Abstract
Objective
To examine the extent to which a common genetic pathway is also involved in the relationship between depressive symptoms, in the absence of major depressive disorder (MDD), and inflammation. Recent data suggested that MDD and inflammation share common genes.
Methods
We recruited 188 male twins from the Vietnam Era Twin Registry who were free of symptomatic coronary artery disease and MDD, with mean ± standard deviation (SD) age of 55 ± 2.75 years, including 54 monozygotic and 40 dizygotic twin pairs. These pairs were assessed for two inflammatory markers, interleukin (IL)-6 and C-reactive protein (CRP). Current depressive symptoms were measured with the Beck Depression Inventory-II. Generalized estimating equations were used to examine the phenotypic association between depression and inflammatory markers. Biometrical genetic modeling was performed to estimate the genetic and environmental contributions to this association.
Results
An association was observed between severity of current depressive symptoms and increased levels of inflammatory markers (p < .001 for IL-6 and p = .005 for CRP). After adjustment for other factors, the association was slightly attenuated but remained statistically significant for IL-6 (p = .002). The heritability of IL-6, CRP, and depressive symptoms were estimated as 0.37, 0.65, and 0.48, respectively. Genetic modeling found a significant genetic correlation between IL-6 and depressive symptoms (rG = 0.22, p = .046), indicating that about 66% of the covariance between them can be explained by shared genetic influences.
Conclusions
Current depressive symptoms are significantly correlated with inflammatory markers. This covariation is due, in large part, to genes that are common to depressive symptoms and inflammation.
Keywords: depression, inflammation, common genes, twin study, middle-aged
INTRODUCTION
The relationship between depression and the incidence of coronary artery disease (CAD) is now well established (1–3). Adverse lifestyle behaviors, lower heart rate variability, and enhanced platelet activation have long been considered potential explanations for this association (4–7). Of more recent interest is the role of inflammation in the development and progression of atherosclerosis (8,9) and its potential association with depression (10–12). Major depression and depressive symptoms have been associated with higher levels of inflammatory biomarkers, including interleukin-6 (IL-6) and C-reactive protein (CRP) (11,13–24). However, the causal direction of this association remains unclear and may actually be bidirectional, such that neurobiological correlates of depression may result in enhanced inflammation, and the latter, in turn, may increase the risk of depression (25,26).
It is also plausible that a common genetic vulnerability accounts for the observed association between depression and inflammation because both phenotypes are heritable. We have recently reported evidence for a common genetic pathway linking major depressive disorder (MDD) and inflammation (27). In this report, we examine if shared genetic vulnerability also plays a role in the link between depressive symptoms in the absence of MDD and inflammation.
METHODS
Subjects
The Twins Heart Study (THS) is an investigation of psychological, behavioral, and biological risk factors for subclinical cardiovascular disease, using twins. Twins were selected from the Vietnam Era Twin (VET) Registry (28), which includes 7369 middle-aged male-male twin pairs—both of whom served in the United States military during the time of the Vietnam War. The characteristics of the VET Registry have been reported elsewhere (29–31).
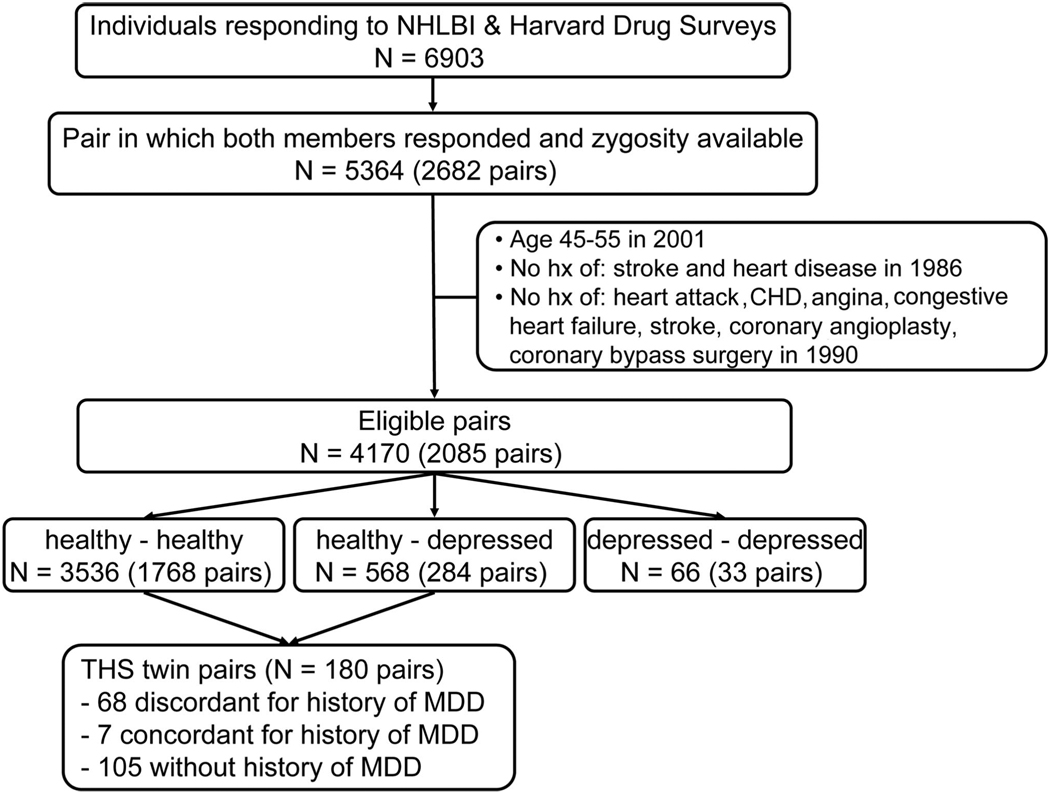
THS included 360 twins from the VET Registry all born between 1946 and 1956 (>90% of the twins in the VET registry fall into this range). The methods of construction of this sample were shown in Figure 1 and were also described previously (32–34). The twins were free of a self-reported previous diagnosis of cardiovascular disease based on survey data collected in 1990 (35), including a previous diagnosis of myocardial infarction, coronary heart disease, angina, congestive heart failure or stroke, or previous coronary angioplasty, or coronary bypass surgery. From this group, we randomly sampled two groups of twin pairs: one group included MDD-discordant twins, where one member of the pair had a lifetime history of MDD assessed with the Diagnostic Interview Schedule around the same time and the other did not; the second group of twins included pairs where neither had a history of MDD. Once selected, twin pairs came together but were examined separately at the Emory University General Clinical Research Center between March 2002 and March 2006, where the twins had a comprehensive history and physical examination and were queried again about previous diagnoses of cardiovascular diseases that might have occurred from the time of the initial screen in 1990. We also administered the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (36) to classify subjects based on a lifetime history of MDD. Only eight subjects met the criteria for current depressive episode, and of the remaining cases of MDD, 78% had their last episode >1 year before study entry. Because our focus for the current analysis was depressive symptoms in the absence of MDD, we excluded twin pairs where one or both twins reported a history of MDD, leaving a sample of 105 pairs. Exclusion of these twins also allowed unbiased heritability estimation because twin pairs discordant for MDD were over-sampled in THS, and therefore their inclusion may cause an artificially higher prevalence of twin pairs discordant for depressive symptoms. Furthermore, to avoid the potential influences on depression and inflammation due to CAD, individuals who reported a history of myocardial infarction, angina pectoris, coronary angioplasty, or coronary bypass surgery from 1990, as well as their co-twins, were excluded (11 pairs). The present analyses, therefore, included 54 monozygotic (MZ) and 40 dizygotic (DZ) twin pairs. Informed consent was obtained from each subject, and the study was approved by the Institutional Review Board of the Emory University School of Medicine.
Fig. 1.
Flow chart showing the construction of the Twins Heart Study sample. NHLBI = National Heart, Lung, and Blood Institute; CHD = coronary heart disease; MDD = major depressive disorder.
Measures
All measurements were performed in the morning after an overnight fast, and both twins in a pair were tested at the same time. A medical history and a physical examination were obtained on all twins. Weight and height were used to calculate body mass index, and waist and hip circumference were used to calculate the waist-hip ratio (WHR). Systolic blood pressure and diastolic blood pressure were measured by mercury sphygmomanometer on the right arm with the subject in sitting position after 10 minutes of rest. The average of two measurements 5 minutes apart was used in the statistical analyses. Venous blood samples were drawn for the measurement of glucose and lipid profile after an overnight fast. The Emory Lipid Research Laboratory, a participant in the Centers for Disease Control/National Heart, Lung and Blood Institute Lipid Standardization Program, performed all analyses from freshly isolated ethylenediaminetetraacetic acid (EDTA) plasma. Direct high-density lipoprotein (HDL) and direct low-density lipoprotein (LDL) cholesterol were obtained using homogeneous assays (Equal Diagnostics, Exton, Pennsylvania). Glucose was measured (CX7 chemistry autoanalyzer, Beckman Coulter Diagnostics, Fullerton, California). Physical activity was assessed by means of a modified version of the Baecke Questionnaire of Habitual Physical Activity used in the Atherosclerosis Risk in Communities Study (37), a 16-question instrument documenting the level of physical activity at work, during sports and nonsports activities. Cigarette smoking was classified into current smoker (any number of cigarettes) versus never or past smoker. Pack-years of smoking were calculated as the number of packs of cigarettes smoked per day times the number of years smoked. Diabetes mellitus was defined as having a fasting glucose level of > 126 mg/dL or being treated with antidiabetic medications.
Assessment of Depressive Symptoms
We administered the Beck Depression Inventory-II (BDI-II) (38), a standardized scale providing a continuous measure of depressive symptoms. This self-report instrument includes 21 items, and subjects rate the severity of each symptom from 0 to 3. It has been used extensively in community samples and has satisfactory test-retest and internal consistency reliability (39).
Markers of Inflammation
IL-6 was assessed using commercially available high-sensitivity ELISA kits obtained from R and D Systems. All samples were run in duplicate. Inter- and intra-assay variability were reliably <10%. CRP was measured with the Beckman Coulter High Sensitivity C-Reactive Protein assay on the Synchron LX-20 analyzer.
Statistical Analyses
In initial descriptive analyses, we compared means and percents of study factors between MZ and DZ twins. The generalized estimating equation (GEE) model was used to assess if there was an association between depressive symptoms and inflammatory markers. Analyses were repeated after adjusting for potential confounding factors. To avoid model overfitting, we constructed a “base” model that included the Framingham Risk Score, a commonly employed summary index of CAD risk that incorporates information on the presence and severity of the following CAD risk factors: age, LDL-cholesterol, HDL-cholesterol, blood pressure, diabetes mellitus, and current smoking. In a separate step, we considered additional a priori specified potential confounders or mediators, including WHR and physical activity. Further adjustment for pack-years of smoking, marital status, and education was also performed, although none of these factors materially changed the study estimates when included in the models. To improve the distributional properties of inflammatory markers, the values were log-transformed and the geometric means were shown in the tables. A two-tailed p < .05 was considered significant, which was corrected for the correlation between co-twins using GEE. These analyses were performed using the statistical software package SAS, Inc. version 9.0 (SAS, Inc., Cary, North Carolina).
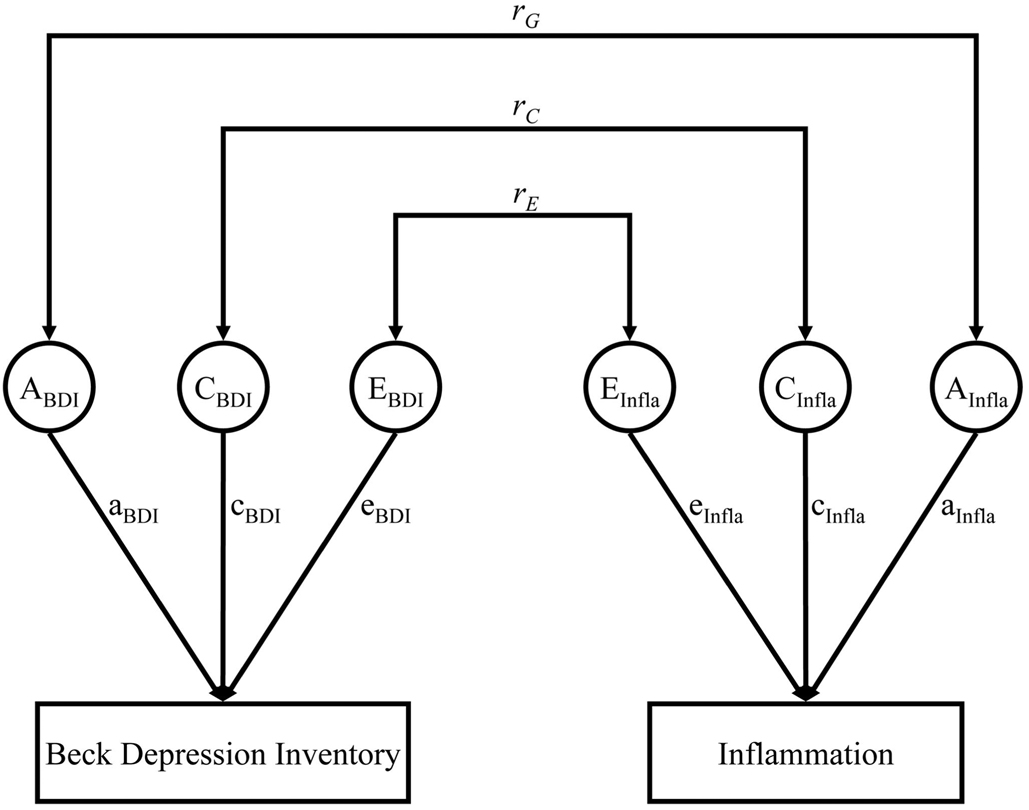
To estimate the relative contributions of genetic and environmental influences on the correlation between depressive symptoms and inflammation, structural equation models were constructed, using the software package Mx (40). For any trait of interest, the phenotypic variation can be divided into three components: additive genetic effects (A), shared (familial) environmental effects (C), and unique (unshared) environmental effects (E). The assumptions under these models are that MZ twins share 100% of their genes whereas DZ twins share 50%. The shared (or familial) environment is assumed to be the same between twins because they were reared together, whereas unique environmental effects were those not shared between twin brothers, either MZ or DZ. The latter includes, for example, environmental exposures in adult life that were not shared by the twins. A greater similarity of phenotype(s) in MZ twins as compared with DZ twins, as indicated by a higher intraclass correlation in MZ than DZ twins, suggests a genetic effect.
The univariate genetic models were fit to estimate the heritability for inflammatory markers and depressive symptoms before and after adjusting for covariates. Furthermore, a bivariate Cholesky decomposition was used to estimate the additive genetic effects (A), shared environmental effects (C), and unique environmental effects (E) between depressive symptoms and inflammatory markers (Figure 2) (41). The correlation between the two traits was similarly partitioned into components resulting from additive genetic influences, shared environmental influences, and unique environmental influences plus measurement error (Figure 2). A series of models were fitted to bivariate variance-covariance matrices. The significance of each component (A, C, and E) was tested by removing them sequentially in specific submodels and comparing them with the full model. Standard likelihood-ratio tests between models were used to assess the importance of each variance component on the fit of the model. Another statistic, the Akaike’s information criterion (AIC), was also used to determine the optimal fitting model, where a lower AIC indicates a more parsimonious and thus a better fitting model.
Fig. 2.
A schematic diagram for the bivariate biometric model examining the relationship between depressive symptoms (Beck Depression Inventory, BDI) and inflammation (Infla). Factors influencing BDI and inflammation include genetic factors (A), shared environment (C), and unique environment (E). Correlations between these factors across traits are represented as rG, rC, and rE, respectively.
RESULTS
Of the 210 THS twins without lifetime history of MDD, 22 were excluded because one or both of twins were found to have a history of CAD at the time of our examination (previous myocardial infarction, angina pectoris, coronary angioplasty, or coronary bypass surgery), leaving 188 for the analysis. The mean ± SD age was 55 ± 2.75 years (range = 47–60 years). Table 1 shows the demographic characteristics and risk factors in MZ and DZ twins. For all study factors, there were no significant differences according to zygosity.
TABLE 1.
General Characteristics of Demographic, Behavioral, and Coronary Risk Factors in Monozygotic (MZ) and Dizygotic (DZ) Twins
| MZ Twins (n = 108) |
DZ Twins (n = 80) |
|
|---|---|---|
| Age, years | 54.9 ± 2.84 | 54.9 ± 2.64 |
| Married, % | 83.3 | 82.5 |
| Current smoker, % | 11.1 | 16.3 |
| Mean body mass index, kg/m2 |
28.8 ± 3.97 | 29.3 ± 5.08 |
| Mean waist-to-hip ratio |
0.94 ± 0.06 | 0.94 ± 0.06 |
| Mean physical activity (Baecke) score |
7.47 ± 1.41 | 7.68 ± 1.41 |
| Hypertension, % | 44.4 | 41.3 |
| Diabetes, % | 3.7 | 7.5 |
| Mean Framingham | 5.91 ± 1.99 | 5.56 ± 2.10 |
| Risk Score | ||
| Mean Beck | 3.25 ± 4.58 | 4.39 ± 5.46 |
| Depression | ||
| Inventory score | ||
| Mean interleukin-6, pg/mLa |
1.69 ± 1.82 | 1.69 ± 1.94 |
| Mean C-reactive protein, mg/La |
1.14 ± 3.01 | 1.23 ± 2.57 |
Mean ± standard deviation values and percentage are shown.
Geometric means calculated from means of log-transformed values.
As shown in Table 2, current depressive symptoms were significantly associated with increased plasma levels of inflammatory markers (p < .001 for IL-6 and p = .005 for CRP). Adjustment for the Framingham Risk Score did not alter the results. After adjusting for WHR and physical activity, the association between BDI and IL-6 was attenuated but remained statistically significant (p = .002), whereas the association between depressive symptoms and CRP became no longer significant. These results suggest that abdominal obesity and lower physical activity may confound the association between inflammation and depressive symptoms, particularly for CRP. Of these two variables, WHR seemed the most important confounder, because it was significantly related to CRP in the multivariable model (p = .001), whereas physical activity showed a borderline association (p = .08). Further adjustment for pack-years, marital status, and education did not change the study estimates.
TABLE 2.
Unadjusted and Adjusted Associations Between Current Depressive Symptom Levels and Inflammatory Markers
| Ln IL-6 | Ln CRP | |||
|---|---|---|---|---|
| β | p | β | p | |
| Unadjusted | ||||
| BDI score | 0.040 | <.001 | 0.034 | .005 |
| Adjusted model 1a | ||||
| BDI score | 0.042 | <.001 | 0.036 | .001 |
| Adjusted model 2b | ||||
| BDI score | 0.032 | .002 | 0.017 | .16 |
| Adjusted model 3c | ||||
| BDI score | 0.026 | .046 | −0.001 | .95 |
IL-6 = interleukin-6; CRP = C-reactive protein; Ln = logarithm transformation; BDI = Beck Depression Inventory.
Adjusted for Framingham Risk Score.
Adjusted for Framingham Risk Score, waist-hip ratio, and physical activity.
Further adjusted for pack-years of smoking, marital status, and education. Regression coefficient (β) and p values were derived from generalized estimating equation models.
For both inflammatory markers and depressive symptoms, the intraclass correlations within MZ twin pairs were consistently higher than those within DZ twin pairs, indicating genetic influence (Table 3). This was confirmed by univariate analysis. The best fitting models for all traits included only genetic and unique environmental contributions. The heritability of IL-6 was estimated as 37% (95% Confidence Interval (CI) = 11% to 57%), whereas the heritability of CRP was 65% (95% CI = 46% to 77%). For depressive symptoms, the heritability was estimated as 48% (95% CI = 23% to 66%) and the remaining variance was explained by unique environmental factors. After adjusting for covariates, the heritability estimations remained similar.
TABLE 3.
Correlations of MZ and DZ Twins and Univariate Model Fitting Results for Depressive Symptoms and Inflammatory Markers
| Ln IL-6 | Ln CRP | BDI Score | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| MZ | 0.37 | 0.40 | 0.69 | 0.66 | 0.48 | 0.45 |
| DZ | 0.15 | 0.15 | 0.11 | 0.21 | 0.12 | 0.05 |
| a2 (95% CI) | 0.37 (0.11–0.57) | 0.39 (0.13–0.60) | 0.65 (0.46–0.77) | 0.63 (0.44–0.76) | 0.48 (0.23–0.66) | 0.47 (0.21–0.67) |
| e2 (95% CI) | 0.64 (0.43–0.89) | 0.61 (0.40–0.87) | 0.35 (0.23–0.54) | 0.37 (0.24–0.57) | 0.52 (0.34–0.77) | 0.53 (0.33–0.79) |
MZ = monozygotic; DZ = dizygotic; Ln = logarithm transformation; IL-6 = interleukin-6; CRP = C-reactive protein; BDI = Beck Depression Inventory; CI = Confidence Interval; a2 = heritability, e2 = unique environmental variance component.
Adjusted for Framingham Risk Sore, waist-hip ratio, and physical activity.
Further adjustment for pack-years, marital status, and education did not substantially affect the study estimates.
Next, we estimated the relative contributions of genetic and environmental factors to the association between current depressive symptoms and IL-6. The bivariate model fitting results are presented in Table 4. Both before and after adjustment for covariates, the most parsimonious model was fit by allowing for additive genetic (A) and unique environmental (E) influences, whereas the CE model was significantly (or borderline) worse compared with the full ACE model (p = .065 and p = .04, respectively). Table 5 shows the parameter estimations for the best fitting AE models. Before adjusting for covariates, the genetic correlation between depressive symptoms and IL-6 was significant (rG = .22, p = .046), whereas the environmental correlation was not significant (rE = .11, p = .17). Thus, in unadjusted analysis, of the overall correlation between depressive symptoms and IL-6 (r = .33), about 66% was due to the same genetic factors. After adjustment for covariates, the genetic correlation remained significant (rG = .27, p = .02), whereas the environmental correlation decreased dramatically (rE = .02, p = .80). As a result, after adjustment for other risk factors, genetic factors accounted for about 93% of the covariation between depressive symptoms and IL-6.
TABLE 4.
Genetic Model-Fitting Comparisons for Full and Reduced Bivariate Models of Additive Genetic, Common and Unique Environmental Influences for Depressive Symptoms and IL-6 Among 94 Twin Pairs
| Phenotypes | Model | AIC | −2LL | df | χ2 | p |
|---|---|---|---|---|---|---|
| Unadjusted | ACE | 723.1 | 1447.1 | 362 | ||
| BDI and Ln IL-6 | AE | 719.8 | 1449.8 | 365 | 2.70 | .44 |
| CE | 724.3 | 1454.3 | 365 | 7.23 | .065 | |
| E | 733.6 | 1469.6 | 368 | 22.5 | .001 | |
| Adjusted a | ACE | 612.8 | 1244.8 | 316 | ||
| BDI and Ln IL-6 | AE | 608.8 | 1246.8 | 319 | 1.98 | .58 |
| CE | 615.0 | 1253.0 | 319 | 8.20 | .04 | |
| E | 622.0 | 1266.0 | 322 | 21.2 | .002 |
AIC = Akaike’s Information Criterion; −2LL = −2 × Log (Likelihood); BDI = Beck Depression Inventory; IL = interleukin.
Best fitting model in bold text.
Adjusted for Framingham Risk Score, waist-hip ratio, and physical activity. Further adjustment for pack-years, marital status and education did not substantially affect the study estimates.
TABLE 5.
Parameter Estimates From the Best Fitting Bivariate Models for Depressive Symptoms and IL-6
| Traits | a2 (95% CI) | e2 (95% CI) | rG (95% CI) | rE (95% CI) |
|---|---|---|---|---|
| Unadjusted | ||||
| BDI | 0.49 (0.24–0.68) | 0.51 (0.32–0.76) | 0.22 (0.002–0.53) | 0.11 (−0.03–0.35) |
| IL-6 | 0.39 (0.13–0.59) | 0.61 (0.41–0.87) | ||
| Adjusteda | ||||
| BDI | 0.51 (0.25–0.70) | 0.49 (0.30–0.75) | 0.27 (0.03–0.62) | 0.02 (−0.08–0.25) |
| IL-6 | 0.44 (0.17–0.64) | 0.56 (0.36–0.83) |
BDI = Beck Depression Inventory; IL = interleukin; CI = Confidence Interval; a2 = additive genetic influence (heritability); e2 = unique environmental influence.
rG = correlation between BDI and IL-6 due to genetic factors.
rE = correlation between BDI and IL-6 due to unique environmental factors.
Adjusted for Framingham Risk Score, waist-hip ratio, and physical activity. Further adjustment for pack-years, marital status, and education did not substantially affect the study estimates.
DISCUSSION
In a sample of predominantly healthy twins, we found a robust correlation between the severity of depressive symptoms and increased plasma levels of IL-6, which withstood adjustment for potential confounders. Bivariate genetic analyses indicated that a shared genetic vulnerability explains most of this association, even after adjustment for other risk factors. These results suggest that depressive symptoms and inflammation may be the expression of a common biological pathway that is genetically modulated.
There has been growing evidence that both inflammation and depression play important roles in the development and progression of cardiovascular disease. Thus, it is perhaps not surprising that depression is accompanied by increase of inflammation in patients with CAD (17,26,42–44). Even among presumably healthy individuals (20–23), however, depressive symptoms are associated with elevated cytokine production (e.g., IL-6) and acute-phase proteins (e.g., CRP). Several community-based studies have found a positive correlation between plasma IL-6 and depressive symptoms in the elderly (11,19,45). In healthy young adults, increased levels of inflammatory markers were also associated with severity of depressive symptoms or a more recent episode of depression in a number of studies (16,46,47). In contrast, among 226 healthy volunteers aged 47 to 59 years, Steptoe et al. (48) found no correlations between depressive symptoms and markers of immune and vascular inflammation.
We found a positive correlation between depressive symptoms and plasma levels of IL-6 in our middle-aged male twins (aged 47–60 years). A possible reason why previous studies on general population samples have been inconsistent may be related to confounding, which is difficult to control in community settings. By using a twin design and by adjusting for lifestyle factors in statistical analyses, our study controlled more completely for potential confounders than previous investigations. For example, family influences and unmeasured lifestyle factors deriving from a shared environment while growing up are successfully controlled in the twin design. In addition to IL-6, we found an association between depressive symptoms and CRP (p = .005). This association was no longer statistically significant after adjustment for cardiovascular and lifestyle risk factors. Specifically, our results indicated that the relationship between depressive symptoms and CRP may be confounded by WHR. Our finding is consistent with several previous studies (11,15,18,49) in which the association between CRP and depression was ultimately explained by other variables. Recently, Miller et al. (46) suggested a model in which depressive symptoms promote weight accumulation, which in turn activates an inflammatory response. This hypothesis may provide a pathway underling the interrelations among depression, adiposity, and CRP.
Several mechanisms linking depression and inflammation have been proposed (25,26,50,51). Hypersecretion of the corticotrophin-releasing hormone (CRH) has been demonstrated in depressed patients as manifested by increased cerebrospinal fluid concentrations of CRH (52,53). CRH is a primary regulatory hormone in response to stress, secondary to activation of hypothalamic-pituitary-adrenal (HPA) axis, and has been found to stimulate the release of proinflammatory cytokines (54). However, a reverse relationship has also been suggested, such that inflammatory cytokines have profound stimulatory effects on HPA axis hormones and CRH, which in turn contribute to depression (55).
In addition to the bidirectional relationship between depression and inflammation, another possible explanation for this relationship is that these two processes may share a common underlying mechanism, such as a common genetic vulnerability. The role of genetic factors on depression and inflammation has been well established by family, twin, and adoption studies (56–60). Heritability is estimated to account for 37% to 75% of the liability for MDD. Depressive symptoms also show a significant genetic influence (49,52,53). Studies of systemic levels of inflammatory markers in families and twins suggested that a considerable part of the variation in these biomarkers can be explained by genetic factors, with heritability estimations of 0.17 to 0.58 for IL-6 (57,58). Our heritability estimates are consistent with these previous findings (h2 = 0.48 and 0.37 for depressive symptoms and IL-6, respectively). Given the substantial genetic influence on both depressive symptoms and inflammatory biomarkers, it is conceivable that common genes contribute to both these phenotypes. Our study addressed precisely this question. Both before and after adjusting for covariates, we found significant genetic correlations between depressive symptoms and IL-6. These results indicate that common genes contribute substantially to the covariation of the two processes: in unadjusted analysis, 66% of the correlation between depressive symptoms and IL-6 was due to shared genetic factors; after adjustment for other risk factors, about 93% of the correlation was due to shared genes. These results complement and expand our previous finding of a common genetic liability to MDD and inflammation (27), suggesting that such a link may also exist for depressive symptoms in the absence of MDD.
Common genes that may be involved in the regulation of both depressive symptoms and inflammation include those in the HPA axis, or in the autonomic nervous system and serotonin pathways (61–63). For example, a higher prevalence of certain alleles in the glucocorticoid receptor gene has been observed in patients with major depression than controls (64). One recent study also reported that a common haplotype in this gene was related to a more active proinflammatory system and a higher risk of myocardial infarction (65). In addition, genetic polymorphisms in serotoninergic genes, such as the serotonin transporter, serotonin receptors, and monoamine oxidase A, have been associated with depression (12). Serotonin, on the other hand, has been suggested to increase IL-6 synthesis in human vascular smooth muscle cells (66). Clarification of the roles of these common genetic variants in the inflammatory process will improve our understanding of the mechanisms underling brain-immune interactions, and ultimately point to more effective primary prevention strategies for the identification and treatment of individuals at highest risk of CAD.
There are several limitations to this study. First, our sample is derived from a twin registry of military veterans, where the members may be healthier than, or in other ways different from the general population of similarly aged individuals (31). Second, our analyses included only males; thus, one should be cautious to extend our results to females. Finally, twins with MDD were excluded from our analysis, and thus, the results may not be extrapolated to people with MDD. However, our previous co-twin analysis of the MDD-discordant twin pairs suggested that genetic liability may also underlie the association between MDD and inflammation (27). In addition, exclusion of the MDD twins could have biased our sample toward lower levels of depressive symptoms. However, because so few twins with MDD were currently depressed, we do not believe that this exclusion significantly biased our analysis.
In conclusion, we found that depressive symptoms are significantly correlated with IL-6 in apparently healthy individuals and that this covariation is due, in large part, to common genetic influences. These results suggest that depressive symptoms and inflammation are the expression of a common biological abnormality that is genetically modulated. Identification of the underlying genetic factors may be important for CAD risk and for our understanding of the mechanisms underlying brain-immune interactions.
Acknowledgments
Supported by Grants K24HL077506, R01 HL68630 and R01 AG026255 from the National Institutes of Health (V.V.); Grant MO1-RR00039 from the Emory University General Clinical Research Center and Grant 0245115N from the American Heart Association (V.V.).
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Institutes of Health; National Opinion Research Center; the National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution, this research would not have been possible.
Glossary
- CAD
coronary artery disease
- MDD
major depressive disorder
- IL-6
interleukin-6
- CRP
C-reactive protein
- THS
Twins Heart Study
- VET
Vietnam Era Twin
- MZ
monozygotic
- DZ
dizygotic
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- WHR
waist-hip ratio
- BDI-II
Beck Depression Inventory-II
- GEE
generalized estimating equation
- AIC
Akaike’s information criterion
- CRH
corticotrophin-releasing hormone
- HPA
hypothalamic-pituitary-adrenal
- SD
standard deviation
- Ln
logarithm transformation
- CI
Confidence Interval
- −2LL
−2×Log (Likelihood)
REFERENCES
- 1.Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 2.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54:227–240. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 3.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 4.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 5.Musselman DL, Marzec UM, Manatunga A, Penna S, Reemsnyder A, Knight BT, Baron A, Hanson SR, Nemeroff CB. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch Gen Psychiatry. 2000;57:875–882. doi: 10.1001/archpsyc.57.9.875. [DOI] [PubMed] [Google Scholar]
- 6.Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 7.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 10.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 11.Tiemeier H, Hofman A, van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MM. Inflammatory proteins and depression in the elderly. Epidemiology. 2003;14:103–107. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 12.McCaffery JM, Frasure-Smith N, Dube MP, Theroux P, Rouleau GA, Duan Q, Lesperance F. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 13.Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, Wiktorowicz K. Indicators of immune activation in major depression. Psychiatry Res. 1996;64:161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, Scharpé S. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66:1–11. doi: 10.1016/s0165-1781(96)02915-0. [DOI] [PubMed] [Google Scholar]
- 15.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 16.Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65:347–356. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 17.Appels A, Bar FW, Bar J, Bruggeman C, de Baets M. Inflammation, depressive symptomtology, and coronary artery disease. Psychosom Med. 2000;62:601–605. doi: 10.1097/00006842-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP. Inflammation and coagulation factors in persons >65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–424. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 19.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 20.Suarez EC. Joint effect of hostility and severity of depressive symptoms on plasma interleukin-6 concentration. Psychosom Med. 2003;65:523–527. doi: 10.1097/01.psy.0000062530.94551.ea. [DOI] [PubMed] [Google Scholar]
- 21.Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65:362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- 22.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 23.Ladwig KH, Marten-Mittag B, Lowel H, Doring A, Koenig W. C-reactive protein, depressed mood, and the prediction of coronary heart disease in initially healthy men: results from the MONICA-KORA Augsburg cohort study 1984–1998. Eur Heart J. 2005;26:2537–2542. doi: 10.1093/eurheartj/ehi456. [DOI] [PubMed] [Google Scholar]
- 24.Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol. 2005;95:317–321. doi: 10.1016/j.amjcard.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Shimbo D, Chaplin W, Crossman D, Haas D, Davidson KW. Role of depression and inflammation in incident coronary heart disease events. Am J Cardiol. 2005;96:1016–1021. doi: 10.1016/j.amjcard.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 26.Kop WJ, Gottdiener JS. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom Med. 2005;67 Suppl 1:S37–S41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- 27.Vaccarino V, Brennan M, Miller AH, Bremner JD, Ritchie JC, Lindau F, Veledar E, Su S, Murrah NV, Jones L, Jawed F, Dai J, Goldberg J, Hazen SL. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry. 2008;64:476–483. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam era twin registry. Twin Res. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 29.Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam era twin (VET) registry: method of construction. Acta Genet Med Gemellol (Roma) 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- 30.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 31.Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam era twin registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- 32.Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW, Vaccarino V. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: a twin study. Circulation. 2008;117:169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su S, Snieder H, Miller AH, Ritchie J, Bremner JD, Goldberg J, Dai J, Jones L, Murrah NV, Zhao J, Vaccarino V. Genetic and environmental influences on systemic markers of inflammation in middle-aged male twins. Atherosclerosis. 2008;200:213–220. doi: 10.1016/j.atherosclerosis.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J, Cheema FA, Reddy U, Bremner JD, Su S, Goldberg J, Snieder H, Vaccarino V. Heritability of flow-mediated dilation: a twin study. J Thromb Haemost. 2007;5:2386–2392. doi: 10.1111/j.1538-7836.2007.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. 2003;65:548–557. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- 36.First MBSR, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV-Patient Edition (SCID-P) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 37.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr, Leon AS. Ability of the atherosclerosis risk in communities (ARIC)/Baecke questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. BDI-II. Beck Depression Inventory: Second Edition. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 39.Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck depression inventory-II with adolescent psychiatric inpatients. Psychol Assess. 2004;16:120–132. doi: 10.1037/1040-3590.16.2.120. [DOI] [PubMed] [Google Scholar]
- 40.Neale MC. Statistical Modeling with Mx. Richmond, VA: Department of Human Genetics, Medical College of Virginia, Virginia Commonwealth University; 1991. [Google Scholar]
- 41.Neale MC, Cardon LR. Methodologies for Genetic Studies of Twins and Families. Dordrecht, Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 42.Goodkin K, Appels A. Behavioral-neuroendocrine-immunologic interactions in myocardial infarction. Med Hypotheses. 1997;48:209–214. doi: 10.1016/s0306-9877(97)90308-x. [DOI] [PubMed] [Google Scholar]
- 43.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 44.Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 45.Dentino AN, Pieper CF, Rao MK, Currie MS, Harris T, Blazer DG, Cohen HJ. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriatr Soc. 1999;47:6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 46.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 47.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 48.Steptoe A, Kunz-Ebrecht SR, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med. 2003;33:667–674. doi: 10.1017/s0033291702007250. [DOI] [PubMed] [Google Scholar]
- 49.Douglas KM, Taylor AJ, O’Malley PG. Relationship between depression and C-reactive protein in a screening population. Psychosom Med. 2004;66:679–683. doi: 10.1097/01.psy.0000138132.66332.85. [DOI] [PubMed] [Google Scholar]
- 50.Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999;4:317–327. doi: 10.1038/sj.mp.4000586. [DOI] [PubMed] [Google Scholar]
- 51.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol. 1992;2:107–113. doi: 10.1016/0924-977x(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 53.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 54.Miller AH, Pearce BD, Pariante CM. Immune System and Central Nervous System Interactions. Philadelphia: Lippincott & Williams; 2000. [Google Scholar]
- 55.Silverman MN, Pearce BD, Biron CA, Miller AH. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18:41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gatz M, Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. Importance of shared genes and shared environments for symptoms of depression in older adults. J Abnorm Psychol. 1992;101:701–708. doi: 10.1037//0021-843x.101.4.701. [DOI] [PubMed] [Google Scholar]
- 57.Grunnet L, Poulsen P, Klarlund Pedersen B, Mandrup-Poulsen T, Vaag A. Plasma cytokine levels in young and elderly twins: genes versus environment and relation to in vivo insulin action. Diabetologia. 2006;49:343–350. doi: 10.1007/s00125-005-0080-8. [DOI] [PubMed] [Google Scholar]
- 58.Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN, Eckfeldt JH, Tracy RP. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–689. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 59.Silberg JL, Heath AC, Kessler R, Neale MC, Meyer JM, Eaves LJ, Kendler KS. Genetic and environmental effects on self-reported depressive symptoms in a general population twin sample. J Psychiatr Res. 1990;24:197–212. doi: 10.1016/0022-3956(90)90010-n. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 61.Eskandari F, Webster JI, Sternberg EM. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res Ther. 2003;5:251–265. doi: 10.1186/ar1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karalis KP, Kontopoulos E, Muglia LJ, Majzoub JA. Corticotropin-releasing hormone deficiency unmasks the proinflammatory effect of epinephrine. Proc Natl Acad Sci U S A. 1999;96:7093–7097. doi: 10.1073/pnas.96.12.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menard G, Turmel V, Bissonnette EY. Serotonin modulates the cytokine network in the lung: involvement of prostaglandin E2. Clin Exp Immunol. 2007;150:340–348. doi: 10.1111/j.1365-2249.2007.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, Salyakina D, Lamberts SW, Holsboer F. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 65.van den Akker EL, Koper JW, van Rossum EF, Dekker MJ, Russcher H, de Jong FH, Uitterlinden AG, Hofman A, Pols HA, Witteman JC, Lamberts SW. Glucocorticoid receptor gene and risk of cardiovascular disease. Arch Intern Med. 2008;168:33–39. doi: 10.1001/archinternmed.2007.41. [DOI] [PubMed] [Google Scholar]
- 66.Ito T, Ikeda U, Shimpo M, Yamamoto K, Shimada K. Serotonin increases interleukin-6 synthesis in human vascular smooth muscle cells. Circulation. 2000;102:2522–2527. doi: 10.1161/01.cir.102.20.2522. [DOI] [PubMed] [Google Scholar]