Abstract
Drugs that increase central noradrenergic activity have been shown to enhance the rate of recovery of motor function in preclinical models of brain damage. Less is known about whether noradrenergic agents can improve the extent of motor recovery and whether such improvement can be sustained over time. This study was designed to determine if increasing central noradrenergic tone using atipamezole, an alpha2 adreneceptor antagonist, could induce a long-term improvement in motor performance in rats subjected to ischemic brain damage caused by permanent middle cerebral artery occlusion. The importance of pairing physical “rehabilitation” with enhanced noradrenergic activity was also investigated. Atipamezole (1 mg/kg, s.c.) or vehicle (sterile saline) was administered once daily on Days 2 – 8 post-operatively. Half of each drug group was housed under enriched environment conditions supplemented with daily focused activity sessions while the other half received standard housing with no focused activity. Skilled motor performance in forelimb reaching and ladder rung walking was assessed for 8 weeks post-operatively. Animals receiving atipamezole plus rehabilitation exhibited significantly greater motor improvement in both behavioral tests as compared to vehicle-treated animals receiving rehabilitation. Interestingly, animals receiving atipamezole without rehabilitation exhibited a significant motor improvement in the ladder rung walk test, but not the forelimb reaching test. These results suggest that a short-term increase in noradrenergic activity can lead to sustained motor improvement following stroke, especially when paired with rehabilitation.
Keywords: middle cerebral artery occlusion, physical therapy, rat, norepinephrine
1. Introduction
Stroke is a devastating neurological event that often results in permanent deficits of motor function(Langhorne et al., 2009). Physical therapy (PT) is the mainstay of rehabilitative treatment aimed at improving motor disability after stroke, but there is still a need for improved rehabilitative strategies. A great deal of investigation has focused on pharmacological means of improving long-term motor outcome. To this end, drugs that enhance central noradrenergic activity have shown some promise for promoting motor recovery following brain injury (Berends et al., 2009;Boyeson and Feeney, 1990;Feeney et al., 2004;Goldstein, 2006).
Clinical observations indicate that norepinephrine re-uptake inhibitors lead to improved acquisition of motor skills in healthy volunteers and in stroke patients (Plewnia et al., 2004;Foster et al., 2006;Zittel et al., 2007). Preclinical studies indicate that a variety of drugs that enhance central noradrenergic activity promote motor recovery following brain damage while drugs that block noradrenergic activity impair motor recovery. For example, blockade of alpha-1 adrenoceptors or depletion of brain norepinephrine significantly slows recovery of function (Feeney et al., 1982;Feeney and Westerberg, 1990;Feeney et al., 2004;Boyeson et al., 1992;Goldstein and Bullman, 2002). In contrast, the psychomotor stimulant amphetamine, which induces the neuronal release of norepinephrine, dopamine and to a lesser extent serotonin, improves forelimb motor function following brain injury (Adkins and Jones, 2005;Feeney et al., 1981;Gilmour et al., 2005;Hovda and Fenney, 1984;Papadopoulos et al., 2009). Clinical studies also suggest that amphetamine can improve motor function, although results are more controversial (Crisostomo et al., 1988;Goldstein, 2009;Martinsson et al., 2007).
One means of increasing central noradrenergic activity that has shown promise in preclinical motor rehabilitative strategies is through alpha2-adreneceptor antagonists. Alpha2-adrenoceptors serve as cell body and nerve terminal inhibitory autoreceptors, the blockade of which can lead to enhanced synaptic availability of norepinephrine (Gobert et al., 2004). Relatively non-selective alpha2 adrenoceptor antagonists, such as yohimbine, did not show great rehabilitative potential following sensorimotor cortex lesions in early pre-clinical studies (Feeney and Westerberg, 1990). However, the more selective alpha2-adrenoceptor antagonist atipamezole (ATI) has recently been shown to improve the rate at which motor performance returns to baseline levels following transient middle cerebral artery occlusion (MCAO) in rats, albeit with some variability (Newman-Tancredi et al., 1998;Pertovaara et al., 2005;Butovas et al., 2001;Haapalinna et al., 1997;Karhunen et al., 2003;Puurunen et al., 2001;Jolkkonen et al., 2000). Despite these promising results in improving the rate of recovery there is no understanding as to whether atipamezole could improve the extent of motor improvement following stroke, because motor deficits were not persistent in these earlier studies. Thus, it remains to be determined whether alpha2-adrenoceptor antagonists can lead to sustained motor improvement after stroke.
The aim of the present study was to determine how sub-acute (i.e. 1 week) treatment with the α2-adrenoceptor antagonist atipamezole affects long-term (i.e. 8 weeks) motor performance following permanent middle cerebral artery occlusion as a model of stroke. Additionally the importance of combining rehabilitation with atipamezole treatment was studied. We chose two behavioral tests, the forelimb reaching test and the ladder rung walk test, which require skilled forelimb use and which show consistent impairment through the course of at least 8 weeks in this stroke model (Whishaw, 2000;Papadopoulos et al., 2009;Ramic et al., 2006).
2. Results
2.1. Lesion analysis
All lesions impinged upon the forelimb area of the sensorimotor cortex ipsilateral to the occluded MCA with no obvious signs of damage to underlying subcortical tissue. Despite the trend for animals receiving atipamezole and/or rehabilitation to exhibit larger lesions, no significant difference was found in infarct volume among the 4 different treatment groups (Table 1, F3,36 = 1.73, p = 0.18).
Table 1.
Experimental Groups and Lesion Analysis
| Group (n) |
Drug/Housing+Activity conditions | Stroke Volume (% of contralesional hemisphere volume) |
|---|---|---|
| VEH/CON (9) | Vehicle-treated animals singly housed under control conditions – no rehabilitation |
8.7 ± 1.5 |
| ATI/CON (9) | Atipamezole-treated animals singly housed under control conditions – no rehabilitation |
11.5 ± 1.7 |
| VEH/REHAB (9) | Vehicle-treated animals group- housed in enriched environment plus focused activity sessions |
12.2 ± 2.2 |
| ATI/REHAB (10) | Atipamezole-treated animals group- housed in enriched environment plus focused activity sessions |
15.2 ± 2.7 |
After training, animals were randomly allocated to the different treatment groups depicted above and subjected to MCAO. Details of drug treatment, housing and activity conditions are described in more detail in Experimental Procedures. No significant difference in lesion size among groups was observed (F3,36 = 1.73, p = 0.18). Data represent the mean ± SEM of the indicated number of animals per group.
2.2. Skilled forelimb reaching
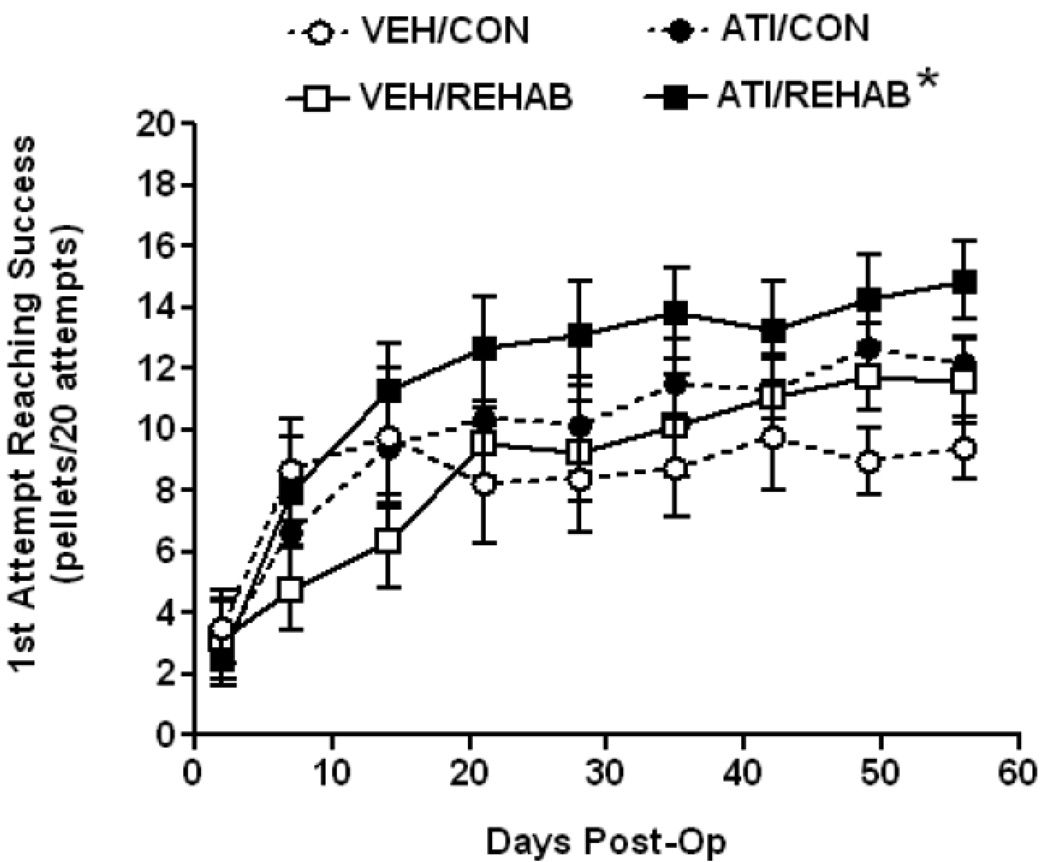
On Postoperative Day 2, before administering any drug or focused activity sessions, reaching performance in all groups showed a marked deficit resulting in a range of mean reaching success of 2.5 – 3.5 pellets/ 20 attempts with no significant difference among groups (F3,36 = 0.14, p = 0.93). Over the course of 8 weeks of testing all treatment groups improved in reaching performance, but to varying degrees (Fig. 1). A one-way ANOVA on Ranks established a significant treatment effect (H = 20.6 with 3 degrees of freedom, p<0.001). Post hoc analysis showed that overall reaching performance in rats receiving short-term atipamezole combined with rehabilitation (ATI/REHAB) was significantly better than rats receiving vehicle plus rehabilitation (VEH/REHAB) or vehicle controls (i.e. no rehabilitation). The difference between ATI/REHAB and animals receiving atipamezole without rehabilitation (ATI/CON) did not reach significance.
Figure 1. Short-term atipamezole enhances rehabilitation-aided motor improvement in skilled forelimb reaching after permanent MCAO.
Time course of motor performance- All animals enrolled in the study achieved the preoperative criteria of an average of 16 successes in 20 attempts for 3 days prior to surgery. At Day 2 Post-op, prior to any treatment, the mean deficit in reaching was not significantly different among groups (F3,36 = 0.14, p = 0.93). A one-way ANOVA on Ranks established an overall treatment effect (H = 20.6 with 3 degrees of freedom, p<0.001) and Dunn’s post-hoc test established that ATI/REHAB produced a significant overall enhancement in reaching performance compared to either vehicle-treated group (*, p<0.05). Data represent the mean ± SEM for following number of animals/group: VEH/CON = 9, VEH/REHAB = 9, ATI/CON = 9, ATI/REHAB = 10.
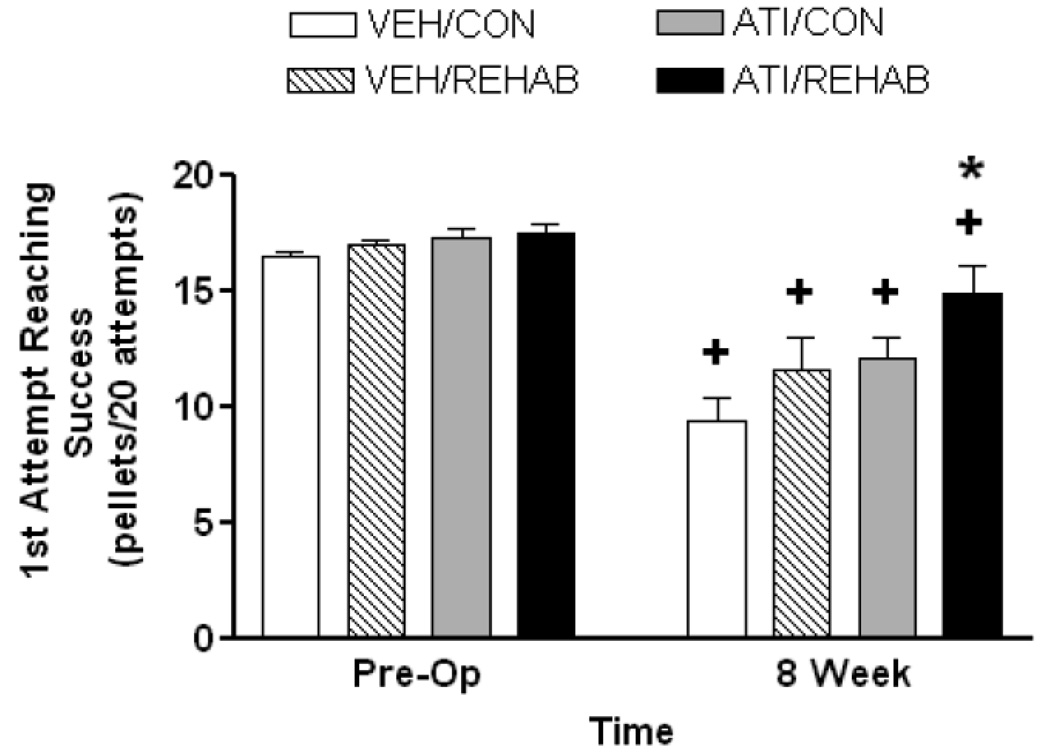
At 8 weeks a comparison between the endpoint observation and pre-operative performance was carried out to determine whether recovery to baseline performance had occurred (Fig. 2). Two-way ANOVA revealed a significant effect of MCAO surgery (F1,33 = 77.97, p<0.001), a significant effect of treatment (F3,33 = 4.46, p = 0.01), but no significant interaction (F3,33 = 2.69, p = 0.06). Post-hoc comparison revealed that endpoint performance in all groups was significantly lower than pre-operative performance (p<0.001 for all groups except ATI/REHAB, p=0.02). However, post-hoc comparison of treatment effect at the 8 week endpoint revealed that the ATI/REHAB group performed significantly better than all other groups (p<0.05).
Figure 2. Short-term atipamezole leads to long term enhancement, but not complete recovery, in skilled forelimb reaching after permanent MCAO.
End point analysis – At eight weeks following MCAO, reaching performance in the ATI/REHAB groups was significantly better than all other groups (*, p<0.05, two-way ANOVA followed by Student-Newman-Keuls). A two-way repeated measures ANOVA indicated that all treatment groups still displayed significant deficits in reaching when compared to pre-operative performance (+, p<0.05, Student-Newman-Keuls), which demonstrates that full recovery to baseline performance in the forelimb reaching task was not achieved. Data represent the mean ± SEM for following number of animals/group: VEH/CON = 9, VEH/REHAB = 9, ATI/CON = 9, ATI/REHAB = 10.
2.3. Ladder rung walk
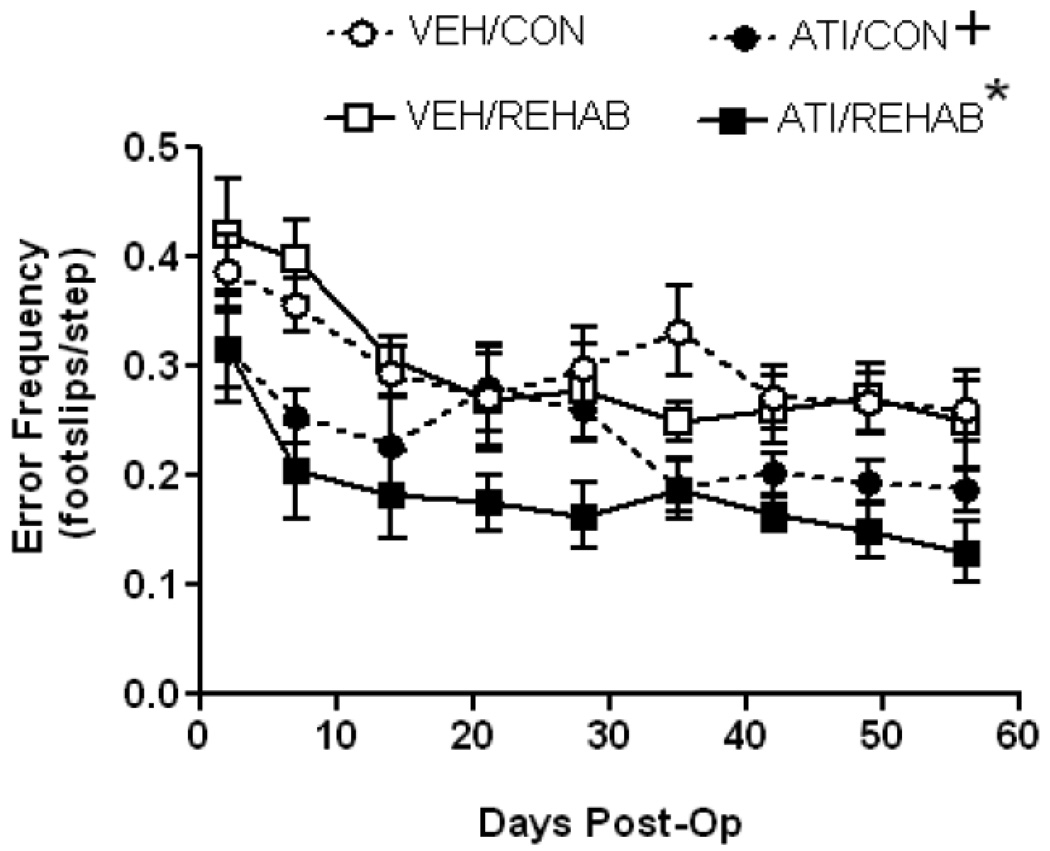
On Postoperative Day 2 all groups showed a marked deficit in accurate placement of the contralesional forelimb, resulting in a range of mean performance of 2.9 – 4.2 errors/10 steps across all groups. Although there was a trend toward fewer errors by animals allocated to the atipamezole groups compared to those in the vehicle groups the mean difference in performance did not reach statistical significance (F3,36 = 2.47, p = 0.08). Fig. 3 shows that, similar to reaching performance, ladder walk performance improved to some extent in all groups over 8 weeks. A one-way ANOVA on Ranks demonstrated a significant treatment effect (H = 63.3 with 3 degrees of freedom, p<0.001). Post-hoc analysis revealed a number of statistical differences among groups. Importantly, overall performance in the ATI/REHAB group was significantly better than any other group. Unlike the results for the reaching task, overall ladder walk performance of the ATI/CON group was significantly better than vehicle-treated animals regardless of the presence of rehabilitation (i.e better than VEH/CON or VEH/REHAB group).
Figure 3. Short-term atipamezole enhances motor improvement in ladder rung walking after permanent MCAO regardless of physical rehabilitation.
Time course of motor performance- At Day 2 Post-op, prior to any treatment, the mean deficit in skilled forelimb placement was not significantly different among groups (F3,36 = 2.47, p = 0.08). A one-way ANOVA on Ranks established an overall treatment effect (H = 63.3 with 3 degrees of freedom, p<0.001) and Dunn’s post-hoc test established that ATI/REHAB produced a significant overall enhancement in ladder rung walking performance compared to all other treatment groups (*, p<0.05). Post-hoc analysis also revealed a significant overall enhancement in performance in ATI/CON compared to either vehicle-treated group (+, p<0.05). Data represent the mean ± SEM for following number of animals/group: VEH/CON = 9, VEH/REHAB = 9, ATI/CON = 9, ATI/REHAB = 10.
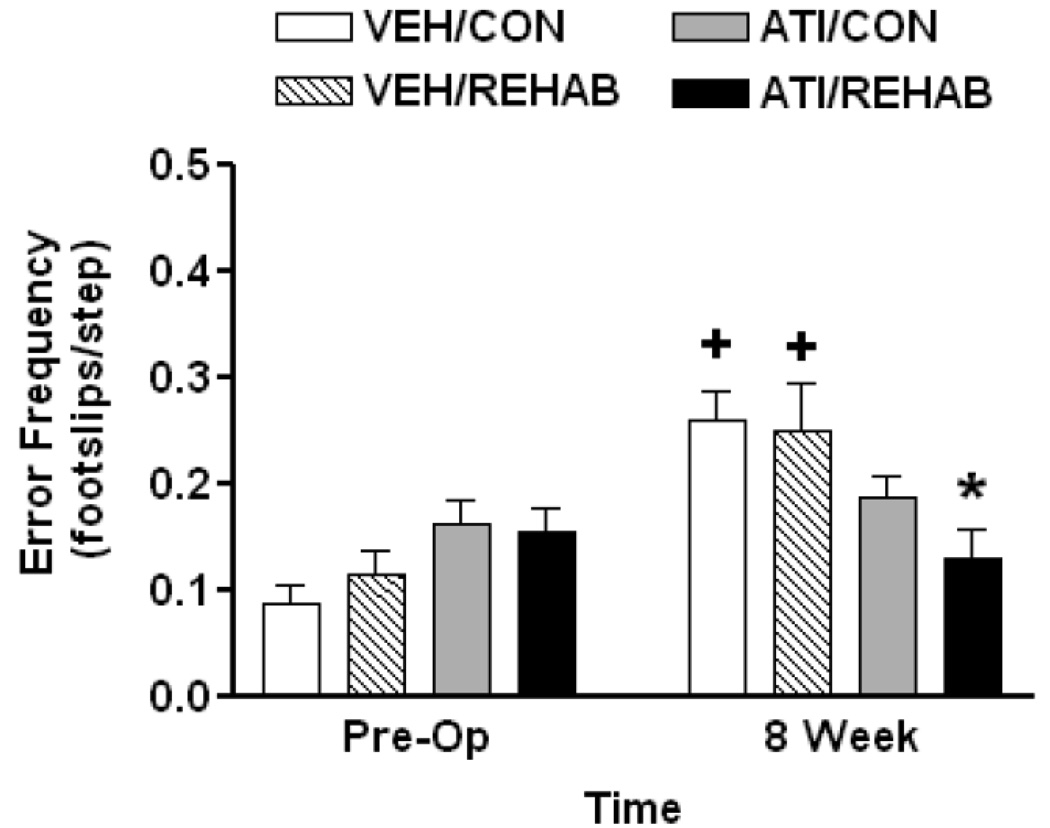
At 8 weeks a comparison between the endpoint observation and pre-operative performance was carried out to determine whether recovery to baseline performance in the ladder rung walk had occurred (Fig. 4). Two-way ANOVA comparing endpoint (8 week) performance vs. preoperative performance revealed a significant effect of MCAO surgery (F1,33 = 14.28, p<0.001), and a significant interaction (F3,33 = 5.13, p = 0.005), but no significant treatment effect (F3,33 = 1.13, p = 0.35). Neither vehicle-treated group recovered to baseline (i.e. pre-operative) performance by 8 weeks post-op. By comparison, ladder rung walk performance at 8 weeks had recovered to baseline (i.e. pre-operative) performance in animals receiving atipamezole regardless of whether or not they received rehabilitation (Fig. 4). Post-hoc comparison of treatment effect at the 8 week endpoint revealed that the ATI/REHAB group performed significantly better than VEH/CON or VEH/REHAB (p<0.006, Student-Newman-Keuls).
Figure 4. Short-term atipamezole leads to recovery of motor performance in ladder rung walking after permanent MCAO regardless of physical rehabilitation.
End point analysis – At eight weeks following MCAO, ladder walk performance following ATI/REHAB was significantly better than either vehicle-treated group (*, p<0.05, two-way ANOVA followed by Student-Newman-Keuls). A two-way repeated measures ANOVA indicated that the ATI/CON and ATI/REHAB groups achieved end-point performance that was not different from pre-operative levels while both vehicle-treatment groups still displayed significant deficits when compared to pre-operative performance (+, p<0.05, Student-Newman-Keuls). Data represent the mean ± SEM for following number of animals/group: VEH/CON = 9, VEH/REHAB = 9, ATI/CON = 9, ATI/REHAB = 10.
3. Discussion
The main finding of this study is that subacute administration of atipamezole, in combination with physical rehabilitation (i.e. focused activity plus enriched environment), led to a significantly greater degree of motor improvement following stroke as compared to rehabilitation alone. Importantly, motor improvement in both the forelimb reaching task and ladder rung walk was apparent at 8 weeks following stroke (7 weeks following cessation of atipamezole), indicating a long-lasting effect of drug treatment. These results are similar to what we found in a previous study using the catecholamine releasing agent D-amphetamine sulfate (Papadopoulos et al., 2009). When paired with physical rehabilitation amphetamine led to enhanced motor improvement following stroke that was significantly greater than rehabilitation alone. Interestingly, amphetamine/rehabilitation induced a recovery to baseline (i.e. pre-operative) performance in both motor tasks at 8 weeks. In the present study, atipamezole paired with physical rehabilitation led to motor recovery to baseline at 8 weeks only in the ladder rung walk. This minor difference in outcome between the two studies may reflect differences in mechanism of action of the two drugs and the degree to which noradrenergic activity is enhanced. Atipamezole is an alpha2-adrenergic antagonist that blocks the activation of presynaptic autoreceptors on noradrenergic neurons (Gobert et al., 2004;Haapalinna et al., 1997;Newman-Tancredi et al., 1998;Pertovaara et al., 2005). Thus the degree to which atipamezole increases the synaptic availability of norepinephrine depends, in part, on the level of activity of noradrenergic neurons. Amphetamine induces the neuronal release of norepinephrine regardless of the level of noradrenergic neuronal activity and it might be expected to cause greater release of norepinephrine (Geranton et al., 2003). Additionally, atipamezole is more selective than amphetamine in its ability to increase the synaptic availability of norepinephrine without affecting the dopaminergic system (Scheinin et al., 1988;Gobert et al., 2004). Preliminary studies from our lab (not shown) and published reports have shown that dopamine D2 blockade with haloperidol can impair motor recovery or prevent amphetamine-induced motor improvement, suggesting that dopamine may also be an important contributor to rehabilitative drug action (Feeney and Hovda, 1983;Goldstein and Bullman, 2002). Along these lines, it is interesting that the noradrenergic releasing agent, methylphenidate, which has greater selectivity but lower efficacy for releasing norepinephrine, has not been shown to have a profound impact on motor recovery in preclinical or clinical studies of brain damage, but may improve cognitive function (Grade et al., 1998;Kajs-Wyllie, 2002;Kline et al., 1994;Liepert, 2008). It would be interesting to determine whether more robust improvement in performance on the reaching task by atipamezole (and other drugs that rely more exclusively on noradrenergic enhancement) could be achieved if paired with physical activity that requires more forelimb and digit dexterity such as the pasta handling test.
Our findings extend the work of previous studies in which it was shown that atipamezole can improve the rate of motor functional recovery following ischemic brain damage. The data from these studies suggest that noradrenergic-enhanced motor recovery following stroke is best achieved in tests requiring use-dependent practice (Jolkkonen et al., 2000;Puurunen et al., 2001;Butovas et al., 2001;Karhunen et al., 2003). In these studies rats received a transient middle cerebral artery occlusion (MCAO) followed by daily motor testing for the first week following stroke and then semi-regular testing (every 3–5 days) for up to 3 more weeks. Administration of atipamezole prior to motor testing increased the rate at which motor scores in limb placing and foot-slip tests returned to pre-operative values. However, the motor deficits induced by transient MCAO were not shown to persist and all experimental groups recovered to baseline performance by the end of each study. Moreover, there was little indication of atipamezole-induced improvement in forelimb use asymmetry in the cylinder test, which was performed only once during the first week post-stroke and once again at 3 weeks post-stroke. Limb use asymmetry in the cylinder test is relatively long lasting and there is little evidence that animals develop compensatory motor strategies (Schallert et al., 2000). These previous findings are important in that they suggest that noradrenergic enhancement of motor function following stroke may require the learning of new motor strategies or repeated motor testing. Accordingly, clinical studies have shown that noradrenergic enhancement improves the acquisition of motor skills (Plewnia et al., 2004;Foster et al., 2006;Zittel et al., 2007), providing the basis for suggesting that faster motor improvement caused by enhanced noradrenergic activity is the result of enhanced acquisition of motor skills. However the previous preclinical studies with atipamezole could not address the issue of whether such treatment would be effective in reversing persistent motor deficits. Our present study addresses this matter by employing a permanent MCAO model of stroke that leads to long lasting deficits in skilled forelimb use. Using this model, motor deficits lasted throughout the observation period of 8 weeks post-stroke. Our design was to administer atipamezole prior to focused activity sessions to avoid the potential confound of a direct influence on performance in the motor assessment tasks. Atipamezole was administered after behavioral assessment and 15 min prior to the start of focused activity sessions in order to coincide with the timing of increased synaptic norepinephrine (Gobert et al., 2004;Pertovaara et al., 2005). Under these conditions atipamezole improved motor performance to an extent greater that that seen in respective vehicle-treated groups. While our experimental design rules out a direct effect of atipamezole on motor performance during the assessment tasks, it should be noted that the focused activity consisted of exercises with similar forelimb motor-specific requirements (i.e. grasping, grip strength, placement, coordination) as the behavioral tests employed. These tasks are similar to the complex motor skills training (i.e. “acrobatic task”) that induces structural plasticity in the intact motor cortex of rats receiving a sensorimotor cortex lesion on the other side (Jones et al., 1999). Following lesion, acrobatic rats showed evidence of increased synaptogenesis compared to rats that were simply required to run in an alley for a similar duration as the acrobatic task. These data suggest that the level of complexity of physical training, which may introduce a learning component to the task, is an important variable to be considered. Given the discussion above related to noradrenergic enhancement of motor skills, it is possible that atipamezole enhanced the learning of compensatory motor strategies, which could then be generalized to the behavioral tests (e.g. see Whishaw, 2000;Bury and Jones, 2002).
The present finding of sustained (i.e. lasting 8 weeks) motor improvement following short-term atipamezole suggests that, like amphetamine, atipamezole may be capable of inducing profound and persistent changes in intracellular signaling, gene transcription as well as altered synaptic morphology (Robinson and Kolb, 1999;Nestler, 2001;Park et al., 2002). Several studies have shown that norepinephrine reuptake inhibiting antidepressants alter gene transcription related to synaptic plasticity in a similar manner (Laifenfeld et al., 2002;Laifenfeld et al., 2005;Schmidt et al., 2008). Additionally, drugs that enhance central noradrenergic activity have a profound effect on the expression of a variety of neurotrophic factors such as basic fibroblast growth factor (bFGF), brain derived neurotrophic factor (BDNF) and glial cell derived neurotrophic factor (Chen et al., 2007;Garcia et al., 2003;Juric et al., 2008;Mannari et al., 2008;Rizk et al., 2006;Schmidt et al., 2008;Bachis et al., 2008;Flores et al., 1998;Riva et al., 1998). Of special interest is bFGF, which is a potent neurotrophic factor that has been shown to enhance recovery of motor function following ischemic brain damage, presumably by contributing to synaptogenesis (Baird, 1994;Patel and McNamara, 1995;Kawamata et al., 1997a;Kawamata et al., 1997b;Kawamoto et al., 1999;Rowntree and Kolb, 1997). Following brain damage, bFGF expression shows a persistent increase that can last for weeks (Takami et al., 1993;Reilly and Kumari, 1996) and which can be localized to both neuronal and astrocyte populations (Chadi et al., 1993;Wei et al., 2000). Activation of astrocytes may be one mechanism by which noradrenergic activity induces upregulation of growth factors. It has long been known that both alpha1- and beta-adrenergic receptor subtypes reside on astrocytes and mediate an increase in neurotrophins such as bFGF and BDNF (Junker et al., 2002;Juric et al., 2008;Kimelberg, 1995;Hertz et al., 2004). The ability of noradrenergic agents to stimulate neurite outgrowth in cell culture has been shown (Laifenfeld et al., 2002;Kwon et al., 1998;Park et al., 2002) and our previous study demonstrated that amphetamine treatment paired with rehabilitation induced neurite outgrowth from corticoefferent pathways of the contralesional hemisphere (Papadopoulos et al., 2009). Unfortunately, technical difficulties in the present study prevented us from determining whether improved motor outcome with atipamezole plus rehabilitation was associated with corticoefferent axonal sprouting. Nevertheless, studies are currently underway to delineate the possible neurobiological mechanisms mediating noradrenergic-enhanced neurite outgrowth in vitro as well as in vivo.
In summary the present findings indicate that short-term treatment with an alpha2 adrenoceptor antagonist can lead to enhanced long-term motor recovery following ischemic stroke when paired with focused physical therapy. These findings, combined with the observation that moderate blockade of alpha2 adrenoceptors appears to induce less profound sympathetic activation than amphetamine (Pertovaara et al., 2005;Schmidt et al., 1997;Martinsson et al., 2007), suggest that increasing central noradrenergic tone through inhibition of alpha2-adrenergic inhibitory autoreceptors may be a useful adjunct in the treatment of stroke. However, care should be taken when extrapolating our results in animals to the design of patient clinical trials. Studies are underway to delineate the pharmacological and neurobiological mechanisms involved.
4. Experimental Procedures
4.1. Animals
Adult male Long Evans, black-hooded rats (250–300 g) were maintained in a temperature and humidity controlled room under a 12:12-h light/dark cycle. Food intake was moderately restricted throughout the study to maintain body weight at 95% of ad libitum weight. Water was available ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committees.
4.2. Housing/Rehabilitation/Drug Administration
Rats were randomly allocated to different experimental groups with differing housing conditions as described below. Control housing conditions (CON) consisted of singly housed animals in a standard Plexiglas cage (24cm × 36cm × 15cm) with no additions. “Rehabilitation” (REHAB) consisted of housing animals in an enriched environment with supplementary daily sessions of focused activity. The enriched environment consisted of group-housed animals (three per cage) in larger Plexiglas cages (81.5cm × 61cm × 45cm) furnished with inclined ladders, hanging toys, chewable material, and tunnels. Once a week novel objects were introduced.
Focused activity sessions began two days following permanent middle cerebral artery occlusion (MCAO) and continued twice daily for the first three weeks. For the remaining 5 weeks focused activity was conducted once daily. Individual sessions were 20 minutes in length and began after completion of daily behavioral testing and on post-op days 2–8 fifteen minutes following drug or vehicle administration. Focused activity consisted of activities that relied heavily on the use of forelimbs, but were distinct from the specific tasks being assessed. During sessions, cage mates (3 animals/session) were placed simultaneously in a 5’x5’ enclosed space containing a 45° inclined ladder (200cm long × 5cm wide), vertical rope (100cm × 4cm diam), and vertical cylindrical grid (100cm × 10cm diameter; 1cm2 mesh). Participation among the rats was equalized by having two investigators follow the rats and physically place each animal on a different piece of exercise equipment at approximately 1 min intervals during the twenty minute sessions.
Atipamezole HCl was obtained as a sterile injectable solution (Antisedan 5 mg/ml, Orion Corporation) and diluted to 1 mg/ml with 0.9% sterile saline. All animals received either atipamezole (1 mg/kg, s.c.) or vehicle (0.9% sterile saline) in a volume of 1 ml/kg on post-op days 2 – 8 following MCAO. Animals receiving focused activity began the session fifteen minutes following injection, which coincides with maximal serum concentration of the drug (Pertovaara et al., 2005).
4.3. Training/Behavioral Testing
All animals were trained on the skilled forelimb reaching task and familiarized with the ladder rung walk test to assess deficits in performance of the impaired (i.e. contralateral to stroke) forelimb as previously described (Papadopoulos et al., 2009). Investigators performing behavioral testing were blinded to the treatment groups. Skilled forelimb reaching involved placing an animal in a transparent Plexiglas chamber (30 × 36 × 30 cm) and training it to reach through a window (1.5 × 3 cm) to retrieve small sucrose pellets (45 mg; Bilaney Consultants, Frenchtown, NJ) placed on a platform at a distance of 1 cm. During the initial days of training, limb preference was determined and placement of pellets was adjusted to favor the use of the preferred forelimb. Prior to surgery, baseline performance (defined as the average of the last three testing sessions of the preoperative testing) was established. Success was defined as an animal grasping the pellet on the first attempt and placing it into the mouth (i.e. “first reach success”). The pre-operative criterion was at least 16 successes in 20 attempts for 3 consecutive days. Animals that reached criterion were randomly allocated to experimental groups (see Table 1) prior to MCAO surgery. Animals that failed to reach criterion were not used. Following MCAO surgery animals were tested on the skilled forelimb reaching task beginning the first day post-operatively and then daily (Mon – Fri) for 8 weeks. Each testing session consisted of 20 reaching opportunities using the preferred/impaired forelimb. Attempts using the non-preferred forelimb were not included in analyses. A maximum time limit of 5 minutes/testing session was given.
In the ladder rung walk animals were scored for their ability to cross a 1 m long horizontal metal-rung runway with varying gaps of 1–2 cm between the rungs (Metz and Whishaw, 2002). All animals underwent 3 familiarization sessions with the apparatus prior to pre-operative baseline testing. Sessions were then videotaped and scored at 1 day pre-op to determine baseline performance. Following MCAO surgery and allocation to experimental groups (see Table 1) animals were videotaped and scored on the ladder rung walk on the 2nd day post-op and then weekly for 8 weeks. A forelimb foot error was defined as a complete miss or slip from the rung. The mean preoperative scores for all experimental groups were approximately 1 foot error per 10 steps. Baseline and post-operative testing sessions consisted of 3 runway crossings. The total number of errors and steps by the preferred forelimb in each session was counted. An error frequency was calculated by dividing the sum total of steps for all 3 crossings in a session by the sum total of errors for the same 3 crossings in that session. Only data representing the performance of the impaired forelimb were analyzed.
4.4. Stroke surgery
All animals underwent MCAO on the side opposite the preferred reaching limb as described previously (Papadopoulos et al., 2009). Animals were anesthetized with sodium pentobarbital (50 mg/kg; i.p.). Bilateral common carotid arteries (CCA) were isolated, a vertical 2 cm long incision was made between the eye and ear, and the temporalis muscle was retracted. A burr hole was made to expose the MCA and it was permanently occluded with a 10-0 suture. The CCA ipsilateral to the MCAO was permanently occluded with a 4-0 suture and the contralateral CCA was temporarily occluded for 45 min. The wounds were then closed and animals warmed under a heating lamp until they awoke.
4.5. Lesion analysis and exclusion criteria
At the end of the study animals were overdosed with sodium pentobarbital (100mg/kg; i.p.) and perfused transcardially with 4% paraformaldehyde. Brains and spinal cords were removed, placed in 30% sucrose for 1–2 days, embedded in OCT freezing compound (Miles, Inc), frozen, and stored at −80° C. Coronal cryosections (50 µm thick) were processed with Nissl stain. Stroke volume was quantitatively analyzed on Nissl stained sections (+4.7 to −5.2 mm from bregma according to Paxinos and Watson) using NIH image as described previously (Papadopoulos et al., 2009). Stroke size was expressed as the percent difference in volume between the infarcted hemisphere and the contralesional intact hemisphere. The entire hemisphere including subcortical tissue was included in the analyses. Animals were excluded from all statistical analyses if the lesion was found not to impinge on the forelimb region of the sensorimotor cortex, and/or if subcortical damage was observed.
4.6. Statistics
Data was analyzed using SigmaStat (Systat Software Inc.). Stroke size was analyzed by one-way ANOVA. Skilled forelimb reaching and ladder rung walking were analyzed independently using a one-way ANOVA on ranks for overall treatment effect since data failed either the normality test, equal variance test or both. Specific post-hoc comparisons were made using Dunn’s Multiple Comparison procedure. A two-way repeated measures ANOVA was used to compare the mean performance values among all groups at end-point (i.e., 8 weeks post-op) vs. baseline (pre-operative). Post-hoc comparisons using Student-Newman-Keuls test were used to compare performance among treatment groups at 8 weeks. In all cases a p value less than or equal to 0.05 was considered significant. All data are presented as mean values ± standard error of the mean (SEM).
Acknowledgements
We would like to thank Veronica Guillen and Juan Ortega for their skillful technical assistance during this project. This work was supported by PHS Grant HD 44772 (W.A.W.) and the Department of Veterans Affairs. This research represents work that was carried out in partial fulfillment of the requirements for the degree of Master’s in Science (E.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins DL, Jones TA. D-Amphetamine Enhances Skilled Reaching After Ischemic Cortical Lesions in Rats. Neurosci Lett. 2005;380:214–218. doi: 10.1016/j.neulet.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I. Chronic Antidepressant Treatments Increase Basic Fibroblast Growth Factor and Fibroblast Growth Factor-Binding Protein in Neurons. Neuropharmacology. 2008;55:1114–1120. doi: 10.1016/j.neuropharm.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A. Potential Mechanisms Regulating the Extracellular Activities of Basic Fibroblast Growth Factor (FGF-2) Mol Reprod Dev. 1994;39:43–48. doi: 10.1002/mrd.1080390108. [DOI] [PubMed] [Google Scholar]
- Berends HI, Nijlant JM, Movig KL, Van Putten MJ, Jannink MJ, Ijzerman MJ. The Clinical Use of Drugs Influencing Neurotransmitters in the Brain to Promote Motor Recovery After Stroke; a Cochrane Systematic Review. Eur J Phys Rehabil Med. 2009;45:621–630. [PubMed] [Google Scholar]
- Boyeson MG, Callister TR, Cavazos JE. Biochemical and Behavioral Effects of a Sensorimotor Cortex Injury in Rats Pretreated With the Noradrenergic Neurotoxin DSP-4. Behav Neurosci. 1992;106:964–973. doi: 10.1037//0735-7044.106.6.964. [DOI] [PubMed] [Google Scholar]
- Boyeson MG, Feeney DM. Intraventricular Norepinephrine Facilitates Motor Recovery Following Sensorimotor Cortex Injury. Pharmacol Biochem Behav. 1990;35:497–501. doi: 10.1016/0091-3057(90)90279-q. [DOI] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Unilateral Sensorimotor Cortex Lesions in Adult Rats Facilitate Motor Skill Learning With the "Unaffected" Forelimb and Training-Induced Dendritic Structural Plasticity in the Motor Cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovas S, Lukkarinen J, Virtanen T, Jolkkonen J, Sivenius J. Differential Effect of Alpha2-Adrenoceptor Antagonist, Atipamezole, in Limb-Placing Task and Skilled Forepaw Use Following Experimental Stroke. Restor Neurol Neurosci. 2001;18:143–151. [PubMed] [Google Scholar]
- Chadi G, Tinner B, Agnati LF, Fuxe K. Basic Fibroblast Growth Factor (BFGF, FGF-2) Immunoreactivity Exists in the Noradrenaline, Adrenaline and 5-HT Nerve Cells of the Rat Brain. Neurosci Lett. 1993;160:171–176. doi: 10.1016/0304-3940(93)90406-b. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA. Norepinephrine Induces BDNF and Activates the PI-3K and MAPK Cascades in Embryonic Hippocampal Neurons. Cell Signal. 2007;19:114–128. doi: 10.1016/j.cellsig.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Crisostomo EA, Duncan PW, Propst M, Dawson DV, Davis JN. Evidence That Amphetamine With Physical Therapy Promotes Recovery of Motor Function in Stroke Patients. Ann Neurol. 1988;23:94–97. doi: 10.1002/ana.410230117. [DOI] [PubMed] [Google Scholar]
- Feeney DM, De Smet AM, Rai S. Noradrenergic Modulation of Hemiplegia: Facilitation and Maintenance of Recovery. Restor Neurol Neurosci. 2004;22:175–190. [PubMed] [Google Scholar]
- Feeney DM, Gonzales A, Law WA. Amphetamine Restores Locomotor Function After Motor Cortex Injury in the Rat. Proc West Pharmacol Soc. 1981;24:15–17. [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, Law WA. Amphetamine, Haloperidol, and Experience Interact to Affect Rate of Recovery After Motor Cortex Injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Hovda DA. Amphetamine and Apomorphine Restore Tactile Placing After Motor Cortex Injury in the Cat. Psychopharmacology (Berl) 1983;79:67–71. doi: 10.1007/BF00433018. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Westerberg VS. Norepinephrine and Brain Damage: Alpha Noradrenergic Pharmacology Alters Functional Recovery After Cortical Trauma. Can J Psychol. 1990;44:233–252. doi: 10.1037/h0084243. [DOI] [PubMed] [Google Scholar]
- Flores C, Rodaros D, Stewart J. Long-Lasting Induction of Astrocytic Basic Fibroblast Growth Factor by Repeated Injections of Amphetamine: Blockade by Concurrent Treatment With a Glutamate Antagonist. J Neurosci. 1998;18:9547–9555. doi: 10.1523/JNEUROSCI.18-22-09547.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Good DC, Fowlkes A, Sawaki L. Atomoxetine Enhances a Short-Term Model of Plasticity in Humans. Arch Phys Med Rehabil. 2006;87:216–221. doi: 10.1016/j.apmr.2005.08.131. [DOI] [PubMed] [Google Scholar]
- Garcia C, Chen MJ, Garza AA, Cotman CW, Russo-Neustadt A. The Influence of Specific Noradrenergic and Serotonergic Lesions on the Expression of Hippocampal Brain-Derived Neurotrophic Factor Transcripts Following Voluntary Physical Activity. Neuroscience. 2003;119:721–732. doi: 10.1016/s0306-4522(03)00192-1. [DOI] [PubMed] [Google Scholar]
- Geranton SM, Heal DJ, Stanford SC. Differences in the Mechanisms That Increase Noradrenaline Efflux After Administration of D-Amphetamine: a Dual-Probe Microdialysis Study in Rat Frontal Cortex and Hypothalamus. Br J Pharmacol. 2003;139:1441–1448. doi: 10.1038/sj.bjp.0705396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour G, Iversen SD, O'Neill MF, O'Neill MJ, Ward MA, Bannerman DM. Amphetamine Promotes Task-Dependent Recovery Following Focal Cortical Ischaemic Lesions in the Rat. Behav Brain Res. 2005;165:98–109. doi: 10.1016/j.bbr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Gobert A, Billiras R, Cistarelli L, Millan MJ. Quantification and Pharmacological Characterization of Dialysate Levels of Noradrenaline in the Striatum of Freely-Moving Rats: Release From Adrenergic Terminals and Modulation by Alpha2-Autoreceptors. J Neurosci Methods. 2004;140:141–152. doi: 10.1016/j.jneumeth.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Goldstein LB. Neurotransmitters and Motor Activity: Effects on Functional Recovery After Brain Injury. NeuroRx. 2006;3:451–457. doi: 10.1016/j.nurx.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LB. Amphetamine Trials and Tribulations. Stroke. 2009;40:S133–S135. doi: 10.1161/STROKEAHA.108.533703. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Bullman S. Differential Effects of Haloperidol and Clozapine on Motor Recovery After Sensorimotor Cortex Injury in Rats. Neurorehabil Neural Repair. 2002;16:321–325. doi: 10.1177/154596830201600402. [DOI] [PubMed] [Google Scholar]
- Grade C, Redford B, Chrostowski J, Toussaint L, Blackwell B. Methylphenidate in Early Poststroke Recovery: a Double-Blind, Placebo-Controlled Study. Arch Phys Med Rehabil. 1998;79:1047–1050. doi: 10.1016/s0003-9993(98)90169-1. [DOI] [PubMed] [Google Scholar]
- Haapalinna A, Viitamaa T, MacDonald E, Savola JM, Tuomisto L, Virtanen R, Heinonen E. Evaluation of the Effects of a Specific Alpha 2-Adrenoceptor Antagonist, Atipamezole, on Alpha 1- and Alpha 2-Adrenoceptor Subtype Binding, Brain Neurochemistry and Behaviour in Comparison With Yohimbine. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:570–582. doi: 10.1007/pl00005092. [DOI] [PubMed] [Google Scholar]
- Hertz L, Chen Y, Gibbs ME, Zang P, Peng L. Astrocytic Adrenoceptors: a Major Drug Target in Neurological and Psychiatric Disorders? Curr Drug Targets CNS Neurol Disord. 2004;3:239–267. doi: 10.2174/1568007043337535. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Fenney DM. Amphetamine With Experience Promotes Recovery of Locomotor Function After Unilateral Frontal Cortex Injury in the Cat. Brain Res. 1984;298:358–361. doi: 10.1016/0006-8993(84)91437-9. [DOI] [PubMed] [Google Scholar]
- Jolkkonen J, Puurunen K, Rantakomi S, Harkonen A, Haapalinna A, Sivenius J. Behavioral Effects of the Alpha(2)-Adrenoceptor Antagonist, Atipamezole, After Focal Cerebral Ischemia in Rats. Eur J Pharmacol. 2000;400:211–219. doi: 10.1016/s0014-2999(00)00409-x. [DOI] [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor Skills Training Enhances Lesion-Induced Structural Plasticity in the Motor Cortex of Adult Rats. J Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker V, Becker A, Huhne R, Zembatov M, Ravati A, Culmsee C, Krieglstein J. Stimulation of Beta-Adrenoceptors Activates Astrocytes and Provides Neuroprotection. Eur J Pharmacol. 2002;446:25–36. doi: 10.1016/s0014-2999(02)01814-9. [DOI] [PubMed] [Google Scholar]
- Juric DM, Loncar D, Carman-Krzan M. Noradrenergic Stimulation of BDNF Synthesis in Astrocytes: Mediation Via Alpha1- and Beta1/Beta2-Adrenergic Receptors. Neurochem Int. 2008;52:297–306. doi: 10.1016/j.neuint.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Kajs-Wyllie M. Ritalin Revisited: Does It Really Help in Neurological Injury? J Neurosci Nurs. 2002;34:303–313. doi: 10.1097/01376517-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Karhunen H, Virtanen T, Schallert T, Sivenius J, Jolkkonen J. Forelimb Use After Focal Cerebral Ischemia in Rats Treated With an Alpha 2-Adrenoceptor Antagonist. Pharmacol Biochem Behav. 2003;74:663–669. doi: 10.1016/s0091-3057(02)01053-5. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, Finklestein SP. Intracisternal Basic Fibroblast Growth Factor Enhances Functional Recovery and Up-Regulates the Expression of a Molecular Marker of Neuronal Sprouting Following Focal Cerebral Infarction. Proc Natl Acad Sci U S A. 1997a;94:8179–8184. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Speliotes EK, Finklestein SP. The Role of Polypeptide Growth Factors in Recovery From Stroke. Adv Neurol. 1997b;73:377–382. [PubMed] [Google Scholar]
- Kawamoto Y, Nakamura S, Kawamata T, Akiguchi I, Kimura J. Cellular Localization of Brain-Derived Neurotrophic Factor-Like Immunoreactivity in Adult Monkey Brain. Brain Res. 1999;821:341–349. doi: 10.1016/s0006-8993(99)01082-3. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Receptors on Astrocytes--What Possible Functions? Neurochem Int. 1995;26:27–40. doi: 10.1016/0197-0186(94)00118-e. [DOI] [PubMed] [Google Scholar]
- Kline AE, Chen MJ, Tso-Olivas DY, Feeney DM. Methylphenidate Treatment Following Ablation-Induced Hemiplegia in Rat: Experience During Drug Action Alters Effects on Recovery of Function. Pharmacol Biochem Behav. 1994;48:773–779. doi: 10.1016/0091-3057(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Kwon JH, Vogt Weisenhorn DM, Downen M, Ruan K, Roback L, Joshi H, Wainer BH. Beta-Adrenergic and Fibroblast Growth Factor Receptors Induce Neuronal Process Outgrowth Through Different Mechanisms. Eur J Neurosci. 1998;10:2776–2789. doi: 10.1111/j.1460-9568.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- Laifenfeld D, Karry R, Grauer E, Klein E, Ben Shachar D. Antidepressants and Prolonged Stress in Rats Modulate CAM-L1, Laminin, and PCREB, Implicated in Neuronal Plasticity. Neurobiol Dis. 2005;20:432–441. doi: 10.1016/j.nbd.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Laifenfeld D, Klein E, Ben Shachar D. Norepinephrine Alters the Expression of Genes Involved in Neuronal Sprouting and Differentiation: Relevance for Major Depression and Antidepressant Mechanisms. J Neurochem. 2002;83:1054–1064. doi: 10.1046/j.1471-4159.2002.01215.x. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Coupar F, Pollock A. Motor Recovery After Stroke: a Systematic Review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- Liepert J. Pharmacotherapy in Restorative Neurology. Curr Opin Neurol. 2008;21:639–643. doi: 10.1097/WCO.0b013e32831897a3. [DOI] [PubMed] [Google Scholar]
- Mannari C, Origlia N, Scatena A, Del Debbio A, Catena M, Dell'agnello G, Barraco A, Giovannini L, Dell'osso L, Domenici L, Piccinni A. BDNF Level in the Rat Prefrontal Cortex Increases Following Chronic but Not Acute Treatment With Duloxetine, a Dual Acting Inhibitor of Noradrenaline and Serotonin Re-Uptake. Cell Mol Neurobiol. 2008;28:457–468. doi: 10.1007/s10571-007-9254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson L, Hardemark H, Eksborg S. Amphetamines for Improving Recovery After Stroke. Cochrane Database Syst Rev. 2007:CD002090. doi: 10.1002/14651858.CD002090.pub2. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and Subcortical Lesions Impair Skilled Walking in the Ladder Rung Walking Test: a New Task to Evaluate Fore- and Hindlimb Stepping, Placing, and Co-Ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular Basis of Long-Term Plasticity Underlying Addiction. Nat Rev Neurosci. 2001;49(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Nicolas JP, Audinot V, Gavaudan S, Verriele L, Touzard M, Chaput C, Richard N, Millan MJ. Actions of Alpha2 Adrenoceptor Ligands at Alpha2A and 5-HT1A Receptors: the Antagonist, Atipamezole, and the Agonist, Dexmedetomidine, Are Highly Selective for Alpha2A Adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Guillen V, Ortega J, Kartje GL, Wolf WA. Motor Recovery and Axonal Plasticity With Short-Term Amphetamine After Stroke. Stroke. 2009;40:294–302. doi: 10.1161/STROKEAHA.108.519769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Kantor L, Wang KK, Gnegy ME. Repeated, Intermittent Treatment With Amphetamine Induces Neurite Outgrowth in Rat Pheochromocytoma Cells (PC12 Cells) Brain Res. 2002;951:43–52. doi: 10.1016/s0006-8993(02)03103-7. [DOI] [PubMed] [Google Scholar]
- Patel MN, McNamara JO. Selective Enhancement of Axonal Branching of Cultured Dentate Gyrus Neurons by Neurotrophic Factors. Neuroscience. 1995;69:763–770. doi: 10.1016/0306-4522(95)00281-m. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Haapalinna A, Sirvio J, Virtanen R. Pharmacological Properties, Central Nervous System Effects, and Potential Therapeutic Applications of Atipamezole, a Selective Alpha2-Adrenoceptor Antagonist. CNS Drug Rev. 2005;11:273–288. doi: 10.1111/j.1527-3458.2005.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewnia C, Hoppe J, Cohen LG, Gerloff C. Improved Motor Skill Acquisition After Selective Stimulation of Central Norepinephrine. Neurology. 2004;62:2124–2126. doi: 10.1212/01.wnl.0000128041.92710.17. [DOI] [PubMed] [Google Scholar]
- Puurunen K, Jolkkonen J, Sirvio J, Haapalinna A, Sivenius J. An Alpha(2)-Adrenergic Antagonist, Atipamezole, Facilitates Behavioral Recovery After Focal Cerebral Ischemia in Rats. Neuropharmacology. 2001;40:597–606. doi: 10.1016/s0028-3908(00)00182-9. [DOI] [PubMed] [Google Scholar]
- Ramic M, Emerick AJ, Bollnow MR, O'Brien TE, Tsai SY, Kartje GL. Axonal Plasticity Is Associated With Motor Recovery Following Amphetamine Treatment Combined With Rehabilitation After Brain Injury in the Adult Rat. Brain Res. 2006;1111:176–186. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- Reilly JF, Kumari VG. Alterations in Fibroblast Growth Factor Receptor Expression Following Brain Injury. Exp Neurol. 1996;140:139–150. doi: 10.1006/exnr.1996.0124. [DOI] [PubMed] [Google Scholar]
- Riva MA, Molteni R, Racagni G. Differential Regulation of FGF-2 and FGFR-1 in Rat Cortical Astrocytes by Dexamethasone and Isoproterenol. Brain Res Mol Brain Res. 1998;57:38–45. doi: 10.1016/s0169-328x(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Rizk P, Salazar J, Raisman-Vozari R, Marien M, Ruberg M, Colpaert F, Debeir T. The Alpha2-Adrenoceptor Antagonist Dexefaroxan Enhances Hippocampal Neurogenesis by Increasing the Survival and Differentiation of New Granule Cells. Neuropsychopharmacology. 2006;31:1146–1157. doi: 10.1038/sj.npp.1300954. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the Morphology of Dendrites and Dendritic Spines in the Nucleus Accumbens and Prefrontal Cortex Following Repeated Treatment With Amphetamine or Cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Rowntree S, Kolb B. Blockade of Basic Fibroblast Growth Factor Retards Recovery From Motor Cortex Injury in Rats. Eur J Neurosci. 1997;9:2432–2441. doi: 10.1111/j.1460-9568.1997.tb01660.x. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS Plasticity and Assessment of Forelimb Sensorimotor Outcome in Unilateral Rat Models of Stroke, Cortical Ablation, Parkinsonism and Spinal Cord Injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Scheinin H, MacDonald E, Scheinin M. Behavioural and Neurochemical Effects of Antipamezole, a Novel Alpha 2-Adrenoceptor Antagonist. Eur J Pharmacol. 1988;151:35–42. doi: 10.1016/0014-2999(88)90689-9. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Banasr M, Duman RS. Future Antidepressant Targets: Neurotrophic Factors and Related Signaling Cascades. Drug Discov Today Ther Strateg. 2008;5:151–156. doi: 10.1016/j.ddstr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ME, Risinger RC, Hauger RL, Schouten JL, Henry M, Potter WZ. Responses to Alpha 2-Adrenoceptor Blockade by Idazoxan in Healthy Male and Female Volunteers. Psychoneuroendocrinology. 1997;22:177–188. doi: 10.1016/s0306-4530(96)00045-5. [DOI] [PubMed] [Google Scholar]
- Takami K, Kiyota Y, Iwane M, Miyamoto M, Tsukuda R, Igarashi K, Shino A, Wanaka A, Shiosaka S, Tohyama M. Upregulation of Fibroblast Growth Factor-Receptor Messenger RNA Expression in Rat Brain Following Transient Forebrain Ischemia. Exp Brain Res. 1993;97:185–194. doi: 10.1007/BF00228688. [DOI] [PubMed] [Google Scholar]
- Wei OY, Huang YL, Da CD, Cheng JS. Alteration of Basic Fibroblast Growth Factor Expression in Rat During Cerebral Ischemia. Acta Pharmacol Sin. 2000;21:296–300. [PubMed] [Google Scholar]
- Whishaw IQ. Loss of the Innate Cortical Engram for Action Patterns Used in Skilled Reaching and the Development of Behavioral Compensation Following Motor Cortex Lesions in the Rat. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Zittel S, Weiller C, Liepert J. Reboxetine Improves Motor Function in Chronic Stroke. A Pilot Study. J Neurol. 2007;254:197–201. doi: 10.1007/s00415-006-0326-5. [DOI] [PubMed] [Google Scholar]