Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a potentially lethal inherited arrhythmia syndrome in which drug therapy is often ineffective. We discovered that flecainide prevents arrhythmias in a mouse model of CPVT by inhibiting cardiac ryanodine receptor–mediated Ca2+ release and thereby directly targeting the underlying molecular defect. Flecainide completely prevented CPVT in two human subjects who had remained highly symptomatic on conventional drug therapy, indicating that this currently available drug is a promising mechanism-based therapy for CPVT.
CPVT is an inherited arrhythmia syndrome characterized by a normal baseline electrocardiogram (ECG), polymorphic ventricular tachycardia induced by adrenergic stress in the absence of structural heart disease, and a high mortality rate in young individuals1. Treatment with β-adrenergic blockers reduces arrhythmia burden and mortality but is not completely effective1, and implantable cardioverter defibrillators (ICDs) are used for the prevention of sudden death. However, painful appropriate or inappropriate defibrillation shocks can trigger further adrenergic stress and arrhythmias, and deaths have occurred despite appropriate ICD shocks2,3. In such instances, stellate ganglionectomy4 or even cardiac transplantation5 have been considered. Two CPVT disease-related genes have been identified: RYR2, encoding the cardiac ryanodine receptor Ca2+ release channel (RyR2), and CASQ2, encoding cardiac calsequestrin6,7. Mutations in these genes destabilize the RyR2 Ca2+ release complex8,9. In mice with CPVT-linked mutations, catecholamines cause spontaneous sarcoplasmic reticulum Ca2+ release resulting in delayed after depolarizations (DADs), and they produce triggered activity in myocytes and polymorphic ventricular tachycardia in vivo10,11. Here we identify a therapy that directly targets the underlying arrhythmia mechanisms: we found that flecainide, an approved antiarrhythmic drug known to block sodium channels, showed remarkable efficacy in suppressing spontaneous sarcoplasmic reticulum Ca2+ release by inhibiting RyR2. Flecainide treatment completely prevented adrenergic stress–induced arrhythmias in a mouse model of CPVT and in humans with CASQ2 or RYR2 mutations that are refractory to standard drug treatment.
The local anesthetic tetracaine has been used to inhibit RyR2 and suppress spontaneous sarcoplasmic reticulum Ca2+ release in isolated myocytes12. However, tetracaine causes a rebound increase in sarcoplasmic reticulum Ca2+ release events during prolonged exposure13, effective inhibitory concentrations14 are too high for clinical use, and systemic administration is contraindicated in humans. We searched among clinically available antiarrhythmic drugs for a more useful RyR2 inhibitor and found that flecainide inhibited RyR2 more potently than tetracaine and by a different mechanism. Whereas tetracaine caused long-lived channel closings, flecainide reduced the duration of channel openings and did not affect closed channel duration (Supplementary Fig. 1 online). Flecainide's inhibitory potency was higher when RyR2 was activated by high luminal Ca2+ concentration mimicking spontaneous sarcoplasmic reticulum Ca2+ releases that trigger premature heart beats (Fig. 1a,b half-maximal inhibitory concentration = 15 ± 3 μM), compared to when RyR2 was activated by high cytosolic Ca2+ concentration, such as would occur during a normal heart beat (Supplementary Fig. 1a; half-maximal inhibitory concentration = 55 ± 8 μM).
Figure 1.
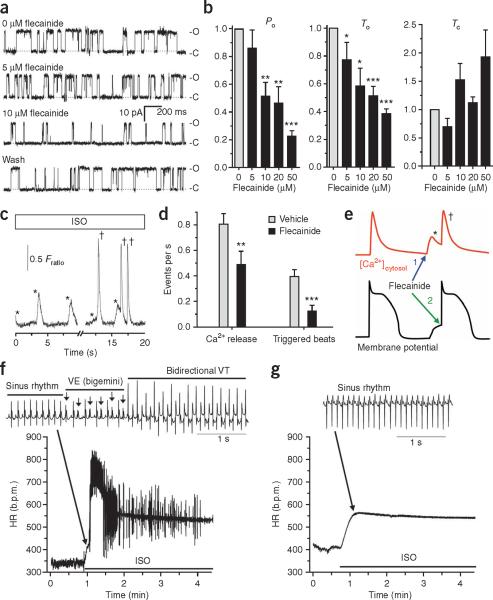
Flecainide inhibits RyR2 Ca2+ release channels, reduces spontaneous Ca2+ release events and triggered beats and prevents ventricular tachycardia in a CPVT mouse model. (a) Representative examples of the activity of single sheep RyR2 channels incorporated in lipid bilayers in response to 0, 5 and 10 μM flecainide followed by washout. C, closed; O, open. (b) Average concentration dependence of flecainide's effect on RyR2 open probability (Po), mean open time (To) and mean closed time (Tc). Data are expressed relative to values at 0 μM flecainide, with absolute Po = 0.12 ± 0.07, To = 13.5 ± 4.4 ms and Tc = 146 ± 56 ms. RyR2 channels were activated by 1 mM Ca2+ on the trans (luminal) side with cis (cytosolic) at 0.0001 Ca2+ mM (to model resting Ca2+ concentration). n = 3–8 experiments per concentration; *P < 0.02, **P < 0.01 and ***P < 0.001. (c,d) Effects of flecainide on isoproterenol (ISO)-stimulated Casq2−/− myocytes. (c) Representative examples of spontaneous sarcoplasmic reticulum Ca2+ release events (*) and triggered beats (†). Fratio, Fura-2 fluorescence ratio, which is proportional to free intracellular Ca2+ concentration (see Supplementary Methods online). (d) Flecainide (6 μM, >10 min incubation) significantly decreased spontaneous sarcoplasmic reticulum Ca2+ release events and triggered beats (n = 44 cells) compared to vehicle (n = 45 cells). **P = 0.0078 and ***P < 0.001. (e) Cartoon illustrating the dual mechanism of flecainide action: (1) inhibition of the sarcoplasmic reticulum Ca2+ release (*) that causes delayed after depolarizations and (2) inhibition of the premature beats (†) that are triggered by delayed after depolarizations. (f) Heart rate response to ISO (1.5 mg kg−1) challenge in a Casq2−/− mouse. As illustrated in the ECG trace, the rapid and irregular heart rate (HR) was the result of numerous repetitive ventricular extrasystoles (VE, arrows) and the induction of ventricular tachycardia (VT). b.p.m., beats per minute. (g) Heart rate of the same mouse after it received flecainide (20 mg kg−1) 15 min before ISO challenge. All data are means ± s.e.m.; for experimental protocols and statistical analysis see Supplementary Methods. Animal experiments were approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee.
We next tested whether RyR2 block by flecainide translates into an inhibition of spontaneous sarcoplasmic reticulum Ca2+ release in ventricular myocytes isolated from mice with gene-targeted deletion of Casq2 (Casq2−/− mice), a model of CASQ2-linked CPVT10. Upon catecholaminergic challenge with isoproterenol, Casq2−/− myocytes exhibit frequent spontaneous sarcoplasmic reticulum Ca2+ releases that can trigger premature beats (Fig. 1c and ref. 10). Flecainide significantly suppressed the rate of spontaneous Ca2+ releases from the sarcoplasmic reticulum by 39% (Fig. 1d), even though Ca2+ content in sarcoplasmic reticulum was not significantly changed (Supplementary Fig. 2a,b online). The reduction of spontaneous Ca2+ releases by flecainide remained significant even after Na+ and Ca2+ were removed from the extracellular bath solution (47% reduction, P = 0.009, Supplementary Fig. 2c,d), indicating that inhibition of trans-sarcolemmal Na+ or Ca2+ fluxes did not contribute to the reduced spontaneous Ca2+ releases; that is, indicating that flecainide does not act by blocking Na+ channels. Compared to vehicle, flecainide significantly decreased diastolic sarcoplasmic reticulum Ca2+ leak in isoproterenol-stimulated Casq2−/− myocytes (Supplementary Fig. 2e,f; P = 0.02). Indeed, in contrast to tetracaine treatment13, flecainide treatment did not result in a compensatory increase in sarcoplasmic reticulum Ca2+ content and prevented spontaneous Ca2+ releases even after prolonged application (Supplementary Fig. 3 online). These findings are consistent with the idea that flecainide inhibits RyR2 by a different mechanism than tetracaine (Supplementary Fig. 1).
A major consequence of spontaneous premature sarcoplasmic reticulum Ca2+ release in intact myocytes is the activation of electro-genic Na+−Ca2+ exchange, which in turn causes membrane depolarizations termed DADs15. DADs of sufficient amplitude activate voltage-gated Na+ channels and trigger full action potentials. Flecainide reduced triggered beats by 69% (Fig. 1d), a higher percent inhibition than its reduction of spontaneous Ca2+ release events (39%; Fig. 1d). These data are consistent with flecainide's known inhibition of Na+ channels to prevent triggered beats16. Taken together, these results indicate a dual mode of flecainide action in CPVT: suppression of spontaneous sarcoplasmic reticulum Ca2+ release events via RyR2 inhibition and suppression of triggered beats via Na+ channel block (Fig. 1e).
We next examined whether this dual mechanism of flecainide action translates into therapeutic efficacy. In a Casq2−/− mouse, catecholamine challenge induced an irregular heart rhythm (Fig. 1f) resulting from frequent ventricular extrasystoles occurring after each normal `sinus' beat (bigemini), which quickly degenerated into polymorphic ventricular tachycardia with alternating upward and downward deflections of the QRS complex (bidirectional ventricular tachycardia), the hallmark of CPVT1. After flecainide treatment, the heart rate remained regular (with a sinus rhythm) and catecholamine challenge did not induce ventricular arrhythmias (Fig. 1g). Flecainide completely suppressed ventricular tachycardia in all 12 mice tested and ventricular extrasystole in 11 out of 12 mice (P < 0.001). In the remaining mouse, flecainide reduced the number of isoproterenol-induced ventricular extrasystoles by 99.5%. Flecainide was equally effective in preventing exercise-induced polymorphic or bidirectional ventricular tachycardia in conscious Casq2−/− mice; a single administration of flecainide (20 mg per kg body weight intraperitoneally) resulted in serum flecainide concentrations of 2.5 ± 0.2 μM (1.2 ± 0.08 mg l−1) 1 h later, when exercise-induced ventricular tachycardia was completely prevented. Protection from ventricular tachycardia persisted for up to 6 h, with no rebound increase in ventricular tachycardia observed during a 20-h follow-up period (Supplementary Fig. 4 online). Lidocaine, a Na+ channel blocker that does not inhibit RyR2 channels14 and lacks clinical efficacy in CPVT17, reduced the number of ventricular extrasystoles, but did not prevent exercise-induced ventricular tachycardia (Supplementary Fig. 5 online), further indicating that RyR2 inhibition is crucial for flecainide's antiarrhythmic efficacy in CPVT.
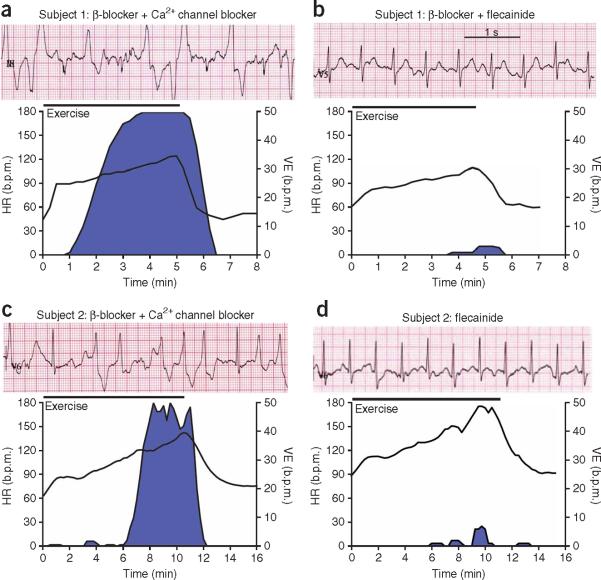
Given flecainide's efficacy in the mouse model, we tested the drug in two subjects with treatment-refractory CPVT. The first subject was a 12-year-old boy, homozygous for a CASQ2 missense mutation18, who suffered from repeated appropriate ICD shocks despite maximal conventional therapy with the β-adrenergic receptor blocker metoprolol and the Ca2+ channel blocker verapamil. ICD shocks were prevented by bed rest, although nonsustained ventricular tachycardia persisted, as documented by examination of ECG recordings stored in the ICD (data not shown). Treadmill exercise reproducibly induced arrhythmias in this subject (Fig. 2a). A therapeutic trial of flecainide (initially 2 mg per kg body weight per day for 6 weeks, then 3 mg per kg body weight per day) was begun, and verapamil was discontinued. Examination of ICD records at 3 weeks and 12 weeks after starting flecainide revealed a complete absence of ventricular tachycardia episodes (data not shown). Repeat exercise tests at 7 weeks and 12 weeks after the start of flecainide treatment showed complete suppression of ventricular tachycardia and marked reduction in the number of ventricular extrasystoles (Fig. 2b).
Figure 2.
Flecainide treatment prevents exercise-induced ventricular arrhythmia in two subjects with CPVT refractory to conventional drug therapy. (a–d) Effect of flecainide treatment in a 12-year-old boy (subject 1) with a CASQ2 mutation (a,b) and in a 36-year-old female (subject 2) with an RYR2 mutation (c,d). Top of each panel, representative ECGs recorded during maximum stress; bottom of each panel, heart rate and rate of ventricular extrasystoles during an exercise test. Blue area indicates arrhythmia burden. Exercise protocol and ventricular extrasystole analysis are as previously described4. Drug therapy during each exercise test: metroprolol 100 mg d−1 plus verapamil 120 mg d−1 (a), metroprolol 100 mg d−1 plus flecainide 150 mg d−1 for 7 weeks (b), bisoprolol 5 mg d−1 plus verapamil 240 mg d−1 (c) or flecainide 150 mg d−1 for 8 weeks (d). Experiments involving human subjects were reviewed and approved by the University of Amsterdam Academic Medical Center Institutional Medical Ethical Review Board. Both human subjects (or their parents) provided informed consent.
The second subject was a 36-year-old woman heterozygous for the CPVT-linked RYR2 S4124G mutation. The subject experienced exercise-induced ventricular tachycardia while on maximum tolerated therapy with a β-blocker and a Ca2+-channel blocker (Fig. 2c). As in the first subject, flecainide substantially reduced arrhythmia burden at rest and with exercise, even after β-blocker and Ca2+-channel blocker treatment was stopped (Fig. 2d). Notably, flecainide prevented ventricular tachycardia even though the subject's maximal heart rate was much higher during the second exercise test (Fig. 2d) than during the previous exercise test (Fig. 2c) owing to the discontinuation of β-blocker therapy.
In summary, we report a previously unrecognized inhibitory action of flecainide on RyR2 channels, which, together with flecainide's inhibition of Na+ channels, allowed us to directly target the underlying mechanism responsible for CPVT11. This targeted therapy with flecainide successfully prevented CPVT in two individuals that had remained highly symptomatic on conventional drug therapy. Flecainide can be proarrhythmogenic in some settings (for example, after myocardial infarction), and thus routine flecainide use cannot be recommended until further clinical studies more precisely define its risks and benefits in humans with CPVT. Our data provide proof of principle for the antiarrhythmic efficacy of inhibiting defective RyR2 Ca2+ release channels in humans and identify a currently available drug as a promising mechanism-based therapy in CPVT.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Huke and S. Kim for their assistance with the data analysis and the preparation of figures, P. Johnson for his assistance with the single-channel recording and N. Masalha and S.A. Clur for their assistance in patient care and management. This work was supported in part by the US National Institutes of Health grants HL88635 and HL71670 (to B.C.K.) and HL49989 (to D.M.R.), by the American Heart Association Established Investigator Award 0840071N (to B.C.K.), by the Australian Research Council grant DP0557780 (to D.R.L.), by an infrastructure grant from New South Wales Health through Hunter Medical Research Institute (to D.R.L.), by the Leducq Foundation grant 05CVD01 (to A.A.M.W. and D.M.R.) and by the Alberta Heart and Stroke Foundation Chair in Cardiovascular Medicine (to H.J.D.).
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
References
- 1.Leenhardt A, et al. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed U, Gollob MH, Gow RM, Krahn AD. Heart Rhythm. 2006;3:1486–1489. doi: 10.1016/j.hrthm.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Pizzale S, Gollob MH, Gow R, Birnie DH. J. Cardiovasc. Electrophysiol. 2008;9:1319–1321. doi: 10.1111/j.1540-8167.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilde AA, et al. N. Engl. J. Med. 2008;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 5.Robinson M, Park J, Raff G. Heart Rhythm. 2007;4(Suppl):S100. [Google Scholar]
- 6.Priori SG, et al. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 7.Lahat H, et al. Am. J. Hum. Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang D, et al. Proc. Natl. Acad. Sci. USA. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.di Barletta MR, et al. Circulation. 2006;114:1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 10.Knollmann BC, et al. J. Clin. Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerrone M, et al. Circ. Res. 2007;101:1039–1048. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venetucci LA, Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Circ. Res. 2006;98:1299–1305. doi: 10.1161/01.RES.0000222000.35500.65. [DOI] [PubMed] [Google Scholar]
- 13.Gyorke S, Lukyanenko V, Gyorke I. J. Physiol. (Lond.) 1997;500:297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoshan-Barmatz V, Zchut S. J. Membr. Biol. 1993;133:171–181. doi: 10.1007/BF00233797. [DOI] [PubMed] [Google Scholar]
- 15.Marban E, Robinson SW, Wier WG. J. Clin. Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen MR, Danilo P., Jr. Circ. Res. 1980;46:117–124. doi: 10.1161/01.res.46.1.117. [DOI] [PubMed] [Google Scholar]
- 17.De Rosa G, et al. Pediatr. Emerg. Care. 2004;20:175–177. doi: 10.1097/01.pec.0000117927.65522.7a. [DOI] [PubMed] [Google Scholar]
- 18.Postma AV, et al. Circ. Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.