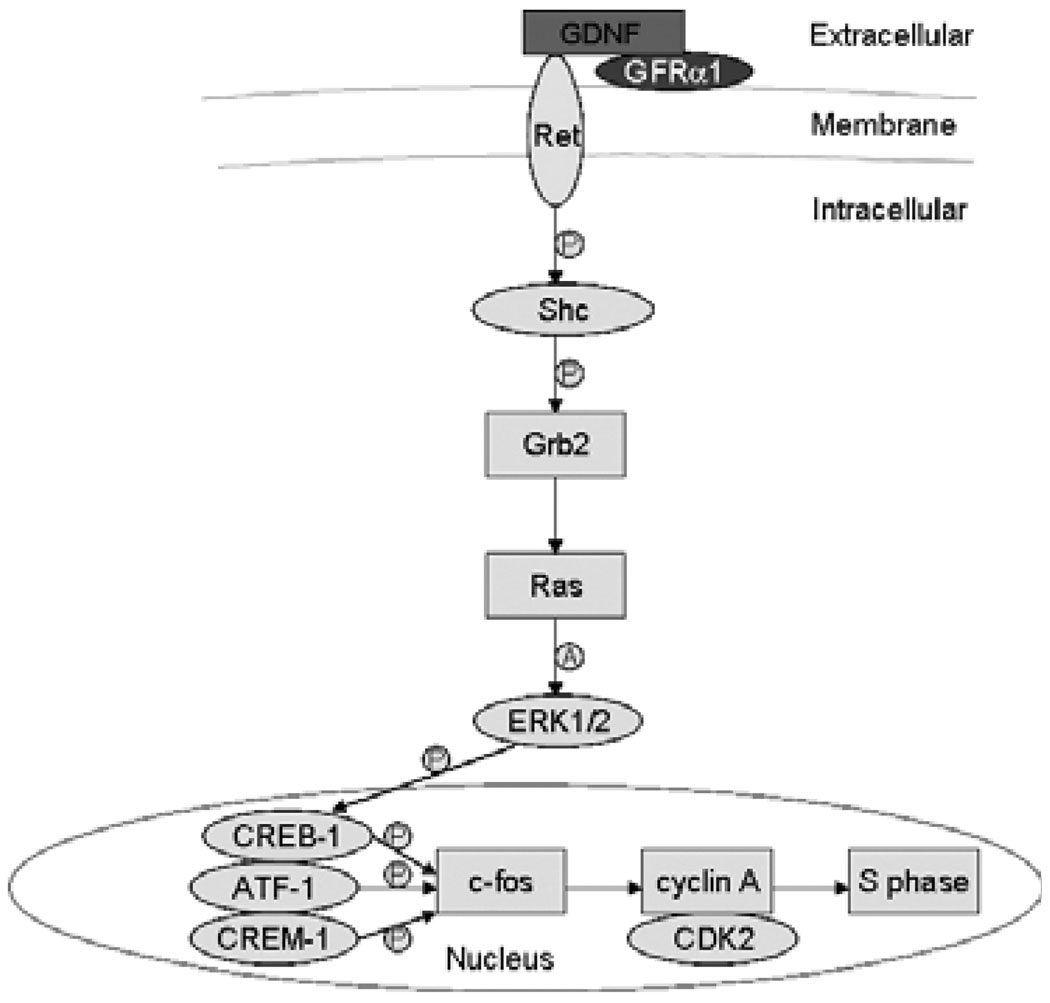
Ret activation by the binding of GDNF also induces the anchoring of the protein adaptors Shc and Grb2 and the activation of Ras in SSCs (He et al. 2008). Ras then activates ERK1/2, which ultimately leads to the phosphorylation and activation of transcription factors such as CREB-1, ATF-1 and CREM-1. Finally, the GDNF/Ret/Ras axis up-regulates the transcription of the immediate-early gene c-fos, the cell cycle activator cyclin A, as well as CDK2 (Fig. 2). This scenario is similar to what is observed in other cell types, where CREB and c-fos enhance the expression of cyclin A, favouring the G1/S cell cycle transition (Desdouets et al. 1995). Therefore, our studies suggest that GDNF induces SSC self-renewal through multiple signalling pathways.
Fig. 2.
Glial cell line-derived neurotrophic factor can signal through a Ras-induced pathway in SSCs. The GDNF can promote cell cycle progression via activation of the Ras/ERK1/2 pathway. Downstream events are also depicted. ‘P’ indicates ‘phosphorylate’, and ‘A’ denotes ‘activate’ (from He et al. 2008)
Other transcription factors have been recently identified, that are crucial for SSC maintenance or self-renewal. These include B cell CLL/lymphoma 6, member b (Bcl6b) (Oatley et al. 2006), TATA box binding protein (TBP)-associated factor 4b (Taf4b) (Falender et al. 2005) and promyelocytic leukaemia zinc-finger (Plzf) (Buaas et al. 2004; Costoya et al. 2004). The exact function of these molecules is not elucidated yet. However, there is evidence that Bcl6b is target of GDNF and plays a role in stem cell maintenance, as mice with a targeted disruption of Bcl6b have an increased incidence of Sertoli cell-only seminiferous tubules (Oatley et al. 2006). In addition, it seems clear that Plzf in WT animals represses SSC differentiation, while its loss in mutant or knockout mice shifts the balance towards differentiation at the cost of self-renewal. In addition, Plzf seems to directly repress the transcription of the receptor c-kit, which is important for spermatogonial differentiation (Filipponi et al. 2007), and undifferentiated spermatogonia isolated from Plzf −/− mice exhibit a marked increase in c-kit expression. Therefore, Plzf might maintain the pool of SSCs through direct repression of c-kit expression.
Regulation of Differentiation
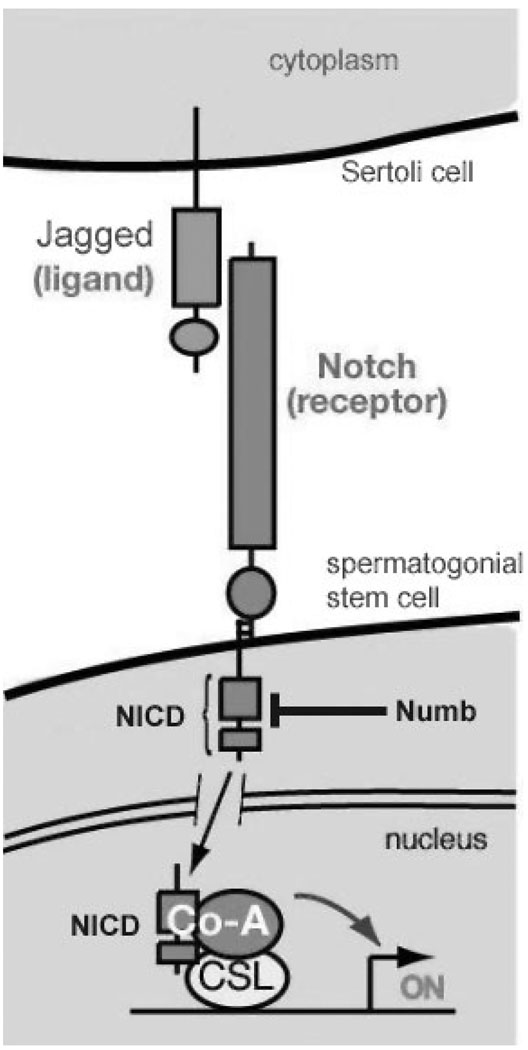
While the exact determinant of the self-renewal/differentiation switch has not been elucidated yet, progress has also been made in our understanding of the control and maintenance of SSCs differentiation. As mentioned above, differentiating spermatogonia express the c-kit receptor, and Kit ligand, produced by Sertoli cells, stimulates their DNA synthesis (Dolci et al. 2001). In addition, a recent study demonstrated that Kit ligand up-regulates the expression of early meiotic genes in these cells (Rossi et al. 2008), emphasizing the importance of this growth factor for germ cell differentiation. In 2001, two groups of researchers described the expression of Notch receptors and their ligands, Jagged-1 and Jagged-2, in the mammalian testis (Dirami et al. 2001; Hayashi et al. 2001). In mammals, the Notch signalling pathway is involved in cell fate decisions during various cellular and developmental processes (Weinmaster 1997). Notch is a transmembrane receptor of approximately 300 kDa in size. Upon binding to its ligand, Notch is proteolytically processed to generate a 180 kDa fragment containing most of the extracellular domain and a 120 kDa fragment containing the transmembrane and cytoplasmic domains. This latter fragment is in turn cleaved, releasing a shorter 80 kDA fragment, called Notch intracytoplamic domain (NICD), which migrates into the cell nucleus and functions as a transcription factor (Fig. 3). In the nucleus, activated Notch (NICD) interacts with other transcription factors to regulate cell fate decision. Because both ligand and receptor are localized in the plasma membrane of the effector and the target cell, respectively, a close cell–cell contact is necessary for the activation of this pathway. In mammals, four Notch genes (Notch1–4) have been isolated (Weinmaster et al. 1991; Lardelli et al. 1994; Uyttendaele et al. 1996). All Notch receptors show complementary and combinatorial expression patterns during the development of various tissues, including the testis (Williams et al. 1995; Mori et al. 2003). Immunocytochemistry revealed that the Notch family proteins are activated in specific germ cell types in the seminiferous tubules during germ cell development, and that their ligands Jagged-1 and Jagged-2 are expressed by Sertoli cells (Dirami et al. 2001). We observed that the expression of the Notch-1 intracytoplasmic domain (N1-ICD) starts before birth in gonocytes, increases as the germ cells proliferate and differentiate into type A and B spermatogonia, and peaks in spermatocytes indicating that activated Notch-1 might function to maintain the proliferation of germ cells as they differentiate. Further, addition of Jagged-1 in the culture media of freshly isolated SSCs increased their differentiation, as the number of Aaligned cells significantly increased in these cultures in comparison with the controls (Fig. 4). Suppression of Notch-1 signalling by using antibodies against Notch-1 or Jagged-2 led to maturation arrest in the rat testis (Hayashi et al. 2001). To evaluate a possible relationship between an alteration of Notch signalling and the pathogenesis of maturation arrest, Hayashi and colleagues examined the expression of Notch-1 and its ligand Jagged-2 in the testis of non-obstructive infertile patients (Hayashi et al. 2004). Patients showed maturation arrest at the spermatogonia, spermatocytes or spermatids stage. Patients with only spermatogonia in their seminiferous tubules lacked expression of both Notch-1 and Jagged-2 surface proteins. Patients exhibiting germ cells that reached the spermatocyte and spermatid stage lacked either Notch-1 or Jagged-2. None of the patients affected with maturation arrest expressed both Notch-1 and Jagged-2. These results indicate that Notch-1 is specifically involved in germ cell differentiation in humans, and that some compensation mechanisms by other Notch family members or Jagged-1 can occur.
Fig. 3.
The Notch signalling pathway. After the binding of Jagged 1/2 to the Notch receptor, the intracellular portion of the Notch receptor is cleaved and becomes Notch intracellular domain (NICD). The NICD is a transcription factor that translocates into the nucleus to activate the expression of target genes (adapted from E. Lai, Memorial Sloan-Kettering Cancer Center, Sloan-Kettering Institute, New York, NY, USA)
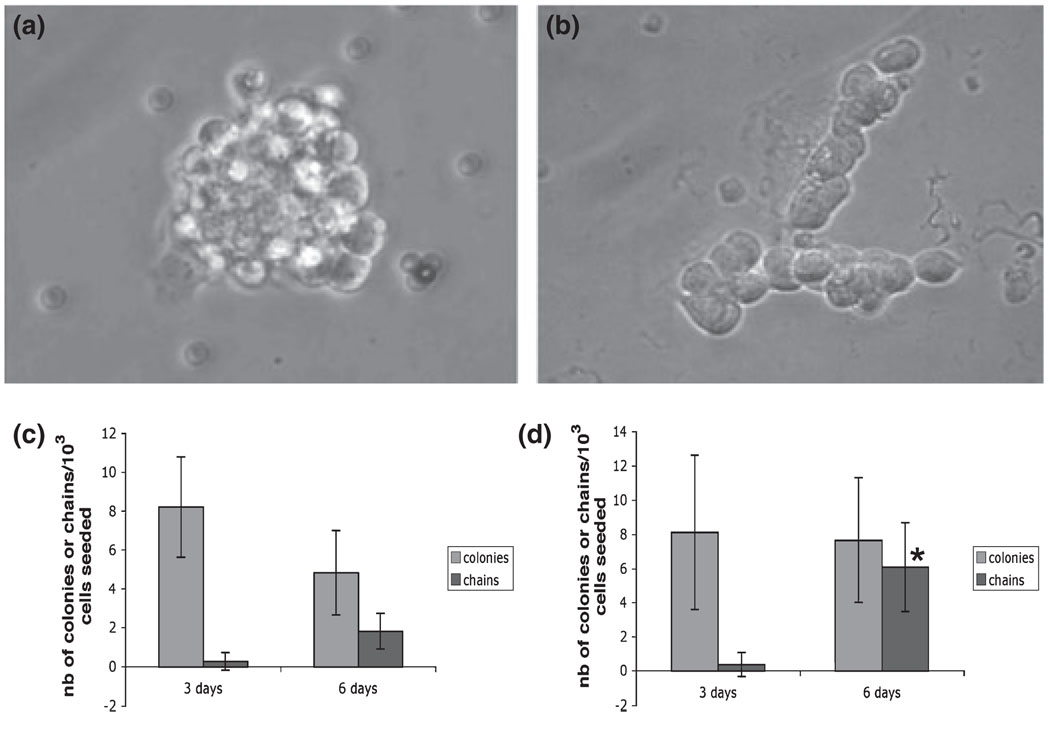
Fig. 4.
Influence of GDNF and Jagged-1 on SSCs in vitro. (a) Example of a colony of SSCs grown in vitro (from Braydich-Stolle et al. 2007). (b) Example of a chain of differentiating spermatogonia grown in vitro (from Hofmann et al. 2006). (c) Number of colonies and chains of spermatogonia obtained after culturing SSCs with 100 ng/ml GDNF for 3 and 6 days. (d) Number of colonies and chains of spermatogonia obtained after culturing SSCs with 10 µg/ml Jagged-1 for 3 and 6 days. A significant increase in the number of chains can be observed, indicating that Jagged-1 mediates SSC differentiation (*p < 0.05, Student’s t-test)
An interesting possibility is that factors induced by GDNF in SSCs would down-regulate Notch-1, and thus favour self-renewal at the expense of differentiation. For example, microarray analysis of GDNF-regulated genes indicated that Numb, an antagonist of Notch signalling, is up-regulated (Braydich-Stolle et al. 2005; Hofmann et al. 2005b). In vitro, we observed that Numb promotes the degradation of Notch-1 at the protein level in germ-line stem cells. Immunocytochemistry of Numb expression in sections of seminiferous tubules parallels the expression of Notch; it is found in type A spermatogonia and peaks in spermatocytes, indicating that Notch-1 is tightly regulated during the first step of spermatogenesis (Corallini et al. 2006).
Regulation of the Spermatogonial Stem Cell Niche
Recent studies have demonstrated that GDNF production by Sertoli cells is regulated by FGF2 (Simon et al. 2007). However, FGF2 knockout mice are viable and fertile, indicating that other factors are necessary. These factors include interleukin-1 beta (IL-1β) and tumour necrosis factor alpha (TNFα), which are produced essentially by monocytes, macrophages and dendritic cells (Simon et al. 2007). It is known that macrophages constitute approximately 20% of the interstitial tissue of the testis, where they are a major source of cytokines (Kern et al. 1995). In addition, GDNF production by Sertoli cells is also regulated by FSH (Tadokoro et al. 2002). Therefore, these data confirm that the stem cell niche is regulated at least in part by the vasculature and cellular components of the interstitium between the seminiferous tubules. The regulation of Jagged-1 /2 in Sertoli cells is not well understood, but studies have suggested that EGF might up-regulate Jagged-2 through interaction with the Sonic Hedgehog/Gli signalling pathway (Kasper et al. 2006).
Another molecule essential for the maintenance of the SSC niche and spermatogenesis is the Ets-related molecule Etv5 (Chen et al. 2005; Hess et al. 2006). The Etv5 is a transcription factor expressed by Sertoli cells and germ cells. While Etv5 expression is under control of GDNF in germ cells, it is driven by FGF2 and EGF in Sertoli cells (Oatley et al. 2006; Simon et al. 2007). The Etv5 binds to other transcription factors to up-regulate the expression of target genes (Gutierrez-Hartmann et al. 2007), and it is likely that its binding partners and function in Sertoli cells and germ cells are different. In mice with a targeted deletion of etv5 (etv5−/−), SSCs fail to renew, and these cells are lost through differentiation. Microarray analysis revealed that Etv-5 expression was essential for the production of several chemokines, including Stromal cell-derived factor (SDF-1, CXCL-12), CXCL5 and CCL7 (Chen et al. 2005). In addition, Etv5 seems important for the expression of the matrix metalloproteinase-12 (MMP-12). As chemokines and MMPs are well known for their involvement in stem cell recruitment, migration and homing (Heissig et al. 2002; Von Luttichau et al. 2005), it appears that in Sertoli cells, Etv5 is responsible for the production of factors that retain stem cells inside their niches. Additional studies also suggest that the blood–testis barrier (Sertoli–Sertoli tight junctional complex) is abnormal in the Etv5(−/−) mice, and several chemokines and MMPs have been shown to be critical in modulating various components that contribute to blood-testes barrier function (Morrow et al. 2008).
Alterations of the spermatogonial stem cell niche will occur with aging, and these changes contribute to deficient stem cell number and activity. This was recently demonstrated by transplanting SSCs from young, fertile male mice into the 1-year and 2-year atrophied testes of old males (Zhang et al. 2006). The results showed that 1-year-old testes are permissive for regeneration of spermatogenesis, while the 2-year-old testes are not, suggesting a gradual change in the SSC microenvironment. Further studies demonstrated a significant decrease of the production of GDNF by Sertoli cells with age, which could in part explain the decline of stem cell numbers (Ryu et al. 2006). However, in reciprocal transplantation experiments, colonization of young testes by 2-year-old SSCs was not as efficient as colonization by 1-year-old SSCs, indicating that stem cell intrinsic factors are also altered as the animal ages (Zhang et al. 2006). Age-related decline of the spermatogonial stem cell niche occurs as well in the Drosophila testis, where the decrease of expression of a key self-renewal signal, Unpaired (Upd), correlates with a decrease in SSCs with aging (Boyle et al. 2007). Conversely, forced expression of Upd by the somatic cells of the niche maintains SSCs in older males. Therefore, similar molecular mechanisms within the testicular niche maintain self-renewal across species, and their alterations contribute to a decline of the stem cell pool and spermatogenesis.
Conclusion
Spermatogonial stem cells are at the origin of spermatogenesis. Their fate is regulated by a complex interplay of growth factors produced by Sertoli cells and other germ cells, the extracellular matrix and vasculature components. The ensemble of these factors is called the spermatogonial stem cell niche. The behaviour of the SSCs is the result of interactions between the signals given from the niche and stem cell intrinsic factors such as kinases, phosphatases and transcription factors. Growth factors provided by the niche that regulate the fate of SSCs include GDNF, stem cell factor (SCF) and Jagged-1/2. There is little known about the mechanisms that regulate the production of these factors by Sertoli cells, but FGF2, FSH and cytokines produced by interstitial cells seem involved. Another molecule that regulates the function of the niche is the Sertoli cell transcription factor Etv5. By maintaining the blood–testis barrier, and by regulating the production of chemokines, and metalloproteinases by Sertoli cells, Etv5 contributes to the homing and physical maintenance of SSCs within the niche.
Acknowledgements
Funded by NIH grants R01-HD044543 and K02-HD054607.
Footnotes
Conflict of interest: The authors declare no conflict of interests.
References
- Adams G, Scadden D. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- Airaksinen M, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones D. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Nolan C, Dym M, Hofmann M. Role of glial cell line-derived neurotrophic factor in germ-line stem cell fate. Ann N Y Acad Sci. 2005;1061:94–99. doi: 10.1196/annals.1336.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, Hofmann M. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. Germline stem cell transplantation and transgenesis. Science. 2002;296:2174–2176. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas F, Kirsh A, Sharma M, Mclean DJ, Morris J, Griswold M, De Rooij DG, Braun R. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe V, Pholpramool C, Orwig K, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao G, Arber S, Kurpios N, Murphy TL, Cheng A, Hassell J, Chandrashekar V, Hofmann MC, Hess R, Murphy KM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corallini S, Fera S, Grisanti L, Falciatori I, Muciaccia B, Stefanini M, Vicini E. Expression of the adaptor protein m-Numb in mouse male germ cells. Reproduction. 2006;132:887–897. doi: 10.1530/REP-06-0062. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs R, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig K, Wolgemuth D, Pandolfi P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- De Rooij DG, Russell L. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Desdouets C, Matesic G, Molina CA, Foulkes N, Sassone-Corsi P, Brechot C, Sobczak-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirami G, Ravindranath N, Achi M, Dym M. Expression of Notch pathway components in spermatogonia and Sertoli cells of neonatal mice. J Androl. 2001;22:944–952. doi: 10.1002/j.1939-4640.2001.tb03434.x. [DOI] [PubMed] [Google Scholar]
- Dolci S, Pellegrini M, Di AS, Geremia R, Rossi P. Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor. J Biol Chem. 2001;276:40225–40233. doi: 10.1074/jbc.M105143200. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett D. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4:195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- Falender A, Freiman R, Geles K, Lo K, Hwang K, Lamb D, Morris PL, Tjian R, Richards J. Maintenance of spermatogenesis requires TAF4b, a gonad-specific subunit of TFIID. Genes Dev. 2005;19:794–803. doi: 10.1101/gad.1290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipponi D, Hobbs R, Ottolenghi S, Rossi P, Jannini E, Pandolfi P, Dolci S. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–6781. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Hartmann A, Duval D, Bradford A. ETS transcription factors in endocrine systems. Trends Endocrinol Metab. 2007;18:150–158. doi: 10.1016/j.tem.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kageyama Y, Ishizaka K, Xia G, Kihara K, Oshima H. Requirement of Notch 1 and its ligand jagged 2 expressions for spermatogenesis in rat and human testes. J Androl. 2001;22:999–1011. doi: 10.1002/j.1939-4640.2001.tb03441.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamada T, Kageyama Y, Negishi T, Kihara K. Expression failure of the Notch signaling system is associated with the pathogenesis of maturation arrest in male infertility patients. Fertil Steril. 2004;81:697–699. doi: 10.1016/j.fertnstert.2003.08.026. [DOI] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann M, Dym M. GDNF up-regulates c-fos transcription via the Ras /ERK1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett N, Crystal RG, Besmer P, Lyden D, Moore M, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R, Cooke P, Hofmann M, Murphy K. Mechanistic insights into the regulation of the spermatogonial stem cell niche. Cell Cycle. 2006;5:1164–1170. doi: 10.4161/cc.5.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Braydich-Stolle L, Dettin L, Johnson E, Dym M. Immortalization of mouse germ line stem cells. Stem Cells. 2005a;23:200–210. doi: 10.1634/stemcells.2003-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005b;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kasper M, Schnidar H, Neill G, Hanneder M, Klingler S, Blaas L, Schmid C, Hauser-Kronberger C, Regl G, Philpott M, Aberger F. Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol. 2006;26:6283–6298. doi: 10.1128/MCB.02317-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Robertson S, Mau V, Maddocks S. Cytokine secretion by macrophages in the rat testis. Biol Reprod. 1995;53:1407–1416. doi: 10.1095/biolreprod53.6.1407. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock M, Brinster R. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock M, Brinster R. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardelli M, Dahlstrand J, Lendahl U. The novel Notch homologue mouse Notch 3 lacks specific epidermal growth factor-repeats and is expressed in proliferating neuroepithelium. Mech Dev. 1994;46:123–136. doi: 10.1016/0925-4773(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Meistrich M, Van Beek M. Spermatogonial stem cells. In: Desjardins C, Ewing LL, editors. Spermatogonial Stem Cells. New York: Oxford University Press; 1993. pp. 266–295. [Google Scholar]
- Meng X, Lindahl M, Hyvonen M, Parvinen M, De Rooij DG, Hess M, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Moore M, Klein R, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt L, Ryan A, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Mori S, Kadokawa Y, Hoshinaga K, Marunouchi T. Sequential activation of Notch family receptors during mouse spermatogenesis. Dev Growth Differ. 2003;45:7–13. doi: 10.1046/j.1440-169x.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- Morrow C, Hostetler C, Griswold M, Hofmann MC, Murphy K, Cooke P, Hess R. ETV5 is required for continuous spermatogenesis in adult mice and may mediate blood testes barrier function and testicular immune privilege. Ann N Y Acad Sci. 2008;1120:144–151. doi: 10.1196/annals.1411.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Brinster C, Orwig K, Ryu B, Avarbock M, Brinster R. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton C, Jain S, Strickland A, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor (GDNF)-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Oakberg E. Spermatogonial stem-cell renewal in the mouse. Anat Rec. 1971;169:515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock M, Telaranta A, Fearon DT, Brinster R. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci USA. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Avarbock M, Brinster R. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Ohmura M, Yumura Y, Sawada H, Kubota Y. Expansion of murine spermatogonial stem cells through serial transplantation. Biol Reprod. 2003;68:316–322. doi: 10.1095/biolreprod.102.004549. [DOI] [PubMed] [Google Scholar]
- Pichel J, Shen L, Sheng H, Granholm A, Drago J, Grinberg A, Lee E, Huang S, Saarma M, Hoffer B, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Rossi P, Lolicato F, Grimaldi P, Dolci S, Di Sauro A, Filipponi D, Geremia R. Transcriptome analysis of differentiating spermatogonia stimulated with kit ligand. Gene Expr Patterns. 2008;8:58–70. doi: 10.1016/j.modgep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Ryu B, Orwig K, Oatley JM, Avarbock M, Brinster R. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira S, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schuchardt A, D’agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Shetty G, Meistrich M. The missing niche for spermatogonial stem cells: do blood vessels point the way? Cell Stem Cell. 2007;1:361–363. doi: 10.1016/j.stem.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Avarbock M, Brinster R. Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Ekman G, Tyagi G, Hess R, Murphy KM, Cooke P. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res. 2007;313:3090–3099. doi: 10.1016/j.yexcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113:29–39. doi: 10.1016/s0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122:2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- Von Luttichau I, Notohamiprodjo M, Wechselberger A, Peters C, Henger A, Seliger C, Djafarzadeh R, Huss R, Nelson PJ. Human adult CD34-progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]
- Watt F, Hogan B. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Weinmaster G. The ins and outs of notch signaling. Mol Cell Neurosci. 1997;9:91–102. doi: 10.1006/mcne.1997.0612. [DOI] [PubMed] [Google Scholar]
- Weinmaster G, Roberts VJ, Lemke G. A homolog of Drosophila Notch expressed during mammalian development. Development. 1991;113:199–205. doi: 10.1242/dev.113.1.199. [DOI] [PubMed] [Google Scholar]
- Williams R, Lendahl U, Lardelli M. Complementary and combinatorial patterns of Notch gene family expression during early mouse development. Mech Dev. 1995;53:357–368. doi: 10.1016/0925-4773(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ebata K, Robaire B, Nagano M. Aging of male germ line stem cells in mice. Biol Reprod. 2006;74:119–124. doi: 10.1095/biolreprod.105.045591. [DOI] [PubMed] [Google Scholar]