Abstract
FACT plays important roles in both gene transcription and DNA replication. However, how this protein complex is targeted to these two distinct cellular processes remains largely unknown. Here we show that ubiquitylation of the Spt16 subunit of FACT by Rtt101, the cullin subunit of an E3 ubiquitin ligase in Saccharomyces cerevisiae, links FACT to DNA replication. We find Rtt101 interacts with and ubiquitylates Spt16 in vitro and in vivo. Deletion of RTT101 leads to reduced association of both FACT and the replicative helicase MCM with replication origins. Loss of Rtt101 also reduces binding of FACT to MCM, but not the association of FACT with Leo1 and Spt5, two proteins involved in transcription. Origin function is compromised in cells lacking Rtt101 or with an Spt16 mutation. These findings identify Spt16 as an Rtt101 substrate, and suggest that Spt16 ubiquitylation is important for FACT to function during DNA replication.
Keywords: Chromatin, DNA replication, FACT, gene transcription, ubiquitylation
In eukaryotic cells, the packaging of DNA into chromatin affects many facets of DNA metabolism, including gene transcription and DNA replication. While it is well documented that these two processes use different machinery for initiation and elongation (Waga and Stillman 1998; Conaway and Conaway 1999; Bell and Dutta 2002), some chromatin remodeling complexes and histone chaperones are known to function in both gene transcription and DNA replication (Morrison and Shen 2009). In most of these cases, it remains unclear how these factors are targeted to these two distinct processes to perform a specific function.
FACT is one of the factors known to be involved in both gene transcription and DNA replication. Human FACT was discovered through its ability to promote transcription by RNA polymerase II (Pol II) on a chromatin template, and consists of two subunits: Spt16 and SSRP1 (Reinberg and Sims 2006). In contrast to ATP-dependent nucleosome remodelers that facilitate transcription by translocating histone octamers relative to DNA (Narlikar et al. 2002), FACT can either assemble or partially disassemble nucleosomes without ATP hydrolysis. In budding yeast, FACT consists of three proteins: a stable heterodimer of Spt16 and Pob3 that is supported by the HMG-like DNA-binding protein Nhp6 (Formosa et al. 2001). More recently, it has been shown that yeast FACT induces global accessibility of nucleosomal DNA without ATP hydrolysis (Xin et al. 2009).
In addition to its role in gene transcription, FACT also functions in DNA replication. Yeast FACT binds physically to DNA polymerase α and interacts genetically with many factors involved in DNA replication (Formosa et al. 2001; Formosa 2003; VanDemark et al. 2006). Moreover, FACT has been found to be an integral component of replisome progression complexes in budding yeast (Gambus et al. 2006), and human FACT interacts with the replicative DNA helicase MCM (Tan et al. 2006). While yeast FACT can be targeted to some promoters by interacting with Swi6 (Takahata et al. 2009), mechanisms for targeting FACT to DNA replication origins are unknown.
Rtt101 is a cullin-based Roc1-dependent E3 ubiquitin ligase (Petroski and Deshaies 2005). Cells lacking Rtt101 are sensitive to DNA-damaging agents and are defective in replication through damaged DNA or natural pause sites (Luke et al. 2006; Zaidi et al. 2008). Recently, it has been shown that Rtt101 binds Mms1, which serves as the adaptor protein to bind substrates (Zaidi et al. 2008). However, the substrates that link Rtt101 to DNA replication have not been identified previously. Here we show that Rtt101 interacts with and ubiquitylates Spt16 in vitro and in vivo in a reaction that is independent of Mms1. Moreover, we show that Rtt101 is required for efficient association of FACT with replication origins as well as the association of FACT with the replicative helicase MCM. In contrast, Rtt101 is not required for the association of FACT with Leo1 or Spt5, two proteins involved in gene transcription. These results demonstrate that Spt16 is a substrate of Rtt101, and suggest that ubiquitylation of FACT by Rtt101 targets FACT to DNA replication.
Results and Discussion
Rtt101 is proposed to function downstream from lysine acetyltransferase Rtt109 to maintain genome stability (Collins et al. 2007). We and others have shown that acetylation of H3 Lys 56 by Rtt109 is important to promote efficient nucleosome assembly following DNA replication and DNA repair (Chen et al. 2008; Li et al. 2008). Therefore, we tested whether Rtt101 ubiquitylates proteins known to be involved in nucleosome assembly. Briefly, tandem affinity purification (TAP)-tagged histone chaperones CAF-1 (Cac2-TAP), Asf1, Rtt106, and FACT (Spt16-TAP) were isolated from wild-type and rtt101Δ mutant cells, and copurified ubiquitylated species were detected by Western blot. We found that deletion of RTT101 had no apparent effect on the amounts of ubiquitylated proteins that copurified with CAF-1, Asf1, and Rtt106 (Supplemental Fig. S1). In contrast, ubiquitylated proteins that copurified with Spt16, the large subunit of the FACT complex, were reduced significantly in the absence of Rtt101 (Fig. 1A; Supplemental Fig. S1).
Figure 1.
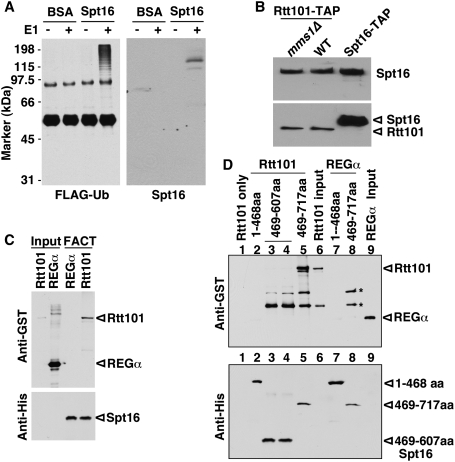
Rtt101 is required for efficient ubiquitylation of Spt16. (A) Ubiquitylation of Spt16 or its associated proteins is reduced in rtt101Δ mutant cells. Spt16-TAP was immunoprecipitated from wild-type (WT) and rtt101Δ mutant cells transformed with a plasmid expressing HA-Ub. Proteins in immunoprecipitated and whole-cell extracts (WCE) were detected by Western blotting using antibodies against the indicated proteins. (B) Ubiquitylated Spt16 was detected in wild-type but not rtt101Δ mutant cells under denaturing conditions. Ubiquitylated proteins from wild-type (spt16-TAP) or rtt101Δ mutant cells (spt16-TAP, rtt101Δ) expressing (His)6-HA-Ub were purified in a buffer containing 8 M urea. Copurified proteins (left panel), as well as proteins in the WCE (right panel), were detected by Western blotting. As a negative control, wild-type (spt16-TAP) cells expressing HA-Ub were used. (C) Ubiquitylation of Pob3 was not detected. Experiments were performed as described in B using wild-type (pob3-TAP) and rtt101Δ (pob3-TAP rtt101Δ) strains, a positive control strain (spt16-TAP), and a negative control strain without the TAP-tagged proteins (No-TAP). (D) The ubiquitylation of Spt16 by Rtt101 depends on the Rtt101–Roc1 interaction. The experiments were performed as in B using the strain (spt16-TAP rtt101Δ) transformed with a plasmid expressing wild-type Rtt101, an Rtt101 mutant lacking residues 680–686 (Δ680–686), or vector. The Spt16-TAP strain transformed with empty vector was used as a control. The band marked by an asterisk represents Rub1-modified Rtt101. (E) Mms1 is not required for efficient Spt16 ubiquitylation. The experiment was performed as described in A except using strains with and without MMS1.
Based on the apparent molecular weight of ubiquitylated proteins purifying with Spt16-TAP, it appeared that Spt16 itself was ubiquitylated by Rtt101. To examine this possibility, we first expressed ubiquitin (Ub) tagged with both histidine and HA epitopes (His-HA-Ub) in wild-type and rtt101Δ mutant cells containing Spt16-TAP. The ubiquitylated proteins were purified using a nickel matrix in a buffer containing 8 M urea, which disrupts protein–protein interactions. Under the denaturing condition, Spt16 copurified with His-HA-Ub from wild-type but not rtt101Δ mutant cells (Fig. 1B). In contrast, Pob3 did not copurify with His-HA-Ub (Fig. 1C). Together, these results demonstrate that Spt16, but not Pob3, is ubiquitylated in an Rtt101-dependent manner in yeast cells.
Cullin-based E3 ligases share a common architecture, including a cullin and a RING finger protein that binds an E2 (Petroski and Deshaies 2005). The RING finger protein for Rtt101 is Roc1, and cells expressing an Rtt101 mutant (rtt101Δ680–686) that cannot bind Roc1 display phenotypes similar to rtt101Δ cells (Supplemental Fig. S2; Michel et al. 2003). We therefore asked whether Roc1 binding to Rtt101 was required for Spt16 ubiquitylation. As shown in Figure 1D, Spt16 ubiquitylation was detectable in rtt101Δ cells ectopically expressing wild-type Rtt101, but not in cells expressing the rtt101Δ680–686 mutant. Thus, Spt16 ubiquitylation is dependent on the Roc1–Rtt101 interaction.
Mms1 and Mms22 function in the same genetic pathway as Rtt101 to maintain genome stability (Collins et al. 2007). It is proposed that Mms1 serves as an adaptor to bind substrates (Zaidi et al. 2008). We confirmed that Rtt101 interacts with Mms1 (Supplemental Fig. S3A). Surprisingly, deletion of MMS1 or MMS22 had no apparent effect on Spt16 ubiquitylation in yeast cells (Fig. 1E; Supplemental Fig. S3B). Thus, Spt16 ubiquitylation by Rtt101 does not appear to depend on Mms1 or Mms22.
Next, we asked whether Rtt101 ubiquitylates Spt16 in vitro. Briefly, Rtt101 was purified from yeast cells and incubated with recombinant Pob3–Spt16 complexes in the presence of E1, E2 (Cdc34), and Flag-tagged ubiquitin (Flag-Ub) in vitro. The ubiquitylated proteins were immunoprecipitated and detected by Western blotting with antibodies against either the Flag epitope or Spt16. Spt16 was ubiquitylated in vitro only in the presence of E1 and Rtt101 (Fig. 2A; data not shown). Moreover, ubiquitylation of BSA was not detected under the same assay conditions. Thus, Rtt101 can modify Spt16 directly in vitro.
Figure 2.
Rtt101 ubiquitylates and binds FACT. (A) Rtt101 ubiquitylates FACT in vitro. Recombinant FACT proteins were incubated with Rtt101, Flag-Ub, and other components for ubiquitylation reactions in the presence or absence of E1. Ubiquitylated species were isolated and detected by Western blotting using antibodies against the Flag epitope and Spt16. (BSA) Bovine serum albumin. (B) Rtt101 interacts with Spt16 in vivo. Rtt101-TAP was purified from wild-type and mms1Δ mutant cells, and copurified proteins were detected by Western blotting with an antibody against Spt16 (top panel) or calmodulin-binding protein (CBP) (bottom panel). Purification of Spt16-TAP was used as a positive control. (C) Rtt101 interacts with Spt16 in vitro. His-tagged FACT proteins immobilized to Ni-NTA agarose were incubated with GST-Rtt101 proteins. Proteins bound to FACT were detected by Western blotting using antibodies against GST-Rtt101 (top panel) and His-Spt16 (bottom panel). GST-REGα, a proteasome-binding protein, was used as a negative control. (D) Rtt101 binds to the region spanning 608–717 of Spt16. The experiment was done as described in C except using His-tagged Spt16 mutant proteins. Asterisks indicate nonspecific bands.
To determine whether Spt16 ubiquitylation by Rtt101 is due to a direct interaction between Spt16 and Rtt101, we first tested whether Rtt101 interacts with Spt16 in yeast cells. As shown in Figure 2B, Spt16 copurified with Rtt101 from yeast cell extracts. Moreover, deletion of MMS1 did not affect the binding of Rtt101 to Spt16. To examine whether Rtt101 binds directly to Spt16, recombinant FACT was immobilized to Ni-NTA beads and incubated with recombinant GST-Rtt101 proteins. The proteins that bound to FACT were detected by Western blotting with antibodies against GST. As shown in Figure 2C, FACT bound GST-Rtt101, but not an unrelated control protein, GST-REGα. Moreover, while the N terminus of Spt16 (1–468) did not bind Rtt101, the Spt16 middle region (469–717) bound Rtt101 in vitro (Fig. 2D). Together, these results demonstrate that Rtt101 interacts directly with FACT in vitro and in vivo, and provide an explanation for the ability of Rtt101 to ubiquitylate Spt16 in the absence of Mms1 or Mms22 (Fig. 1E; Supplemental Fig. S3).
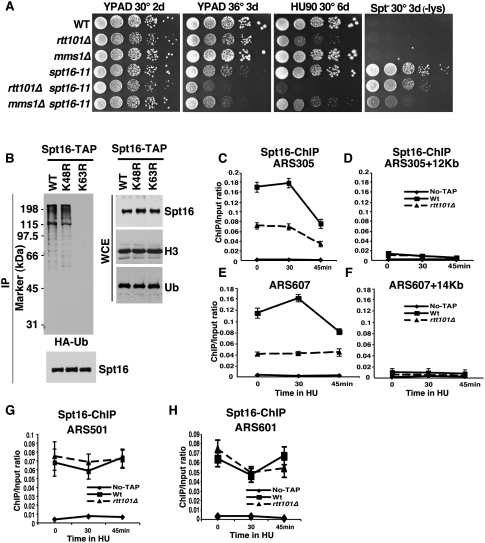
The Spt16–Pob3 complex is involved in both DNA replication and gene transcription (Formosa 2003; Reinberg and Sims 2006). Thus, cells expressing the spt16-11 allele are sensitive to the DNA-damaging agent hydroxyurea (HU), indicating a defect in replication, and they also exhibit the Spt− phenotype, indicating a defect in initiation of transcription (Fig. 3A; Supplemental Fig. S4A; Formosa 2003). To address whether ubiquitylation of Spt16 by Rtt101 affects Spt16's function in replication, gene transcription, or both, we examined the effect of deleting RTT101 in cells with the spt16-11 mutation. As shown in Figure 3A, the rtt101Δ spt16-11 double-mutant cells grew more slowly at 36°C and were more sensitive to replication stress (HU) than either the rtt101Δ or spt16-11 single mutants. In contrast, the rtt101Δ mutation had little or no effect on the Spt− phenotype of the spt16-11 mutant cells. Deletion of RTT101 also enhanced the DNA replication-related phenotypes caused by a mutation in another subunit of FACT, Pob3 (pob3-Q308K), but not the transcription defect (Supplemental Fig. S4B). Interestingly, deletion of MMS1 exhibits a distinct pattern of genetic interactions when combined with either spt16-11 or pob3-Q308K (Fig. 3A; Supplemental Fig. S4B), consistent with biochemical studies indicating that Spt16 ubiquitylation does not require Mms1. Importantly, the genetic analysis suggests that Rtt101 promotes the ability of FACT to function in DNA replication but not in gene transcription in vivo.
Figure 3.
Rtt101 is required for efficient binding of Spt16 to replication origins. (A) SPT16 interacts genetically with RTT101 in a process linked to DNA replication. Tenfold serial dilutions of yeast cells of the genotype indicated at the left were spotted onto YPD medium alone, YPD medium containing HU, or lysine dropout medium to assay the Spt− phenotype. Plates were incubated at the indicated temperature and time (in days). (B) Spt16 ubiquitylation is mediated by K63-linked Ub. The experiment was performed as described in Figure 1A in cells expressing wild-type ubiquitin (WT) or ubiquitin with mutations at Lys 48 (K48A) or Lys 63 (K63A). (C–F) The levels of Spt16 at early replication origins are reduced in cells lacking Rtt101. ChIP assays were performed in wild type (spt16-TAP), rtt101Δ (spt16-TAP rtt101Δ), and a strain without the tag (No-TAP). The ChIP DNA was analyzed by real-time PCR using four sets of primers that amplify two early replication origins (ARS305 and ARS607), a fragment 12 kb away from ARS305 (ARS305 + 12 kb), or a fragment 14 kb away from ARS607 (ARS607 + 14 kb). Similar results were obtained from at least two independent ChIP experiments, with a representative example shown. (G,H) Deletion of Rtt101 had no effect on the binding of Spt16 to two late-firing origins. ChIP assays were performed as described in C–F.
Protein ubiquitylation can serve as a signal for either protein degradation or altered function (Bergink and Jentsch 2009; Bhoj and Chen 2009). Deleting RTT101 only slightly increased the half-life of Spt16 (Supplemental Fig. S5), and the level of Spt16 did not change significantly in the rtt101Δ mutant cells compared with wild-type cells (Fig. 1A,B), suggesting that Spt16 ubiquitylation by Rtt101 does not signal degradation of FACT. To test this idea further, we determined whether Spt16 ubiquitylation uses Lys 63 or Lys 48 of ubiquitin. Ubiquitin chains formed through Ub K48 serve as a proteasome targeting signal, whereas the Ub K63-linked chains play mainly regulatory functions (Bergink and Jentsch 2009; Bhoj and Chen 2009). We found that mutation of Lys 63, but not of Lys 48, of Ub significantly reduced Spt16 ubiquitylation in yeast cells (Fig. 3B), suggesting that ubiquitylation of Spt16 predominantly uses the K63 linkage. This result suggests that Spt16 ubiquitylation by Rtt101 is not involved in proteolysis, and may instead alter its affinity for binding partners and/or other properties.
To find how Spt16 ubiquitylation impacts its function in replication, we used a chromatin immunoprecipitation (ChIP) assay to ask whether Rtt101 is required for Spt16 localization to replication origins. Briefly, wild-type and rtt101Δ mutant cells with TAP-tagged Spt16 and a strain with no TAP tag were synchronized in G1 using α-factor and then released into fresh media containing HU. Samples were collected at different times after release, and ChIP assays were performed using antibodies against the TAP tag. In the presence of HU, early replication origins such as ARS305 and ARS607 fire normally, but replication forks progress slowly (Bell and Dutta 2002). Therefore, the replication forks originating from ARS305 inefficiently reach a fragment 12 kb away from this origin in media containing HU. As shown in Figure 3, C–F, more Spt16 was detected at two early replication origins (ARS305 and ARS607) than at regions distant from the origin in G1 as well as in early S phase. Moreover, Spt16 was associated with two late replication origins (ARS501 and ARS601) in both G1 and early S phase. To our knowledge, this is the first report that Spt16 localizes to replication origins in G1 and early S phase in budding yeast. Remarkably, the amount of Spt16 associated with either early replication origin (Fig. 3C,E), but not the two late replication origins tested (Fig. 3G,H), was significantly reduced in rtt101Δ mutant cells. Furthermore, we found that chromatin binding of Spt16 was not affected in orc2-1 mutant cells at a restrictive temperature (Supplemental Fig. S6). Orc2 is a subunit of the origin recognition complex (ORC), and the ORC is not stable in orc2-1 mutant cells (Bell et al. 1993; Shor et al. 2009). These results suggest that Rtt101 is required for efficient association of Spt16 with a subset of replication origins during G1 and early S phase, and that this association may be independent of a functional ORC.
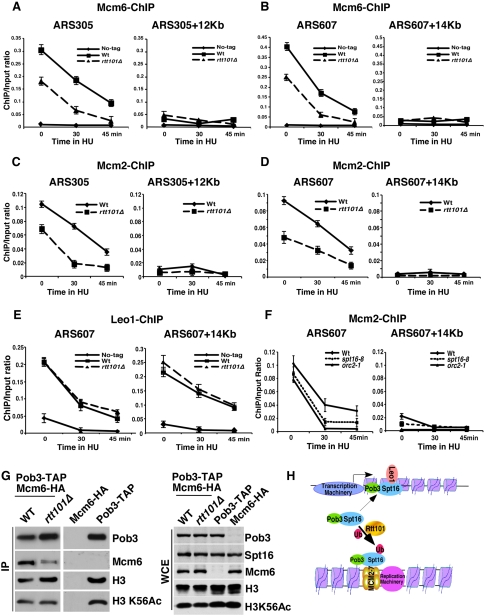
FACT has been found to be part of a large replication progression complex containing the replicative helicase MCM proteins and GINS (Gambus et al. 2006). We next determined whether Rtt101 is required for stable association of MCM with replication origins using a ChIP assay. As shown in Figure 4, A–D, both Mcm6 and Mcm2 were associated with ARS305 and ARS607 in G1 and early S phase, and this association was reduced significantly in cells lacking Rtt101. In contrast, we found that deletion of RTT101 did not affect chromatin association of Leo1, a subunit of the Paf1 complex involved in gene transcription (Squazzo et al. 2002), or Spt5, a protein involved in transcription elongation by Pol II (Fig. 4E; Supplemental Fig. S7; Hartzog et al. 1998). Finally, we determined whether FACT is also required for MCM binding to chromatin using the SPT16 temperature-sensitive allele spt16-8 (see below). We found that Mcm2 binding to the early replication origin ARS607 was reduced in spt16-8 cells compared with wild-type cells, but to a lesser degree than in orc2-1 cells at a nonpermissive temperature (Fig. 4F). Interestingly, the binding of Mcm2 to two late replication origins tested was not affected significantly in spt16-8 cells (Supplemental Fig. S8A,B). Therefore, both Rtt101 and FACT are required for efficient binding of MCM to a subset of replication origins, and Rtt101 is not required for chromatin association of Leo1 and Spt5 (two proteins involved in gene transcription).
Figure 4.
Rtt101 and FACT are required for efficient binding of MCM proteins at replication origins. (A–D) Cells lacking Rtt101 display reduced levels of Mcm2 (A,B) and Mcm6 (C,D) at replication origins during G1 and early S phase. ChIP assays were performed as described in Figure 3, C–F, except that strains with no tag, mcm6-HA (WT), and mcm6-HA rtt101Δ (rtt101Δ), and antibodies against the HA epitope were used. The ChIP DNA was analyzed by real-time PCR using four sets of PCR primers as described in Figure 3, C–F. (E) The chromatin binding of Leo1 is not affected in rtt101Δ mutant cells. ChIP assays were performed using strains No-tag, leo1-HA (WT), and leo1-HA rtt101Δ (rtt101Δ). (F) Mcm2 binding to replication origin ARS607 is reduced in spt16-8 cells. Wild-type, spt16-8, and orc2-1 cells were synchronized in G1 at 25°C, and were released into HU media at 37°C at the indicated time. ChIP assays were performed using antibodies against Mcm2, and ChIP DNA was analyzed as described in Figure 3. (G) The association of Mcm6 with FACT is reduced in rtt101Δ mutant cells. Pob3 was purified from wild-type (pob3-TAP, mcm6-3HA) and rtt101Δ mutant (pob3-TAP, mcm6-3HA rtt101Δ) cells. As controls, the purification procedures were also performed using the mcm6-3HA and pob3-TAP strains. Purified proteins (IP) and proteins in WCE were analyzed by Western blotting. (H) A proposed model for targeting FACT to DNA replication by Rtt101-mediated Spt16 ubiquitylation.
To determine whether decreased association of the MCM complex with origins in rtt101Δ and spt16-8 mutant cells affects origin firing efficiency, we tested plasmid maintenance in these cells. Cells with impaired origin firing efficiency lose plasmids at a high rate, but this can be rescued by providing multiple replication origins on the plasmid to give multiple independent opportunities to initiate replication (Hogan and Koshland 1992). Deletion of RTT101 caused a significant increase in the rate of plasmid loss, and this was suppressed by providing multiple origins (Supplemental Fig. S8C). Similarly, spt16-8 mutants displayed a pattern of plasmid loss that indicates a defect in initiation (Supplemental Fig. S8C), while some other FACT mutants exhibited normal replication initiation (Supplemental Fig. S8C; data not shown). These results demonstrate that both Rtt101 and FACT have roles in DNA replication initiation.
Our results appear to contradict a previously published report that initiation of DNA replication is normal in rtt101Δ mutant cells because deletion of RTT101 does not affect formation of replication intermediates as detected by two-dimensional (2D) agarose gels (Luke et al. 2006). One possible explanation is that analysis of replication intermediates by 2D gels is not sensitive enough to detect subtle defects in initiation of DNA replication in rtt101Δ mutant cells. Consistent with a subtle but important role for Rtt101 in initiation, the chromatin association of Cdc17, the catalytic subunit of DNA polymerase α, was also not affected significantly in rtt101Δ mutant cells (Supplemental Fig. S8D), but, as noted above, initiation was significantly impacted (Supplemental Fig S8C). The number of MCM complexes loaded onto chromatin far exceeds the number of replication origins in the genome (Lei et al. 1996; Ibarra et al. 2008). The additional MCM molecules are proposed to allow initiation of dormant replication origins in response to stresses such as DNA damage (Ibarra et al. 2008). Thus, it is possible that the reduced level of MCM proteins in chromatin that we observed in rtt101Δ mutant cells explains the defects in replication through damaged DNA in rtt101Δ mutant cells (Luke et al. 2006). We suggest that the Rtt101-dependent loading of MCM proteins to replication origins contributes to both initiation of DNA replication and replication through damaged DNA.
Finally, we asked whether Rtt101 is required for FACT to bind its interacting partners involved in both DNA replication (MCM and Sld5) and gene transcription (Leo1 and Spt5). Pob3-TAP or Spt16-TAP were purified from wild-type and rtt101Δ mutant cells, and copurified proteins were detected by Western blotting. Mcm6 and Mcm4 copurified with Pob3 (Fig. 4G; Supplemental Fig. S9A–C), suggesting that FACT interacts with the MCM complex. Importantly, the association of FACT with MCM was reduced significantly in rtt101Δ mutant cells compared with wild-type cells in three distinct experiments (Fig. 4G; Supplemental Fig. S9). In contrast, deletion of RTT101 had no apparent effect on the binding of Spt16 with Sld5, a subunit of the GINS complex, in cell extracts (Supplemental Fig. S10A). This result suggests that FACT–MCM and FACT–GINS interactions are regulated distinctly, and is consistent with the report that the FACT–MCM interaction is independent of GINS (Gambus et al. 2006). In contrast, the association of FACT with Leo1 (Supplemental Fig. S10B) and Spt5 (Supplemental Fig. S10C) was not affected in rtt101Δ mutant cells. These results indicate that Rtt101 is required for efficient binding of Spt16 with the MCM complex in vivo. As shown above, the efficient association of both FACT and the MCM complex with replication origins requires Rtt101. It is possible that ubiquitylation of FACT enhances its recruitment to replication origins, which in turn promotes MCM loading. Alternatively, MCM association with origins might be a consequence of Rtt101 action independent of the modification of Spt16. Additional investigation will be required to determine which is the case.
In summary, we showed that Spt16 is a substrate of the E3 ubiquitin ligase complex containing Rtt101. Rtt101 is required for efficient binding of FACT with MCM, as well as the association of both FACT and MCM with replication origins during G1 and early S phase. Moreover, both FACT and Rtt101 are required for efficient initiation of DNA replication. Together, these results suggest that ubiquitylation of FACT by Rtt101 targets FACT to replication machinery, promoting initiation of DNA replication (Fig. 4H). This is the first report of a mechanism that specifically promotes the function of FACT in DNA replication without affecting its roles in transcription.
Materials and methods
Yeast strains
Yeast strains isogenic to W303-1A (leu2-3, 112 ura3-1 his3-11, trp1-1, and ade2-1 can1-100) were used for all experiments except those in Figure 3A and Supplemental Figures S4B and S8C, which are isogenic with A364a (Supplemental Table 1). Standard yeast media and manipulations were used.
Detection of ubiquitylated species in cell extracts
Two assays were used to detect ubiquitylated species in yeast cells. First, yeast strains containing the TAP-tagged proteins were transformed with the plasmid pGPD416-Ub for expressing HA-Ub or the plasmid pHis415-HA-Ub for expressing His-HA-Ub. A standard TAP purification procedure was then followed to purify TAP-tagged proteins first using IgG sepharose (Li et al. 2008). Proteins that bound to IgG beads were eluted using TEV protease, and were further purified using calmodulin sepharose beads. Alternatively, ubiquitylated proteins were purified under denatured conditions (8 M urea) using Ni-NTA agarose. In both cases, purified protein was resolved by SDS-PAGE and detected by Western blotting using antibodies against the HA epitope or antibodies against the calmodulin-binding protein (CBP).
In vitro ubiquitylation assay
To test whether Rtt101 ubiquitylates FACT in vitro, a 200-μL reaction mixture including 25 ng of yeast E1, 155 ng of Cdc34 (E2), 1 μg of Rtt101 (E3), 2 μg of FACT (substrate), 25 ng of Flag-Ub, and 5 μm of Ub-aldehyde was assembled in a buffer containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 0.6 mM DTT, 2 mM ATP, and 200 μM Na3VO4. Following incubation for 1 h at 30°C, the reaction mixture was mixed with M2 anti-Flag beads (Sigma). Ubiquitin and ubiquitylated proteins were recovered, and ubiquitylated proteins were detected by Western blotting using antibodies against the Flag epitope. Flag-Ub, Ub-aldehyde, and yeast E1 were purchased from BostonBiochem. Recombinant Cdc34 was purified from Escherichia coli. Detailed procedures for TAP and ChIP assays are described in the Supplemental Material.
Acknowledgments
We thank Drs. Deshaies, Tansey, and Callis for plasmids. We also thank Drs. Brian Davies, Dave Katzmann, and Marty Rechsteiner for critical reading of the manuscript. This work is supported by grants from the NIH to Z.Z. (GM719729 and GM 81838) and to T.F. (GM64649). Z.Z. is a scholar of Leukemia and Lymphoma Society. J.H. was supported by the Kendall-Mayo fellowship.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1887310.
Supplemental material is available at http://www.genesdev.org.
References
- Bell SP, Dutta A 2002. DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374 [DOI] [PubMed] [Google Scholar]
- Bell SP, Kobayashi R, Stillman B 1993. Yeast origin recognition complex functions in transcription silencing and DNA replication. Science 262: 1844–1849 [DOI] [PubMed] [Google Scholar]
- Bergink S, Jentsch S 2009. Principles of ubiquitin and SUMO modifications in DNA repair. Nature 458: 461–467 [DOI] [PubMed] [Google Scholar]
- Bhoj VG, Chen ZJ 2009. Ubiquitylation in innate and adaptive immunity. Nature 458: 430–437 [DOI] [PubMed] [Google Scholar]
- Chen CC, Carson JJ, Feser J, Tamburini B, Zabaronick S, Linger J, Tyler JK 2008. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell 134: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, Chu CS, Schuldiner M, Gebbia M, Recht J, Shales M, et al. 2007. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 446: 806–810 [DOI] [PubMed] [Google Scholar]
- Conaway JW, Conaway RC 1999. Transcription elongation and human disease. Annu Rev Biochem 68: 301–319 [DOI] [PubMed] [Google Scholar]
- Formosa T 2003. Changing the DNA landscape: Putting a SPN on chromatin. Curr Top Microbiol Immunol 274: 171–201 [DOI] [PubMed] [Google Scholar]
- Formosa T, Eriksson P, Wittmeyer J, Ginn J, Yu Y, Stillman DJ 2001. Spt16–Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. EMBO J 20: 3506–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, van Deursen F, Edmondson RD, Labib K 2006. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol 8: 358–366 [DOI] [PubMed] [Google Scholar]
- Hartzog GA, Wada T, Handa H, Winston F 1998. Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev 12: 357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan E, Koshland D 1992. Addition of extra origins of replication to a minichromosome suppresses its mitotic loss in cdc6 and cdc14 mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci 89: 3098–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra A, Schwob E, Mendez J 2008. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc Natl Acad Sci 105: 8956–8961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Kawasaki Y, Tye BK 1996. Physical interactions among Mcm proteins and effects of Mcm dosage on DNA replication in Saccharomyces cerevisiae. Mol Cell Biol 16: 5081–5090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhou H, Wurtele H, Davies B, Horazdovsky B, Verreault A, Zhang Z 2008. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell 134: 244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Versini G, Jaquenoud M, Zaidi IW, Kurz T, Pintard L, Pasero P, Peter M 2006. The cullin Rtt101p promotes replication fork progression through damaged DNA and natural pause sites. Curr Biol 16: 786–792 [DOI] [PubMed] [Google Scholar]
- Michel JJ, McCarville JF, Xiong Y 2003. A role for Saccharomyces cerevisiae Cul8 ubiquitin ligase in proper anaphase progression. J Biol Chem 278: 22828–22837 [DOI] [PubMed] [Google Scholar]
- Morrison AJ, Shen X 2009. Chromatin remodelling beyond transcription: The INO80 and SWR1 complexes. Nat Rev Mol Cell Biol 10: 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlikar GJ, Fan HY, Kingston RE 2002. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108: 475–487 [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ 2005. Function and regulation of cullin–RING ubiquitin ligases. Nat Rev Mol Cell Biol 6: 9–20 [DOI] [PubMed] [Google Scholar]
- Reinberg D, Sims RJ 3rd 2006. de FACTo nucleosome dynamics. J Biol Chem 281: 23297–23301 [DOI] [PubMed] [Google Scholar]
- Shor E, Warren CL, Tietjen J, Hou Z, Muller U, Alborelli I, Gohard FH, Yemm AI, Borisov L, Broach JR, et al. 2009. The origin recognition complex interacts with a subset of metabolic genes tightly linked to origins of replication. PLoS Genet 5: e1000755 doi: 10.1371/journal.pgen.1000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squazzo SL, Costa PJ, Lindstrom DL, Kumer KE, Simic R, Jennings JL, Link AJ, Arndt KM, Hartzog GA 2002. The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J 21: 1764–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata S, Yu Y, Stillman DJ 2009. The E2F functional analogue SBF recruits the Rpd3(L) HDAC, via Whi5 and Stb1, and the FACT chromatin reorganizer, to yeast G1 cyclin promoters. EMBO J 28: 3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BC, Chien CT, Hirose S, Lee SC 2006. Functional cooperation between FACT and MCM helicase facilitates initiation of chromatin DNA replication. EMBO J 25: 3975–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDemark AP, Blanksma M, Ferris E, Heroux A, Hill CP, Formosa T 2006. The structure of the yFACT Pob3-M domain, its interaction with the DNA replication factor RPA, and a potential role in nucleosome deposition. Mol Cell 22: 363–374 [DOI] [PubMed] [Google Scholar]
- Waga S, Stillman B 1998. The DNA replication fork in eukaryotic cells. Annu Rev Biochem 67: 721–751 [DOI] [PubMed] [Google Scholar]
- Xin H, Takahata S, Blanksma M, McCullough L, Stillman DJ, Formosa T 2009. yFACT induces global accessibility of nucleosomal DNA without H2A–H2B displacement. Mol Cell 35: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi IW, Rabut G, Poveda A, Scheel H, Malmstrom J, Ulrich H, Hofmann K, Pasero P, Peter M, Luke B 2008. Rtt101 and Mms1 in budding yeast form a CUL4(DDB1)-like ubiquitin ligase that promotes replication through damaged DNA. EMBO Rep 9: 1034–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]