Abstract
Although the physiologic pathways that control regulatory T cells (Foxp3-expressing regulatory T cells, IL-10-secreting Tr1 cells) and Th17 cells in rodents have been defined, the factors that control these differentiation pathways in humans are not well understood. In this study, we show that IL-27 promotes the differentiation of IL-10-secreting Tr1 cells while inhibiting Th17 generation and molecules associated with Th17 function. Furthermore, IL-27 inhibits IL-17-polarizing cytokines on dendritic cells, which in turn decrease IL-17 secretion from T cells. Our results demonstrate that IL-27 plays a key role in human T cells by promoting IL-10-secreting Tr1 cells and inhibiting Th17 cells and thus provides a dual regulatory mechanism to control autoimmunity and tissue inflammation.
The immune system must distinguish not only between self and non-self, but also between innocuous and pathological foreign Ags to prevent unnecessary or self-destructive immune responses. Unresponsiveness to self-Ags is established through central and peripheral processes. Whereas clonal deletion and anergy are mechanisms of peripheral tolerance, active suppression by regulatory T cells (Tregs)3 has emerged as an essential factor in the control of self-reactive cells. Two important classes of Tregs within the CD4+ subset are CD4+CD25+Foxp3+ Tregs and T regulatory type 1 (Tr1) cells (1–4). These two regulatory subsets differ in a number of biological features, including their cytokine profile, cellular markers, transcription factors, and mechanism of immune suppression. Tr1 cells are CD4+ T lymphocytes defined by their production of IL 10 and suppression of helper T cells (5). Tr1 cells are inducible cells, arise from naive precursors, and can be differentiated both ex vivo and in vivo. Stimulation of human CD4+ T cells with allogeneic monocytes or murine CD4+ T lymphocytes with Ag, particularly in the presence of IL-10, leads to the generation of Tr1 clones (4). In addition, Tr1 cell differentiation has also been induced by dexamethasone and vitamin D3 (6). Finally, CD46 activation of T cells in the presence of IL-2 leads to Tr1 differentiation characterized by a massive secretion of IL-10 and bystander CD4+ T cell suppression (7). Altered function of Tr1 cells in the human autoimmune disease condition multiple sclerosis has been reported, implicating the function of this subset in regulation of human autoimmune disease (8).
Contrary to the function of Tr1 cells, Th 17 cells are known to have potent proinflammatory functions. Th17 cells belong to a recently identified Th subset, in addition to the traditional Th1 and Th2 subsets. These cells are characterized as preferential producers of IL-17. Th17 cells and their effector cytokines are associated with the pathogenesis of several human inflammatory and autoimmune diseases including multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus, and psoriasis (9, 10). In mice, TGF-β and IL-6 or TGF-β and IL-21 have been shown to induce the differentiation of naive mouse T cells toward the Th17 phenotype (11–14). The differentiation of Th17 cells that secrete IL-17 (IL-17A) requires the expression of transcription factor retinoid orphan nuclear receptor (RORγt, an orphan nuclear hormone receptor) (15). Although the cytokines described above positively regulate Th17 differentiation, other cytokines in the murine immune system have been shown to negatively regulate differentiation of Th17 cells. The cytokines IL-4, IFN-γ, IL-2, and IL-27 have been shown to inhibit Th17 cell differentiation (16–20).
Although the factors that promote and/or control murine Th17 differentiation have been extensively studied, there has been less information on the regulation of this cytokine in human T cells in terms of differentiation or regulation of committed Th17 cells. Furthermore, the factors involved in the differentiation of murine T cells have not always been paralleled in humans. Initial studies have shown that IL-1β, IL-6, and IL-23 are important in driving human Th17 cell differentiation (21, 22). Most recently, TGF-β in combination with other proinflammatory cytokines (IL-1β, IL-6, IL-23, and IL-21) were required to induce Th17 cells in humans (23–25).
IL-27 is a member of the IL-12 family and is comprised of an IL-12p40-related protein, encoded by the EBV-induced gene 3 (EBI3, also known as IL27), and a unique IL-12p35-like protein, IL-27p28v (26). Initial animal studies on the biology of IL-27 provided evidence of a role for this cytokine in the initiation of the Th1 response (27, 28); however, subsequent work using mouse models of pathogen-induced and autoimmune inflammation have indicated that IL-27 has broad inhibitory effects on Th1, Th2, and Th17 subsets of T cells as well as on APC function (19, 20, 29–31). Most recently, we and others have shown that IL-27 is capable of inducing IL-10 from murine T cells and act as a negative feedback mechanism against proinflammatory immune responses (32–35). However, the role of IL-27 in the human immune system is not well understood. In this study, we show that IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells.
Materials and Methods
Media and reagents
RPMI 1640 was supplemented (complete RPMI with 10% heat-inactivated FCS, 1 mM nonessential amino acids, 45 μg/ml penicillin and streptomycin, and 2 mM L-glutamine (all from Life Technologies.). Recombinant human IL-27, IL-2, IL-1β, IL-6, IL-23, and IL-27 receptor Abs were obtained from R&D Systems. mAbs specific for HLA-DR, CD80, CD86, CD83, CD40, and CCR7 were purchased from BD Biosciences. Isotype control Ab mouse IgG1 and mouse IgG2a were also from BD Biosciences. TLR ligands LPS (Escherichia coli) and peptidoglycan (PGN) (Staphylococcus aureus) were obtained from InvivoGen.
Human T cell stimulation
Peripheral blood was obtained after informed consent from healthy subjects. PBMCs were isolated from heparinized venous blood by Ficoll-Hypaque density gradient centrifugation (Amersham Pharmacia Biotech). Total, naïve, and memory CD4+ T cells were prepared using magnetic beads by negative selection (Miltenyi Biotec). The CD4+ T cells were cultured at a concentration of 1.5 × 106 cells/ml in 24-well plates coated with anti-CD3 and anti-CD28 mAb (1.0 μg/ml) in the presence or absence of 100 ng/ml of recombinant human IL-27. For polarization experiments, naive CD4+ T cells were seeded at a density of 1.5 × 106 cells/ml in 24-well plates coated with anti-CD3 and anti-CD28 (1 μg/ml). In some cases, IL-1β (50 ng/ml), IL-6 (50 ng/ml), IL-21 (25 ng/ml), IL-23 (25 ng/ml), TGF-β1 (2 ng/ml), and IL-27 (100 ng/ml) were added at day 0 and were maintained throughout the experiment. Cell-free culture supernatants were collected on day 5 for ELISA.
Primary and secondary cell stimulation
In vitro primary stimulation was conducted in 96-well culture plates coated with mAbs to CD3 (1.0 mg/ml), CD28 (1.0 mg/ml), and IL-27 (100 ng/ml). The wells were washed and purified CD4+ T cells (2.0–3.0 × 105 cells/well) were added in 200 ml of culture medium. After 3 days of primary stimulation, cells were washed and expanded for 3 days in medium supplemented with 100 U/ml human IL-2. At day 6, cells were counted and subjected to secondary stimulation under conditions similar to those of the primary activation. The experiment had each activation condition in triplicate. For the bystander T cell suppression assay, naive CD4+ T cells were seeded at a density of 2 × 105 cells/well in a 96-well plate coated with mAbs to CD3 (1.0 μg/ml) and CD28 (1.0 μg/ml). Cell-free supernatants from the CD3/28/IL-27- or CD3/28-activated cell populations were transferred to the above culture with or without neutralizing Ab to IL-10 and proliferation was measured at day 3. In some culture conditions, anti-IL-27 Ab was added at a concentration of 20 μg/ml.
Generation of monocyte-derived dendritic cells (DCs)
Human PBMCs were isolated from buffy coats by centrifugation through a Ficoll-Paque Plus (Amersham Pharmacia Biotech) density gradient. Cells were enriched for monocytes (CD14+ cells) by using a monocyte enrichment kit (Miltenyi Biotec). Monocytes were resuspended at 2 × 106 cells/ml in DC medium (RPMI 1640 plus Glutamax (Invitrogen), 5% FBS, 15 mM HEPES, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin) containing IL-4 (50 ng/ml) and GM-CSF (100 ng/ml; BD Biosciences) and cultured for 6 days with replacement of half of the medium and addition of fresh cytokines every alternate day. Nonadherent DCs were harvested by centrifugation and suspended in DC medium without antibiotics. The resulting cells were determined to be >95% CD11c+ by flow cytometry.
DC-T cell coculture assay
Total and naive CD45RA+ Th cells were enriched by immunomagnetic negative selection. They were then cocultured with DCs that had been activated alone with LPS (0.5 μg/ml), PGN (10 μg/ml), or combined with IL-27 (100 ng/ml) for 12 h. The DCs were washed three times before they were cultured with T cells. Cocultures were performed with a DC:T cell ratio of 1:3 in U-bottom 96-well plates. The culture supernatants were assayed for IL-17 levels by ELISA.
Flow cytometric analysis
Cells were resuspended in PBS containing 1% BSA (Sigma-Aldrich) and 0.1% sodium azide (Sigma-Aldrich) and incubated with FITC- or PE-conjugated Abs or isotype control Abs at the recommended dilutions for 30 min on ice.
Cytokine measurement
Supernatants from DCs, DC-T cell cocultures, and IL-27-treated T cells were harvested at the indicated time points and stored at −80°C. Cytokines were quantified by the ELISA using Opt-EIA kits (BD Pharmingen) or by cytokine bead array (inflammation kit; BD Pharmingen) according to the manufacturer's instructions.
Real-time quantitative RT-PCR
Total RNA was extracted with the RNAeasy Mini Kit (Qiagen) according to the manufacturer's instructions. First-strand cDNA synthesis was performed for each RNA sample using TaqMan reverse transcription reagents (Applied Biosystems). Transcripts were quantified by real-time quantitative PCR on an Applied Biosystems 7500 Sequence Detector with Applied Biosystems predesigned TaqMan Gene Expression Assays and reagents according to the manufacturer's instructions. The following probes were used (identified by Applied Biosystems assay identification number): TBX21, Hs00203436_m1; IL-17A, Hs99999082_m1; IL-22, Hs00220924_m1; CCL20, Hs00171125_m1; GATA-3, Hs00231122_m1; FOXP3, Hs00203958_m1; retinoid orphan nuclear receptor (RORC), which encodes the human ortholog of mouse RORγt, Hs01076112_m1; IL-17F, Hs00369400_m1; and CCR6, Hs00171121_m1. For each sample, mRNA abundance was normalized to the amount of the housekeeping gene GAPDH.
Results
IL-27 induces IL-10 production from human CD4+ T cells
We stimulated ex vivo total CD4+ T cells with plate-bound anti-CD3 and anti-CD28 in the presence or absence of recombinant human IL-27 and assessed their cytokine profile. Stimulation in the presence of IL-27 induced large amounts of IL-10 with a moderate increase in IFN-γ (Fig. 1, A and B). By contrast, IL-27 stimulation inhibited anti-CD3 and anti-CD28 induced IL-17 without affecting TGF-β production (Fig. 1, C and D). Neither stimulatory condition induced IL-4. The transcription factors T-bet, GATA-3, RORC, and Foxp3 are required for the generation of Th1, Th2, Th17, and Treg cells, respectively. We found that cells stimulated with anti-CD3 and anti-CD28 induced expression of mRNA encoding Foxp3, GATA-3, T-bet, and RORC. Addition of IL-27 to the above culture condition greatly reduced the expression of GATA-3 and RORC, whereas the expression of T-bet and Foxp3 transcripts were not affected (Fig. 1, E–H). These observations suggest that IL-10 production induced by IL-27 did not depend on GATA-3, a transcription factor required for the generation of Th2 cells (36) and that IL-27-stimulated T cells have a cytokine profile that is distinct from that induced by stimulation with Abs to CD3 and CD28 but similar to that of Tr1 cells.
FIGURE 1.

IL-27 stimulation induces IL-10 production from human peripheral blood CD4+ T cells. ELISA of total CD4+ T cells stimulated with plate-bound anti-CD3 and anti-CD28 in the presence or absence of recombinant human IL-27 (100 ng/ml). A and B, IL-27 stimulation increased anti-CD3- and anti-CD28-induced IL-10 and IFN-γ production. C and D, IL-27 inhibited anti-CD3- and anti-CD28-induced IL-17 without affecting TGF-β production. E–H, Quantitative PCR of the expression of mRNA encoding T-bet, GATA-3, RORC, and Foxp3 in total CD4+ T cells stimulated with plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-27 (100 ng/ml), presented relative to the expression of mRNA encoding GAPDH. Data are from 11 randomly selected healthy donors. Horizontal bars indicate the median.
To determine whether the IL-10-producing cells were derived from the naive or memory T cell population, CD4+ T cells were separated into CD45RA+ and CD45RO+ T cells and were then stimulated with plate-bound anti-CD3 and anti-CD28 in the presence or absence IL-27 (Fig. 2A). Naive CD4+ T cells stimulated with IL-27 responded more robustly in that they produced IL-10 in greater folds (Fig. 2B). Neither naive nor memory CD4+ T cells produced IL-4. However, both cell populations secreted moderate quantities of IFN-γ in response to IL-27 stimulation (Fig. 2B). Consistent with IL-10 secretion, naive CD4+ T cells expressed more IL-27 receptor on their surface (Fig. 2C). In addition, we found that IL-27 did not affect the proliferation of either naive or memory CD4+ T cells (supplemental Fig. 14). The above data suggest that primary activation of naive CD4+ T cells with IL-27 induces an IL-10-producing T cell phenotype. To determine whether these cells are then committed to maintain this phenotype, we analyzed the properties of these CD4+ T cells on secondary stimulation. Purified naive CD4+ T cells were initially stimulated for 3 days and subsequently expanded for 4 days in medium supplemented with rIL-2. These cells were then subjected to secondary stimulation and analyzed for IL-10 production. CD4+ T cells, first activated with CD3/CD28/IL-27, produced large amounts of IL-10 on secondary stimulation (Fig. 2D). By contrast, CD4+ T cells initially activated without IL-27 produced small amounts of IL-10 on secondary stimulation with IL-27 (Fig. 2E). Therefore, primary stimulation through IL-27 is required for IL-10 production in restimulated cells. Of note, secondary stimulation with CD3 or CD3/CD28 alone became sufficient for the induction of IL-10 synthesis from the cells received IL-27 primary stimulation, suggesting these cells are differentiated and committed to the IL-10-producing phenotype (Fig. 2D). In addition, restimulated cells did not produce IL-4 but synthesized small amounts of IL-2 (data not shown).
FIGURE 2.
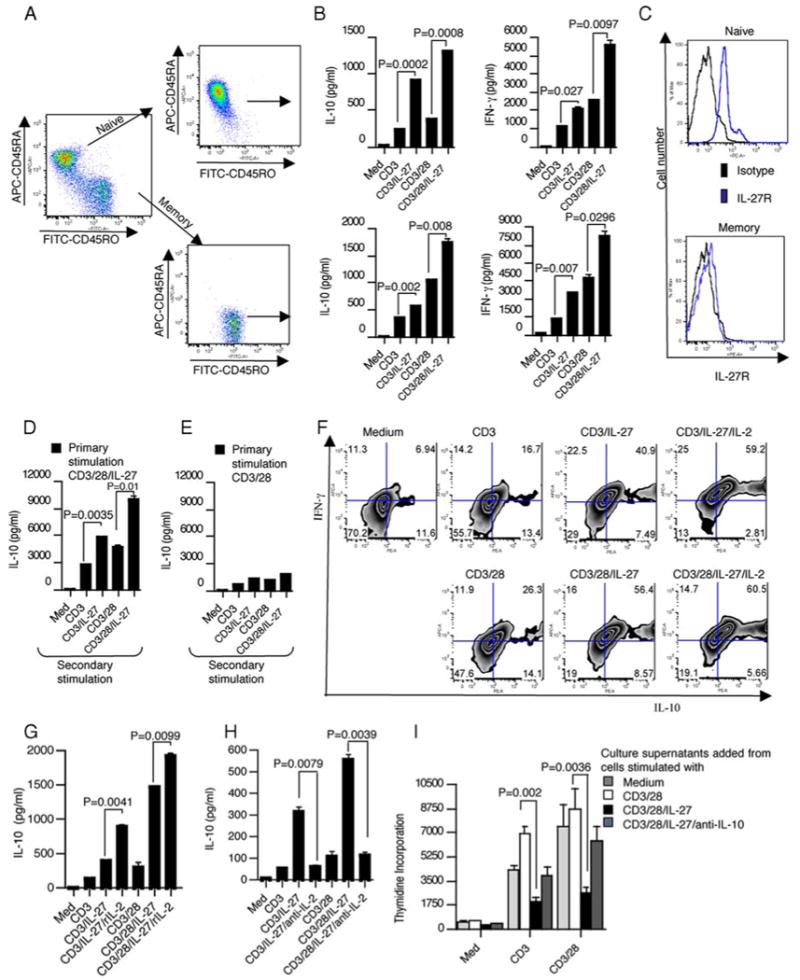
IL-27 stimulation induces Tr1 like cells. A, Purity of naive and memory CD4+ T cells. Flow cytometry of naive CD4+ T cells after purification and staining with anti-CD4 PE, anti-CD45RA allophycocyanin, and anti-CD45RO FITC. B, Analysis of the cytokine expression of naive and memory CD4+ T cells stimulated with anti-CD3 and anti-CD28 in the presence or absence of recombinant human IL-27 (100 ng/ml). C, Naive CD4+ T cells expressed more IL-27 receptor on their surface compared with memory CD4+ T cells as measured by FACS. D and E, Analysis of the cytokine expression of secondary stimulated T cells. CD4+ T cells, first activated with CD3/CD28/IL-27, produced large amounts of IL-10 on secondary stimulation. Cells were stimulated for 3 days with anti-CD3 and anti-CD28 in the presence or absence of IL-27 and 3 days after primary stimulation cells were expanded and subjected to secondary stimulation. IL-10 secretion was measured at day 3. F, IL-27 induces the generation of IFN-γ+ and IL-10+CD4+ T cells. Flow cytometry of naive CD4+ T cells activated with anti-CD3 and anti-CD28 in the presence or absence of IL-27 stained for intracellular IL-10 and IFN-γ. G and H, IL-27-induced IL-10 production is IL-2 dependent. CD4+ T cells were incubated with the plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-27 and IL-10 secretion was measured at day 3. rIL-2 (100 U/ml) or neutralizing Ab to IL-2 (20 μg/ml) was added as indicated. I, IL-27-stimulated T cells inhibit the proliferation of bystander CD4+ T cells in an IL-10-dependent manner. Naive CD4+ T cells were incubated with the plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-27 for 3 days. Cell-free culture supernatants were transferred to freshly purified CD4+ T cells cultured with plate-bound anti-CD3 alone or with anti-CD3 and CD28. Neutralizing anti-IL-10 Ab (20 μg/ml) was added to the indicated condition and proliferation was measured at day 5. Data represent one of four (A–C) experiments or one of three (D and E) or one of two (F–I) experiments involving nine randomly selected donors, and the error bars represent the mean ± SD.
It has been evident from the previous studies that the cytokine production profile of Tr1 cells was their key trait. Tr1 cells, upon activation via the TCR, produce high amounts of IL-10 but are distinct from Th2 cells since they do not produce IL-4 and produce very low levels of IL-2 (5–7). It has been shown that human Tr1 cells also produce IFN-γ in addition to IL-10 (5, 7). Consistent with ELISA data, intracellular cytokine analysis revealed that IL-27 stimulation of naive CD4+ T cells lead to increased coexpression of IL-10 and IFN-γ (Fig. 2F). The addition of IL-2 further enhanced the expression of IL-10 in these cells. It has been shown that IL-10 secretion by human Tr1 cells requires IL-2 (7). Because Tr1 cells are characterized by release of IL-10 without concurrent production of IL-2, we tested the role of IL-2 in IL-27-induced IL-10 secretion. CD4+ T cells activated with either CD3/CD28/IL-27 or CD3/IL-27 in the presence of IL-2 produced large amounts of IL-10 (Fig. 2G). The addition of a neutralizing mAb to IL-2 abrogated IL-10 production, suggesting that IL-10 production is dependent on IL-2 in IL-27-activated T cells (Fig. 2H). The above data suggest that primary activation of naive CD4+ T cells with CD3/IL-27 in the presence of IL-2 generates a Tr1 phenotype. We next examined whether the supernatant from CD3/CD28/IL-27-activated T cells suppresses proliferation of bystander T cells. At day 3, supernatants from the activated cell populations were transferred onto freshly purified CD4+ T cells. These cells were then activated with CD3/CD28 and proliferation was measured at day 3. The supernatants from CD4+ T cells activated by CD3/CD28/IL-27 inhibited proliferation of bystander CD4+ T cells (Fig. 2I). This inhibition was not observed when a neutralizing mAb to IL-10 was added. However, supernatants from CD3/CD28-activated CD4+ T cells did not inhibit proliferation of T cells. Moreover, the suppression was not due to the presence of IL-27 in the culture supernatants since addition of IL-27 to the naive CD4+ T cells did not affect their proliferation. Also, we found that the addition of anti-IL-27 Ab along with the IL-27-stimulated T cell culture supernatants did not affect naive T cell proliferation (supplemental Fig. 2). Collectively, these findings suggest that IL-27-treated CD4+ T cells resemble IL-10-producing Tr1 cells. We have shown that IL-27 in combination with TGF-β induced IL-10 production while inhibiting Foxp3 from murine T cells (32). Thus, we investigated the effect of IL-27 in combination with TGF-β in the generation of IL-10 from CD4+ T cells in humans. Naive CD4+ T cells activated in the presence of IL-27 produced IL-10 and IFN-γ. The addition of TGF-β along with IL-27 further increased IL-10 production without affecting the production of IFN-γ (supplemental Fig. 3, A and B). Because Foxp3 expression in T cells can be induced by TGF-β, we measured Foxp3 expression in T cells cultured with TGF-β and IL-27. We found that, although TGF-β stimulation increased Foxp3 expression, the addition of IL-27 did not inhibit TGF-β- induced Foxp3 expression (supplemental Fig. 3C).
IL-27 inhibits IL-17 production and molecules associated with its effector functions from CD4+ T cells
Although the factors that promote and/or control murine Th17 differentiation have been extensively studied, there has been much less information on the regulation of this cytokine in human T cells. It has been shown that IL-1β, IL-6, and IL-23 are important in promoting IL-17 secretion from human CD4+ T cells (21, 22). The nuclear receptor RORC acts as a key transcription factor in this lineage commitment process. However, the factors that control IL-17 production from human T cells are not known. Therefore, we examined the effect of IL-27 on IL-17 secretion from CD4+ T cells. In agreement with published studies (37, 38), induction of IL-17 from naive T cells was not observed in response to CD3 alone or CD3/CD28 stimulation (data not shown). However, total CD4+ T cells produced IL-17 in response to CD3/CD28 stimulation and was inhibited by the addition of IL-27 (Fig. 3A). Furthermore, IL-27 induced inhibition of IL-17 expression was associated with substantial reduction of RORC (Fig. 3B). It has been reported that IL-17A, IL-22, and IL-17F are mainly produced by Th17 cells (21–25). IL-23 is involved in the generation and maintenance of Th17 cells and constitutive expression of the IL-23 receptor on Th17 cells has been described (39). Thus, we asked whether IL-27 modulates molecules associated with function and maintenance of Th17 cells. We found that IL-27 inhibited CD3/CD28-induced IL-I7F and IL-22 expression from total CD4+ T cells (Fig. 3, C and D). The inhibitory effect of IL-27 was also well pronounced on IL-23 receptor expression as determined by both real-time PCR and flow cytometry (Fig. 3E). Human IL-17-producing cells also express the chemokine receptor CCR6 and its ligand CCL20 (40). We found that the addition of IL-27 inhibited the expression of both CCR6 and CCL20 (Fig. 3, F and G). We next tested whether the inhibition of IL-17 production by IL-27 was dependent on IL-10. We found that the addition of neutralizing IL-10 Ab did not reverse IL-27-mediated inhibition of IL-17. However, addition of neutralizing IFN-γ Ab completely reversed the IL-27-mediated suppression of IL-17 (Fig. 3H). Furthermore, IL-27-induced inhibition of IL-17 expression was associated with a substantial reduction of RORC, and neutralizing IFN-γ Ab restored the IL-27-mediated suppression of RORC (Fig. 3H). The addition of recombinant IFN-γ led to significant inhibition of IL-17 production from CD3/CD28-stimulated total CD4+ T cells. Furthermore, IFN-γ-induced inhibition of IL-17 expression was associated with a substantial reduction in RORC expression (Fig. 3I).
FIGURE 3.

IL-27 inhibits IL-17 production from total CD4+ T cells. A, Total CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence or absence of IL-27 for 3 days and cell-free culture supernatants were assayed for IL-17A by ELISA. B, IL-27 inhibits RORC in total CD4+ T cells. Quantitative PCR of the expression of mRNA total CD4+ T cells stimulated with plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-27 (100 ng/ml). C–G, IL-27 inhibits molecules associated with Th17 effector molecules in total CD4+ T cells. Quantitative PCR of the expression of mRNA encoding IL-17F, IL-22, IL-23R, CCR6, and CCL20 from total CD4+ T cells stimulated with plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-27 (100 ng/ml). Data are from five randomly selected healthy donors. Horizontal bars indicate the median. H and I, IL-27-mediated IL-17 suppression was mediated by IFN-γ. Total CD4+ T cells stimulated with plate-bound anti-CD3 and anti-CD28 in the presence or absence of IL-27 (100 ng/ml). To some conditions, neutralizing anti-IL-10 Ab (20 μg/ml) or anti-IFN-γ (20 μg/ml) was added as indicated. Data represent one of three independent experiments with cells from two randomly selected donors.
Next, we tested whether IL-27 is capable of inhibiting IL-17 secretion under Th17-polarizing conditions. We stimulated naive CD4+ T cells with IL-17-polarizing cytokines IL-1β and IL-23 with or without IL-27. In agreement with previous studies, IL-17 was produced under this condition; moreover, the addition of IL-27 suppressed IL-17 secretion (Fig. 4A). Furthermore, IL-27-induced inhibition of IL-17 expression was associated with a substantial reduction of RORC (Fig. 4B) and Th17-associated cytokines IL-17F and IL-22 (supplemental Fig. 4, A and B). IL-27 also showed its inhibitory effect on the surface expression of the IL-23 receptor and chemokine receptor CCR6 and its ligand CCL20 (supplemental Fig. 4, C–E). It has been recently shown that TGF-β in combination with other proinflammatory cytokines (IL-1β, IL-6, IL-21, and IL-23) are capable of driving IL-17 secretion from naive T cells (21–25). We found that even under these strong Th17-polarizing conditions, IL-27 markedly inhibited IL-17 secretion (Fig. 4C). In accordance with IL-17 secretion, the RORC mRNA expression was lower in these conditions (Fig. 4D). In this system, the addition of IL-27 consistently up-regulated IL-10 in all culture conditions, demonstrating a wide effect of IL-27 on IL-10 expression (Fig. 4E). Furthermore, IL-27 showed an inhibitory effect on memory cell IL-17 production and molecules associated with its effector function and maintenance of the Th17 phenotype (supplemental Fig. 5). Collectively, these data indicated that IL-27 inhibits IL-17 and molecules associated with function and maintenance of Th17 cells.
FIGURE 4.

IL-27 inhibits IL-17 production from Th17-polarized cells. Naive CD4+ T cells were activated with plate-bound anti-CD3 and anti-CD28 in the presence of Th17-polarizing cytokines IL-1β (50 ng/ml) and IL-23 (50 ng/ml) with or without IL-27 (100 ng/ml). A, ELISA of IL-17 in cell-free culture supernatants. B, Real-time quantitative RT-PCR of transcript expression of RORC at 24 h after activation. C, IL-27 inhibits TGF-β in combination with other proinflammatory cytokine-induced IL-17 secretion. ELISA of IL-17 secretion by Th17-polarized cells by TGF-β in combination with IL-1β (50 ng/ml), IL-6 (50 ng/ml), IL-21 (12.5 ng/ml), and IL-23 (50 ng/ml) with or without IL-27. D, IL-27 inhibits RORC expression as determined by real-time quantitative RT-PCR. E, IL-27 induces IL-10 secretion from CD4+ T cells stimulated with TGF-β in combination with other proinflammatory cytokines. ELISA of IL-10 secretion from T cells stimulated with TGF-β in combination with IL-1β (50 ng/ml), IL-6 (50 ng/ml), IL-21 (25.0 ng/ml), and IL-23 (50 ng/ml) with or without IL-27. Data represent one of three independent experiments with cells from three randomly selected donors.
IL-27 inhibits IL-17-polarizing cytokines from DCs
It is well established that the development of Th cell subsets is orchestrated by cytokines produced by DCs that differentially sense archetypical structures defining different classes of pathogens. For example, DC-secreted IL-12 has been shown to favor Th1 development while DC-secreted IL-10 has been shown to induce IL-10-producing Tr1 cells (41, 42). In addition, TGF-β secretion by DCs can modulate Foxp3+ Treg cell development (43). Likewise, DC-secreted IL-1β and IL-6 appear to synergize with IL-23 in the induction of IL-17 by human CD4+ T cells. It has been shown that human DCs primed by TLR agonists produce enhanced amounts of IL-1β, IL-6, and IL-23(21). Therefore, we tested whether IL-27 modulates IL-17-polarizing cytokine secretion by DCs. For this, we stimulated DCs with TLR ligands such as LPS and PGN in the presence or absence of IL-27 and analyzed cytokine expression. Stimulation of DCs with TLR ligands induced the production of the IL-17-polarizing cytokines IL-1β, IL-6, and IL-23 (Fig. 5, A–C). Conversely, the addition of IL-27 inhibited these cytokines (Fig. 5, A–C). The observed IL-27 effect on DCs was found to be mediated through IL-27R as DCs expressed IL-27R on their surface (Fig. 5D). Next we tested whether culturing these DCs with CD4+ T cells would have an impact on IL-17 production from T cells. Stimulation of total CD4+ T cells with either LPS- or PGN-activated DCs lead to significantly enhanced IL-17 production. In contrast, IL-17 production was inhibited when the T cells were cultured with IL-27-preactivated DCs (Fig. 5E). We also tested the effect of IL-27-stimulated DCs on IL-17 production from naive and memory CD4+ T cells. Stimulation of naive T cells with either LPS- or PGN-activated DCs did not significantly enhance IL-17 production (supplemental Fig. 6A). In contrast, coculture of memory CD4+ T cells with LPS- or PGN-activated DCs led to a marked increase in IL-17 production, which was dramatically reduced by IL-27-preactivated DCs (supplemental Fig. 6B). These observations suggest that in addition to a direct inhibitory effect of IL-27 on T cell IL-17 production, IL-27 could suppress Th17- polarizing cytokines from DCs, thereby leading to inhibition of IL-17 production by T cells.
FIGURE 5.

IL-27 inhibits IL-17 polarizing cytokines from monocyte derived dendritic cells. A–C, Cytometric bead assay of IL-1β and IL-6 and ELISA of IL-23 in monocyte-derived DCs stimulated with LPS or PGN alone or in the presence of IL-27 (100 ng/ml). D, DCs express IL-27R on their surface. Monocyte-derived DCs used in our experiments were >95% CD11c+ and these cells expressed IL-27R on their surface as determined by flow cytometry. E, Total CD4+ T cells were cultured with a 1: 3 ratio of DCs prestimulated with LPS or PGN alone or in the presence of IL-27. The DCs were washed three times before they were cultured with T cells. After 4 days of coculture, the cell-free culture supernatants were assayed for IL-17 by ELISA. Data are representative of three experiments with three donors.
Discussion
In this study, we show that in humans IL-27 induces the generation of T cells that secrete large amounts of IL-10 and suppress T cell proliferation in an IL-10-dependent manner. On the contrary, IL-27 inhibited IL-17 secretion and the molecules associated with function and maintenance of the Th17 phenotype. Control of self-reactive T lymphocytes by Tr1 cells has been proposed to maintain tolerance and prevention of autoimmunity (4, 5). These cells suppress immune responses through either direct cell-cell interactions or the release of inhibitory cytokine IL-10. The differentiation of CD4+ T cells into Tr1 cells is poorly defined, in part because of difficulties in inducing and culturing such cells. Tr1 cells produce large amounts of IL-10 and moderate amounts of IFN-γ but no IL-4 and are able to suppress in vitro T cell responses (5). In agreement with these features, our results suggest that IL-27-induced CD4+ T cells produced large amounts of IL-10 and moderate amounts of IFN-γ but no IL-4 and are able to suppress in vitro T cell responses. Tr1 cells are characterized by the release of IL-10 without concomitant production of IL-2 (7). Our results suggest that in humans activation of CD4+ T cells with IL-27 in the presence of IL-2 enhanced the production of IL-10, whereas neutralizing anti-IL-2 Ab abrogated the effect, suggesting that IL-10 production is dependent on IL-2 in IL-27-activated T cells.
IL-17 has been shown to have a pathogenic role in autoimmunity. IL-17 expression has been detected in the target tissue during the progression of various human autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, psoriasis, and Crohn's disease (9). Consistent with these observations, IL-17-deficient mice or mice treated with an IL-17 receptor antagonist are resistant to the development of adjuvant-induced arthritis (44, 45). Similarly, IL-17-deficient animals develop experimental autoimmune encephalomyelitis with delayed onset and reduced severity (46). Given the pathogenic relevance of IL-17, it is important to understand how IL-17 is controlled in human T cells.
Despite a great deal of information on the regulation of IL-17 in the mouse, until recently there has been a paucity of information on the differentiation pathways that lead to IL-17 generation in humans. It has been recently shown that the cytokines IL-1β, IL-6, and IL-23 are capable of driving IL-17 secretion from human CD4+ T cells (21, 22). Most recently, TGF-β in combination with other proinflammatory cytokines has been shown to induce Th17 differentiation from cord blood-derived naive CD4+ T cells (23–25). However, the factors that negatively regulate IL-17 production in humans are not known. In this study, we show that IL-27 is capable of inhibiting IL-17 production both from total and memory CD4+ T cells. In addition, IL-27 exerted its suppressive role on IL-17 production even under strong Th17-polarizing conditions. Also, IL-27 showed a broad inhibitory effect on the Th17 subset-associated effector cytokines such as IL-17F and IL-22. It has been proposed that IL-23 is required to shape a stable Th17 population in the secondary lymphoid tissue and to maintain a pathogenic Th17 population at the site of inflammation in animals (47). Furthermore, constitutive expression of IL-23R on IL-17-producing T cells was observed in humans (27). We found that anti-CD3/CD28 stimulation induced IL-23R on total and memory CD4+ T cells. The addition of IL-27 markedly inhibited the expression of IL-23R on these cells, suggesting that IL-27 can inhibit expansion of the IL-17-producing cells. Th17 cells in humans have been reported to express chemokine receptor CCR6 and its ligand CCL20, indicating that Th17 cells might regulate their own recruitment to inflamed tissues in an autocrine manner. We found that addition of IL-27 inhibited the expression of both CCR6 and CCL20, suggesting a role for IL-27 in limiting the molecule associated with Th17 recruitment to the site of inflammation. Thus, our results suggest that IL-27 inhibits not only IL-17 production from T cells but also molecules associated with function and maintenance of the Th17 phenotype.
The differentiation of CD4+ T cells into effector population is profoundly influenced by cytokine produced by DCs. DC-secreted IL-1β and IL-6 appear to synergize with IL-23 in the induction of IL-17 by human CD4+ T cells. It has been shown that human DCs primed by TLR agonists produce enhanced amounts of IL-1β, IL-6, and IL-23 and that culturing these DCs with T cells induced IL-17 production from T cells (21). Our results suggest an additional mechanism for IL-27 in suppressing IL-17 production from T cells. IL-27 inhibited TLR ligand induced IL-17-polarizing cytokines IL-1β, IL-6, and IL-23. Moreover, culturing IL-27-pretreated DCs with T cells showed reduced IL-17 secretion, suggesting that IL-27 is capable of inhibiting IL-17-inducing cytokines from DCs and thereby inhibiting IL-17 production from T cells.
Our results demonstrate that IL-27 plays a key role in human T cells by promoting a specific subset of Tregs (IL-10 secreting) and inhibiting Th17 cells and thus provides a dual regulatory mechanism to control autoimmunity and tissue inflammation.
Supplementary Material
Acknowledgments
We thank Dr. Vijay K. Kuchroo for comments on this manuscript.
Footnotes
This work was supported by the National Institutes of Health Grants (NS038037, AI043458, and NS23132), the National MS Society, and the Nancy Davis Foundation. A.M. was supported by the National Research Service Award Fellowship (Grant F32AI075761) from the National Institute of Allergy and Infectious Diseases.
Abbreviations used in this paper: Treg, regulatory T cell; DC, dendritic cell; PGN, peptidoglycan; RORC, retinoid orphan nuclear receptor (RORC), which encodes the human ortholog of mouse RORγt.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Martinez C. Mechanisms of self tolerance. Immunol Today. 1992;13:401–404. doi: 10.1016/0167-5699(92)90090-t. [DOI] [PubMed] [Google Scholar]
- 3.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 4.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 5.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 6.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 8.Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 10.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 13.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Lubberts E, Joosten LA, Chabaud M, van Den Bersselaar L, Oppers B, Coenen De-Roo CJ, Richards CD, Miossec P, van Den Berg WB. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest. 2000;105:1697–1710. doi: 10.1172/JCI7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. Cutting edge: IFN-γ regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 18.Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, Zou W. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 19.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 20.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 22.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 23.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor ROR γt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 27.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 28.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 32.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 34.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 35.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 36.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 37.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci USA. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, Moles JP, Danger Y, Ravon E, Lesaux S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 41.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 42.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 43.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 45.Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 46.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 47.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


