Abstract
Although mechanical ventilation (MV) is a life-saving intervention for patients with acute respiratory distress syndrome (ARDS), it can aggravate or cause lung injury, known as ventilator-induced lung injury (VILI). The biophysical characteristics of heterogeneously injured ARDS lungs increase the parenchymal stress associated with breathing, which is further aggravated by MV. Cells, in particular those lining the capillaries, airways and alveoli, transform this strain into chemical signals (mechanotransduction). The interaction of reparative and injurious mechanotransductive pathways leads to VILI. Several attempts have been made to identify clinical surrogate measures of lung stress/strain (e.g., density changes in chest computed tomography, lower and upper inflection points of the pressure–volume curve, plateau pressure and inflammatory cytokine levels) that could be used to titrate MV. However, uncertainty about the topographical distribution of stress relative to that of the susceptibility of the cells and tissues to injury makes the existence of a single ‘global’ stress/strain injury threshold doubtful.
Keywords: acute lung injury, acute respiratory distress syndrome, esophageal pressure, lung strain, lung stress, mechanical ventilation, mechanotransduction, plateau pressure, ventilator-induced lung injury
Acute respiratory distress syndrome (ARDS), as defined by the American–European Consensus Conference Committee, is a syndrome of inflammation and increased permeability associated with acute onset of hypoxemia (partial arterial oxygen pressure/fractional inspired oxygen [PaO2/FiO2] ≤200 mmHg) accompanied by bilateral infiltrates on chest radiograph that cannot be explained by, but may coexist with, left atrial or pulmonary capillary hypertension (pulmonary artery occlusion pressure ≤18 mmHg) [1]. The term acute lung injury (ALI) implies a milder degree of hypoxemia (PaO2/FiO2 ≤300 mmHg) [1]. It was first described by Ashbaugh et al. in 1967 in 12 patients, seven (58%) of whom died [2]. Mechanical ventilation (MV) is an integral part of ARDS therapy, but like all treatments, inappropriate application can lead to side effects, namely ventilator-induced lung injury (VILI). To date, limiting VILI through lower tidal volumes is the only intervention proven to reduce mortality in ARDS [3]. Patients randomized to a lower tidal volume (6 ml/kg) ventilation strategy had a 22% relative reduction in mortality compared with patients receiving 12 ml/kg [3]. Although mortality in patients with ALI/ ARDS in large randomized controlled trials in the last decade is reported to have fallen by almost half [3–5], a recent meta-analysis questions whether this is representative of real-world practice [6,7]. In this meta-analysis by Phua et al., mortality did not decrease between 1994 and 2006 [6]. Moreover, mortality was lower in the controlled environment of randomized trials than observational studies [6], implying a contribution of potentially modifiable iatrogenic factors.
Since the first report by Greenfield et al. demonstrated that MV with large tidal volumes and pressures alters the surface properties of canine lung extracts [8], it became obvious in both in vitro and in vivo studies that although MV is a lifesaving tool in patients with ARDS, it can aggravate pre-existing or even cause de novo lung injury [9]. The central role of mechanotransduction (i.e., the conversion of a mechanical stimulus into chemical activity) in the pathogenesis of VILI is now well recognized. However, despite the progress made in the understanding of the mechanisms through which cells decorating the blood–gas barrier and lining the airways respond to mechanical stress and produce a local and systemic response [10,11], the factors that define the stress and the actual stress to which cells are exposed is still unclear. The major reason for this uncertainty stems from the inherent limitations of currently available imaging techniques to provide real-time information on the mechanics of individual alveoli. This article will focus on current knowledge and controversies regarding the properties of injured lungs that contribute to the generation of stress from MV and render them sensitive to VILI; efforts undertaken to define and measure lung stress and the effects of ventilatory management on its distribution; the interconnectedness of different mechanotransductive responses and their potential role (protective/reparative vs injurious) and, finally, will cover evidence in support of injury mechanisms from large clinical trials.
Biophysical properties of injured lungs
Baby lung
For almost two decades, ARDS was considered a homogeneous process associated with relatively uniform lung injury. Quantitative assessment of chest computed tomography (CT) images, however, revealed that the ARDS lung consists of normally aerated, poorly aerated and nonaerated tissue, and the amount of normally aerated tissue is roughly equivalent to that of a healthy male child of 5/6 years [12–14]. This finding gave birth to the concept of ‘baby lung’ by Gattinoni [14,15]. The same group of authors also found that respiratory compliance is any well correlated only with the amount of normally aerated tissue and not with the amount of nonaerated tissue, and specific tissue compliance (compliance/normally aerated tissue) of the residual inflated lung is nearly normal [16]. These findings reinforced the idea that the ARDS lung is not stiff but small [15]. Thus, a large proportion of the delivered tidal volume gets distributed to the baby lung, placing it at risk of injury from overdistension [17].
The redistribution of the densities to the dependent portion of the lung after prone positioning indicated that baby lung is not a distinct anatomical structure [18]. It was hypothesized that increased lung weight due to accumulated edema raises superimposed pressure, and gas in the dependent lung regions is squeezed out by the heavy lung parenchyma above [15,16,19]. Dependent regions collapse at lower transpulmonary pressures (Ptp) and reopen abruptly at an elevated opening pressure corresponding to the lower inflection point (LIP) of the pressure–volume (P–V) curve, after which respiratory system compliance increases. The above interpretation of CT and P–V curve findings has since been challenged [20]. Gravitational CT grayscale gradients correspond to gradients in regional air, and can be due to either gradients in alveolar size or gradients in water per alveolus. CT cannot distinguish between collapsed and fluid-filled alveoli and hence provide information on regional volume and stress [20]. In direct measurements of regional volume in edematous lungs, regional volumes in the dependent part of the lung were somewhat larger than the corresponding volumes at the same airway pressure in the control state, even though regional ventilation was much smaller than that in the control state [21]. Moreover, when examining regional ventilation as a function of time, no evidence of abrupt opening, which could correspond to the LIP, was found [21]. The changes in the P–V curve described in ARDS patients are similar to those noted during the inflation of lungs rinsed with fluids of fixed surface tension [22–24]. The knee of the P–V curve corresponds to the pressure where the air–fluid interface enters the alveolus and describes the transition from the fluid-filled to the air-filled high-surface-tension state [25]. The alveolar tissue remains open during this transition. In agreement, a recent study of subpleural alveoli in normal and hydrochloric acid-injured murine lungs using optical coherence tomography by Mertens et al. found that volume increases by alveolar expansion rather than recruitment in both normal and injured lungs [26,27]. Although there is agreement on the existence of airless dependent regions that contribute to the gas exchange abnormalities, atelectasis (derived from the greek ‘ateles ektasis’, which literally means incomplete expansion) of these regions has completely different implications for stress generation than if they are viewed as fluid-filled and expanded [11].
Altered vascular barrier function & surface tension
Altered vascular barrier function is considered the main mechanism of both ARDS and VILI pathophysiology [9,28]. Increased elastance and resistance of edematous lungs is attributed to increased minimal surface tension caused by surfactant decrease and dysfunction [29], airway block caused by air–liquid interfaces and bubble formation in small airways [30–32], bronchoconstriction [33,34], pneumoconstriction [35] and peribronchial edema [31]. Surfactant dysfunction is attributed to alveolar flooding and plasma protein-induced changes in surfactant properties [29]. MV may also specifically affect the endogenous surfactant system by influencing aggregate conversion through changes in alveolar surface area [29]. The increased surface tension promotes alveolar collapse, which is counterbalanced by interdependence mechanisms [36]. The resultant fall in interstitial pressure promotes further alveolar leaking from the malfunctioning vascular barrier, which increases as the mean radius of curvature of the air–liquid interface, and thus the alveolar liquid pressure falls [20,21]. Laser confocal [20] and electron microscopy [37] of edematous animal lungs shows that the alveoli are not collapsed, but rather completely or partially flooded and that they contain air pockets of different sizes. Obstruction of conducting airways by liquid and foam could cause and preserve a nonuniform alveolar gas pressure in trapped air pockets that would explain the lack of increase in the vertical gradient of regional lung volume [21]. Liquid bridges and moving of air–liquid interfaces through conducting airways would increase impedance to regional ventilation, causing a reduction in tidal expansion of dependent regions that is restored after positive end-expiratory pressure (PEEP) application [21]. Moreover, the tension of an advancing air–liquid interface in a channel the size of a small airway and the pressure generated at the air–liquid interface when a collapsed wet tube is opened are capable of deforming and wounding lining cells [38,39]. The energy released during fracture of liquid bridges is also sufficient to injure neighboring cells [40].
Lung heterogeneity & interdependence
Heterogeneity in local impedance to lung expansion has important implications for shear stress generation owing to interdependence. Alveoli on the outside of the lung are exposed to Ptp (alveolar pressure minus pleural pressure), but within the lung the outside wall of one airspace is at the same time the inside wall of its neighboring unit [36]. In a homogeneously expanded lung, gas pressure in all interconnected airspaces is the same and approximates Ptp. In non-uniformly expanded lungs, local distending pressures differ from Ptp in such a manner as to reduce the nonuniformity of expansion [36]. When gas or liquid pressure differences exist between adjacent alveoli, such as in the ARDS lung, air space septa are deformed. Variations in shape or volume change the projected area over which distending forces of tissue attachments are distributed, thus changing local stress (force per unit area). Tissue attachments between neighboring units with different pressure carry a shear stress that is substantially greater than average Ptp [36].
Unravelling the interplay between the topographical distributions of deforming stress and the attributes that render different structures susceptible to deforming stress is paramount for the understanding of VILI and its prevention.
Effects of mechanical ventilation on the topographical distributions of parenchymal stress & strain
Basic definitions & concepts
Engineering terms have traditionally been used to describe the mechanical properties of tissues. More specifically, ‘stress’ is defined as the counterforce per unit of cross-sectional area of a structure that balances and reacts to an external load [41]. It has the same units as pressure, and in fact pressure is one special variety of stress. The associated deformation of the structure relative to its resting state is called ‘strain’ [41]. Although ideally the reference state is the unstressed state, the lung is always pre-stressed (i.e., Ptp is not zero). Therefore, the choice of reference state (volume) is arbitrary and different authors have computed lung strain relative to total lung capacity [42] or, alternatively, relative to relaxation volume [43]. Needless to say, the choice of reference volume is critical if one wishes to interpret strain as a determinant of mechanotransduction. Stress and strain are vectors, which can be expressed as directed orthogonally (normal) or tangentially (shear) to a plane of interest. The observation that the response of cells and tissues to normal stress often differs from that to shear stress is simply a reflection of the complex nonuniform geometrical arrangement of the molecular stress/strain sensors in cells and tissues. Although continuum mechanics and linear elasticity theory provide a useful framework for thinking about lung deformation on a macroscopic scale, they are not suitable theories for modeling mechanotransduction on a cellular and molecular level [44].
The topographical distribution of parenchymal stress and strain of the normal lung is not uniform [45–48] because it is governed by shape-matching constraints between two gravitationally deformed elastic solids: the lungs and the chest wall. The weight of the heart [49,50] and the effect of the abdomen on the diaphragm and thoracic cage [51] also affect lung shape and regional volume distribution, along with small-scale heterogeneity that still has not been explained by any gravitational mechanism [52,53]. The weight of the healthy lung itself is only a minor determinant of the nonuniform Ptp [46]. In the case of the injured ‘heavy’ lung, although the pleural pressure gradient would be expected to increase, how this increase would reflect on regional volume is not straightforward [20].
The major force-bearing element in the lung is the connective tissue, which consists of three different systems interconnected with each other: an axial fiber network extending from the central airways to the alveolar ducts, a peripheral network extending centrally from the visceral pleura, and a parenchymal interstitium that is anchored at the elements of both [54]. The peripheral network is extended by changes in lung volume, whereas the line elements of the central network that form alveolar entrance rings are extended by surface tension on the alveolar walls [55]. The anatomical constituents of the connective tissue of the lung are the collagen and the elastic fibers, and the matrix in which they are embedded [56]. Their complex structural organization and interconnectedness along with surface tension at the air–liquid surface contribute to pulmonary elasticity [55,56]. Distortion of the connective tissue by mechanical forces is transferred through the extracellular matrix to the adhering cells [57].
Measurements of the macromechanical properties of the lung do not offer direct information regarding the micromechanics of the parenchyma. Interalveolar septa have pleats at the sites of septal junctions that unfold as lung volume increases and alveolar walls only get stretched at higher lung volumes [55,56,58,59]. Tschumperlin and Margulies found that, during an inspiratory capacity maneuver, the alveolar basement membrane area increased by 35%, which corresponded to a cell stretch of only approximately 15% [60]. Therefore, strains computed from lung regions greater than 1 cm3 greatly overestimate cell stretch [20].
Strengths & limitations of clinically derived stress/strain surrogates
Imaging techniques
Better insights into ARDS and VILI pathophysiology changed the goals of MV from obtaining normal blood gases to accomplishing the minimum necessary gas exchange while protecting the lung. Clinically derived stress and strain surrogates (Box 1) have to be employed in order to evaluate different ventilation strategies. CT has led the way in enhancing the understanding of the heterogeneity of lung injury in ARDS and has since been used to test the effect of different ventilator settings. The CT density of the lung is defined by the amount of alveolar gas, tissue, blood and water [18]. Density is a ratio of gas/tissue or water and thus, in cases of increased density, CT cannot differentiate between tissue and water. More specifically, CT cannot differentiate between atelectatic (collapsed) and edematous tissue. Moreover, the anatomical structures included in one voxel, the ‘CT pulmonary unit’, may range from one to several acini depending on the resolution of the CT and the status of the lung [18]. CT scans can detect recruitment, defined as the difference in nonaerated tissue under different ventilatory conditions [16], and hyperinflation, defined as an increased gas/tissue ratio [18]. CT has highlighted the importance of the individuality of lung injury distribution in patients with ARDS to potential responses to MV and VILI [61,62]. It has been used extensively to study the effects of different levels of PEEP in an effort to find a ventilatory strategy that confers the most recruitment with the least hyperinflation [62–65]. It is important to stress, however, that it cannot give any information on overdistension, stress or strain. A lung may be overdistended and not hyperinflated and vice versa [18]. Finally, its usefulness in setting the parameters of MV in everyday clinical practice given the levity of ARDS patients and the amount of radiation exposure is limited.
Box 1. Clinical surrogates used for the estimation of lung stress/strain and experimental tools in the investigation of macro- and micro-mechanics
Clinically derived stress/strain surrogates
Imaging techniques
Chest computed tomography
Lung ultrasound
Macromechanical measures
LIP and UIP of static P–V curve
Pplat
- Pes
-
–Change in Ptp during inspiration
-
–Absolute values of Ptp at end-expiration and end-inspiration
-
–
Experimental tools
Lung elastography (macromechanics)
Magnetic resonance elastography
Ultrasound elastography
Intravital microscopy (micromechanics)
Real-time confocal microscopy
Optical coherence tomography
Atomic force microscopy
LIP: Lower inflection point; Pes: Esophageal pressure; Pplate: Plateau pressure; Ptp: Transpulmonary pressure; P–V: Pressure–volume; UIP: Upper inflection point.
Lung ultrasound can also demonstrate loss of aeration and increased amount of lung tissue/water in the form of ‘comet tails’ in critically ill patients with ALI/ARDS [66] and provide information on reaeration in patients with ventilator-associated pneumonia [67]. Given its ease of application, it could perhaps be used to provide information on recruitment after PEEP changes, but it would not reveal any specific information on lung stress/strain.
Macromechanical measurements
In ARDS, ‘one size does not fit all’ [68], and measurements of the macromechanical properties of the respiratory system have been used to estimate global lung stress/strain and adapt ventilator settings to individual characteristics of lung injury. Initially the LIP and upper inflection point (UIP) of the characteristic static P–V curve were considered to represent pressures below which alveolar derecruitment took place and above which overdistension occured, respectively [69]. It was soon demonstrated, however, that alveolar recruitment occurs throughout the P–V curve up to the highest pressure studied [70,71]. Assuming normal chest wall mechanics, the plateau pressure (Pplat; i.e., the airway pressure during an end-inspiratory occlusion maneuver) is a surrogate measure of peak Ptp (i.e., peak lung stress). Based on this reasoning, many experts recommend limiting Ptp to <30 cmH20 [72]. However, there is now ample evidence that chest wall mechanics in patients with ARDS are abnormal [73–75] and that peak lung stress may not be the only or even the most important determinant of VILI [76,77]. For example, a post hoc analysis of the ARDS network’s low tidal volume trial suggested that reducing tidal volume from 12 to 6 ml/kg was beneficial even in patients with Pplat values significantly below 30 cmH2O [78].
Chiumello et al. measured esophageal pressure (Pes) as a surrogate of pleural pressure in order to calculate lung stress; they defined lung strain as the fractional volume change between functional residual capacity (FRC), the volume at zero end-expiratory pressure and end-inspiration [43]. They observed considerable variability between subjects in apparent maximal lung stress and strain because chest-wall mechanics and the volume of nonrecruitable lung varied considerably between patients [43]. On this basis, they suggested that Pplat and tidal volume are inadequate surrogates of lung mechanics on which to base ventilator management decisions [43]. However, the implication that threshold values of peak stress and strain as defined by Chiumello et al. are useful guides of VILI risk have been challenged [79].
In material science, the change in stress is determined by the product of the material’s elastic modulus, which is a measure of its stiffness, and strain (i.e., its corresponding deformation). Accordingly, Chiumello et al. substituted ΔPtp for Δstress and the fractional change in lung volume between end-inflation and relaxed end-expiration for strain. The relationship between stress and strain was fairly linear and, surprisingly, its slope (the elastic/bulk modulus of the lung) was fairly normal. Notwithstanding the uncertain confounding by lung recruitment during inflation, this suggested that the mechanical properties of the ‘open lung’ (the bulk modulus of Gattinoni’s baby lung) were near normal. While it is fair to assume that esophageal manometry yielded a reasonable estimate of the change on Ptp, there is considerable concern about systematic bias (the ‘so-called’ mediastinal artifact) in specific Ptp values, including those at end-inspiration. Therefore, the poor correlation between Pplat and the lung stress estimate at end-inflation could reflect some combination of between-subject variability in chest wall recoil pressure, in measurement bias and in the amounts of recruitable lung.
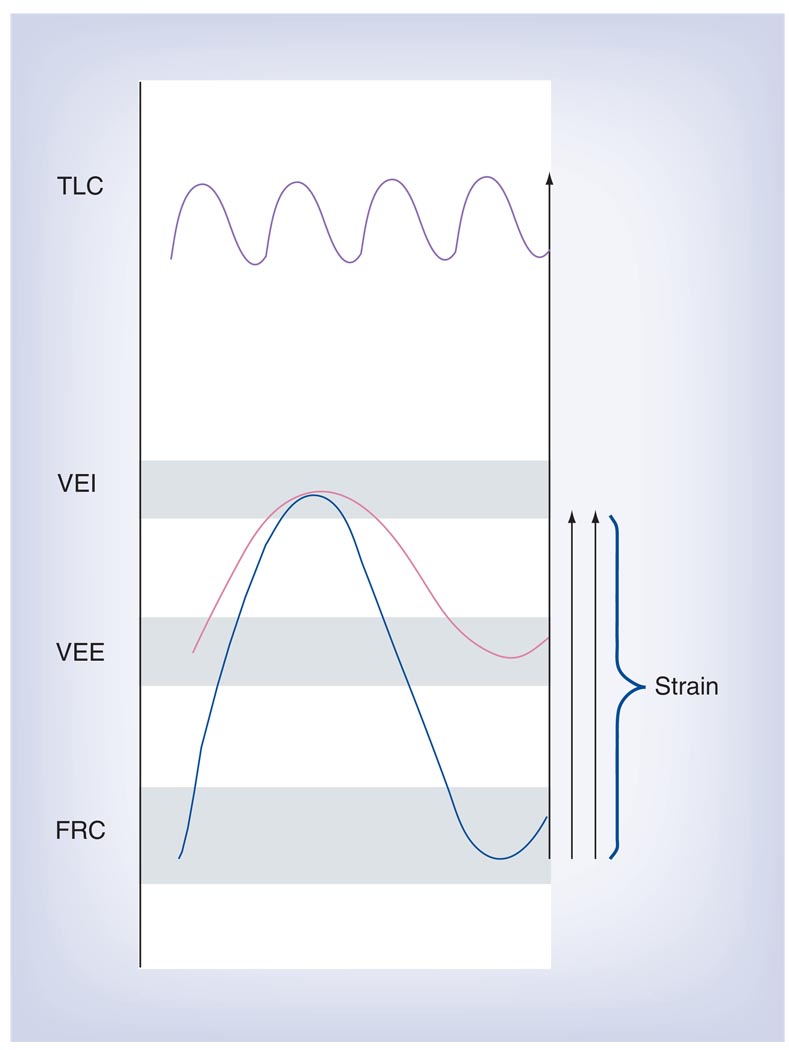
A gas dilution technique for measuring thoracic gas volume in intubated patients has recently been made available for clinical use. Chiumello et al. used a nitrogen washout technique to measure the lung volume of patients with ARDS at zero end-expiratory pressure (FRC) and found that volumes varied a great deal between individuals. This is of interest insofar as current tidal volume setting guidelines are based on predicted body weight (PBW) and as such are relatively insensitive to the severity of lung injury. PBW scales with the size of the normal lung, but not with that of the injured lung [80,81]. Consequently, Chiumello et al. were able to show that using current guidelines the lungs of patients with a small FRC are exposed to much larger deformations than those of patients with a relatively normal FRC. To emphasize this point, Chiumello et al. computed strain as the volume change between FRC and end-inspiration, normalized by FRC (strain = ΔV/FRC), and implied that strains greater than 2 might be injurious. However, this is unfortunate because strain, as defined by Chiumello et al., ignores the independent effects of tidal volume and PEEP on lung injury (FIGURE 1). As early as 1974, Web and Tierney showed that lung injury in rats ventilated with a peak inspiratory pressure of 45 cmH2O and no PEEP was much greater than when they were ventilated to the same peak pressure but with 10 cmH2O PEEP [82].
Figure 1. Chiumello et al. computed strain as the volume change between functional residual capacity and end-inspiration, normalized by functional residual capacity (strain = ΔV/FRC).
They were able to show that by titrating tidal volume (VT) to predicted body weight, the lungs of patients with a small FRC are exposed to much larger deformations than those of patients with relatively normal FRC. The above definition of strain ignores the independent effects of VT and positive end-expiratory pressure on lung injury.
FRC: Functional residual capacity; TLC: Total lung capacity; VEE: Volume at end-expiration with positive end-expiratory pressure; VEI: Volume at end-inspiration.
Data from [43].
In contrast to Chiumello et al., Talmor and Loring have used the absolute values of airway occlusion pressure and Pes to calculate lung stress (Ptp) at end-inspiration and relaxed end-expiration [75–77]. In 70 patients with acute respiratory failure, only 24% of the variance in apparent Ptp was explained by airway pressure, and 52% was due to variation in Pes [75]. Interestingly, the apparent end-expiratory Ptp was often negative [75–77]. This suggested that the injured lung is compressed by pleural effusions and the weight of the abdominal contents and that much higher values of PEEP are required to counterbalance this compressive force than are used in conventional clinical practice. The dilemma arising from this conclusion is the concern for stress injury in those parts of the lung that are not exposed to compressive stress, namely, the already ‘open’ largely nondependent baby lung.
For Pes to accurately reflect pleural pressure, the pressure measured in the esophageal balloon has to accurately represent the pressure in the esophagus; the transmural pressure of the esophagus should be zero; the esophagus should not be compressed by the heart or other mediastinal structures; and the pressure in the mediastinum surrounding the esophagus should be equal to pleural pressure [83,84]. Pes can exhibit variations unrelated to pleural pressure depending on the position of the balloon in the esophagus [85], on the volume of air inside the balloon [86] or on postural artifacts [87]. There is general agreement among experts that, if calibrated and positioned appropriately, Pes may be used to estimate pleural pressure change during breathing in normal man. This is because when near resting volume the normal lung is fluid-like and hence regional variability in changes in pleural pressure is relatively small. But at high volumes and high prestress the lung becomes stiff and even small deviations from the shape that would have been observed in a uniformly inflated state produce large local stress gradients [88]. The stress distribution in the injured lung of the ARDS patient is even more heterogeneous, because altered barrier properties promote alveolar flooding, occlusion of small airways by liquid plugs, small-scale variability in surface tension and varying degrees of gas absorption atelectasis [20]. Pelosi et al. compared Pes and pleural pressure recorded by pressure transducers placed directly in the pleural space in oleic-acid-injured dogs and found that the variations of pleural pressure and Pes were correlated [71]. The absolute values of Pes, however, did not always correlate with pleural pressure for all regions of the lung [71].
Given the greatly increased topographical variability in surface pressure of injured lungs, the chance that the pressure anywhere along the lumen of the esophagus, especially in dependent areas surrounded by pleural effusion, informs about the average lung stress is slim [88]. Negative Ptp may be attributed to airway closure during exhalation that causes alveolar gas trapping and underestimation of alveolar pressure by airway pressure [76,77]. Alternatively, Ptp is biased by units that are closed and extrinsically compressed by pleural fluid and/or the weight of abdominal contents [88]. Pleural pressure is probably lower and Ptp higher in the nondependent lung. The authors consider Pes as an estimate of global pleural pressures [89] and “an indicator, albeit imperfect, of an effective pleural pressure in critically ill patients” [76]. However, those concerned with lung injury attributable to hyperinflation might argue that knowing average pleural pressure and consequently average lung stress misses the point. PEEP should be titrated so that maximal stress, namely that acting on nondependent, already recruited units, can be maintained below some yet to be defined injury threshold. Until we learn how to measure that stress, it is unlikely that the debate about ‘best PEEP’ will arrive at an informed conclusion.
Experimental tools in the investigation of macro- & micro-mechanics
Lung elastography
Efforts have been made to apply imaging techniques for the mapping of the elastic properties of lung tissue (Box 1). Magnetic resonance elastography (MRE) is a phase-contrast technique that encodes synchronized cyclic shear wave displacements into the phase of the MR signal followed by estimation of the spatial frequency of the shear wave [90–92]. The shear modulus is calculated by determining the local shear wavelength from the shear wave displacement maps. It can spatially map shear stiffness (shear stress/shear strain) of tissue-like materials. The intrinsically low signal-to-noise ratio of lung parenchyma makes lung MRE challenging. Moreover, the application of shear waves to the lung is not practical owing to the presence of the chest wall and the pleural space. Recently, the feasibility of using conventional 1H MRE in a small animal model was demonstrated [90]. Although the magnitude of the signal within the lung was low, there remained enough phase coherence to encode phase displacements from the shear wave. This technique was capable of spatially resolving differences in shear modulus associated with changes in inflation pressure and the introduction of fluid into the lung. Both the use of 1H and 3H MRE in animal models have shown that shear stiffness of the lung parenchyma increases with inflation pressure [90–92]. Similar ‘proof of concept’ observations were recently made using an ultrasound-based surface wave method for noninvasively measuring the viscoelasticity of lungs [93].
Lessons from intravital microscopy
Conventional histological techniques may not reflect in vivo architecture. Most fixatives alter tissue architecture [94] and the lung volume history prior to fixation affects the degree of unfolding or stretching of the alveolar epithelium [95]. The extent to which cells undergo mechanical deformation with changes in lung inflation remains elusive, even in the normal lung during tidal breathing [96]. Morphometric studies on perfusion-fixed lung tissue suggest that alveolar walls unfold and stretch only at high tidal volumes [59,60]. Perlman and Bhattacharya applied real-time confocal microsopy in an isolated perfused rat lung to image the alveolar perimeter distention of single alveoli, divided the perimeter into five–eight segments and determined their lengths [97]. Hyperinflation to 20 cmH2O from 5 cmH2O increased mean segment length by only 14%. Segment distention was heterogeneous, even within the same alveolus.
On the contrary, a series of intravital microscopy studies in animals with both normal and injured lungs suggest that the main mechanism of alveolar volume change is recruit-ment/derecruitment [98–103]. Interpretation of the results of those studies is confounded by methodological issues. Lung injury was inflicted by surfactant deactivation, images of subpleural alveoli in the apical nondependent part of the lung were examined, mechanics of subpleural alveoli may have been influenced by their coupling to the visceral pleura, and the pleural surface pressure was altered by thoracotomy and gentle suction applied to hold the lung under the coverslip.
Recently, Mertens et al. examined P–V changes of subpleural alveoli in normal and hydrochloric acid-injured mouse lungs using optical coherence tomography. They concluded that volume increases by alveolar expansion and not recruitment in both models [27]. Preferential loss of compliance in small alveolar clusters redistributed tidal volume to larger alveoli, which increased spatial heterogeneity in alveolar inflation in the injury model. It is worth noting that the optical coherence tomography probe does not need to be in contact with the tissue. Thus, instead of applying suction, a ribcage window was covered by a transparent polyvinylidine membrane and gas was removed from the pleural space with a transdiaphragmatic catheter. This study was also confounded by the examination of subpleural alveoli in the nondependent part of the lung [26].
Apart from the contribution of the heterogeneity of stress distribution in the whole lung and at the individual alveolar level to lung injury, responses to deformation on a cellular or even subcellular level have been investigated. Even though the extracellular matrix is considered to be the main load-bearing element in the lung, mechanical properties of alveolar cells or cellular compartments can influence mechanotransduction. Azeloglu et al. used atomic force microscopy indentation in combination with fluorescence imaging to map the elastic modulus distribution in alveolar epithelial type I and II cells and fibroblasts [104]. They reported phenotypic differences in biomechanical properties of the cells, but also of different cellular compartments. Berrios et al. have previously shown using magnetic twisting cytometry that the state of differentiation and the application of deforming stresses can influence the mechanical properties of type II cells in culture [105]. Therefore, mechanical properties and responses to uniform deformations may be different and modifiable in different cell types or even within different compartments of the same cell type in the alveolus. Despite the existing limitations, intravital imaging techniques (Box 1) may shed some light on stress-related pathology at the alveoli level in the experimental setting.
Mechanotransduction
From macromechanics to mechanotransduction
Mechanotransduction refers to the conversion of mechanical stimuli into chemical signals and is ultimately executed by the cell [106]. The lung is a dynamic organ subjected to varying mechanical forces during normal breathing, and through mechanotransduction those forces influence lung development and metabolism [96]. MV applies a force to the lung and can produce injury both by direct mechanical distortion and by influencing mechanotransduction. The extent of the injury depends on the magnitude and rate of application of the force. In other words, lung stress and strain depend on maximum pressure and volume, but also on the amount and rate of P–V change [17,82,107,108]. For reasons outlined previously, stress distribution is not uniform and therefore individual alveoli, depending on their location and their state of recruitment, are exposed to different levels of stress [36]. Even within the same alveolus, strain may not be uniform [97]. The major load-bearing structures of the alveolus are the extracellular matrix that is composed of collagen, elastic fibers and proteoglycans, each of which is characterized by different mechanical behavior and surface tension [56]. Cells then sense mechanical forces via receptors and adhesion complexes that connect the cytoskeleton to the extracellular matrix [57]. Different cell types and even different compartments of the same cell type not only have different mechanical properties [104], but also the ability to modify these properties in response to various stimuli [11,105] and are therefore subjected to different strains. VILI is then mediated through uncompensated cell deformation [11] and surfactant inactivation [29], which in turn increase alveolar instability [20] and heterogeneity in regional mechanics [36] further raising cell and tissue stress [36]. Even if the stress applied to the lungs of two different patients is the same, the force actually reaching individual cell elements and the corresponding response (mechanotransduction) can be quite heterogeneous and variable. Given the regional variability of stress as well as the variability in the susceptibility of different lung structures to stress, the existence of a single ‘global’ stress/strain injury threshold is questionable [43,76,79].
Mechanotransductive pathways & their significance as surrogate markers of lung injury
The microscopic alterations described in experimental VILI include interstitial and intra-alveolar edema, endothelial lesions, inter- and intracellular gap formations, denuded basement membrane and epithelial cell membrane disruption [11]. Some of these findings could represent an active remodeling response in an effort to adapt to strain and are not necessarily the result of stress failure [11]. Gajic et al. observed that after removal of the injurious stress, 60% of injured cells repair plasma membrane defects [109]. In vitro experiments have shown that living cells attempt to adapt to deformation through matrix and cyto-skeletal remodeling [110–112] and regulation of cell surface area and plasma membrane tension [113–116]. The susceptibility to stress failure varies not only with strain amplitude but also with strain rate [114]. Failure to activate protective mechanisms or failure of those mechanisms to repair injured cells could lead to the release of the intracellular contents, which would in turn initiate an immune and inflammatory response (i.e., biotrauma) [117–120].
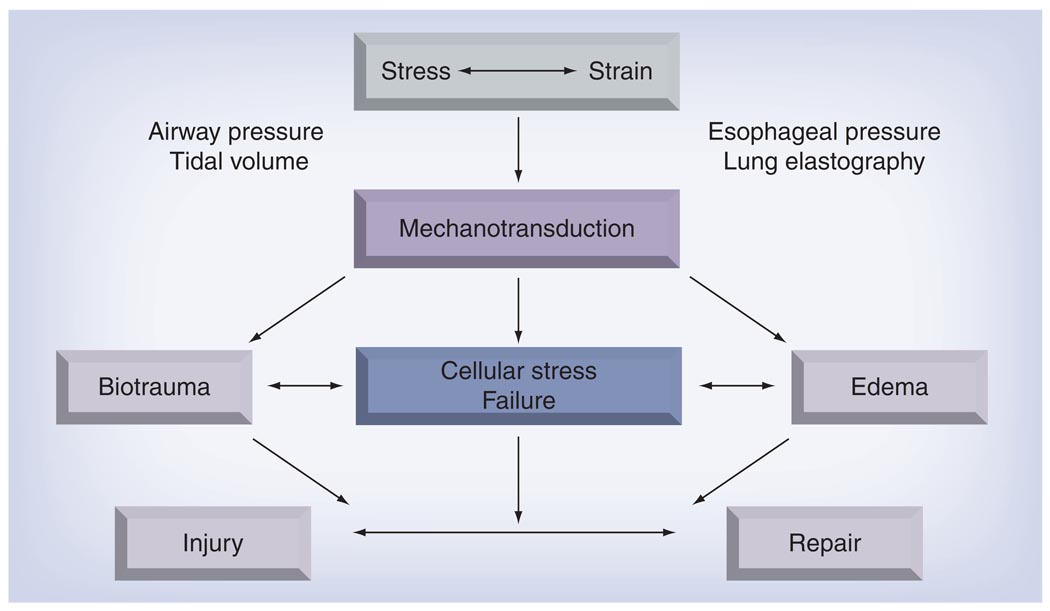
It is noteworthy that not all of these changes are necessarily observed simultaneously. For example, epithelial gaps and endothelial lesions have been described in the absence of cell necrosis [121,122] and increases in the mRNA expression of extracellular matrix proteins have been measured in the absence of alveolar cell breaks [123]. Although plasma membrane wounding causes activation of stress response genes followed by the induction of proinflammatory signaling, stress can also trigger the release of proinflammatory cytokines in the absence of gross cell injury [124–126]. In a model of low-volume injury, D’Angelo et al. found alterations in lung mechanics and an increase in lung water that were not associated with an increase in proinflammatory cytokine TNF-α [127]. Therefore, in different models of stress injury, edema can develop in the absence of cell membrane wounding or inflammation, and inflammation can be upregulated in the absence of gross cell injury. Animal models of experimental lung injury, which are confounded by the differences in immune and inflammatory responses compared with humans, do not fully reproduce the features of human lung injury, exhibiting either impaired vascular permeability and impaired surface tension or inflammatory changes, but seldom all three [128]. Edema, altered tissue mechanics, cellular stress failure and the innate immune response may all act synergistically, but could also be manifestations of different injury pathways (FIGURE 2). Whether they represent different stages of the same response or whether different amounts of stress initiate different mechanisms and which of those are really important for the final clinical picture are questions to be answered [129].
Figure 2. Mechanical ventilation applies an additional stress to the prestressed lung and the resulting strain is transformed by cells into chemical signals.
Edema, cellular stress failure and the innate immune response may act synergistically, but may also be manifestations of different injury pathways. The interaction of injurious and protective/reparative mechanotransductive pathways leads to ventilator-induced lung injury. Airway pressure and tidal volume are widely used in clinical practice as surrogate markers of lung stress/strain. Given their limitations, however, other measures, such as esophageal manometry and lung elastography, are under investigation.
Evidence on VILI from clinical trials
The extensive research that has provided insights on deformation-induced lung injury has mainly involved in vitro and animal experiments. Several studies have tried to translate these findings in clinical practice in order to design ‘protective’ mechanical ventilation strategies. Protective mechanical ventilation has focused on two variables, the choice of tidal volume and ‘best PEEP’. After three negative or inconclusive trials [69,130,131] and a small single-center study that suggested benefit of an open lung strategy [132], the ARDS Network trial unequivocally settled the tidal volume question. Patients ventilated with 6 ml/kg PBW and a Pplat of 30 cmH2O or less compared with 12 ml/kg PBW had a 22% relative mortality reduction [3]. The lack of significant outcome difference in the negative trials was attributed to the smaller difference in tidal volume settings between treatment arms [20]. In a retrospective study of patients who developed ALI after the onset of mechanical ventilation, the use of large tidal volume was an independent risk factor for ALI (odds ratio 1.3 for every ml above 6 ml/kg PBW) [133]. This and a series of subsequent reports focusing on the ventilatory management of patients with normal lungs in the operating room [134–136] strongly suggest that MV with tidal volumes above 10 ml/kg PBW initiates a proinflammatory immune response and is probably harmful in some cases.
The extent to which tidal volume and Pplat should be reduced in patients with ALI remains controversial. Those who consider peak lung stress and overinflation as primary injury mechanisms had argued that tidal volume reductions were less important in patients with low Pplat. However, in a post hoc analysis of the ARDS Network low tidal volume trial, a safe Pplat, below which there would be no beneficial effect of further tidal volume reduction, could not be identified [78]. Until there is greater clarity on the relative risks of overinflation versus under-recruitment, not to mention the confounding influences of alveolar ventilation and local CO2 tension on surfactant function, vascular barrier properties and innate immune responses, this issue will not be settled in the foreseeable future [137–140].
The open lung strategy entails opening the lung with a recruitment maneuver and then keeping it open by the application of PEEP or gradually opening the lung with stepwise increases in PEEP, thus preventing tidal recruitment/derecruitment. The value of PEEP in the treatment of ARDS is even mentioned by Ashbaugh in the original description of ARDS [2]. Nevertheless, higher PEEP levels repeatedly failed to be associated with improved mortality. The lack of beneficial effects has been attributed to suboptimal PEEP titration. Two studies set PEEP according to predetermined tables of PEEP–FiO2 combinations [4,141]. Gas exchange, however, is not the most informative surrogate marker of micromechanical alterations. Limiting tidal volume and Pplat may not be sufficient to avoid hyperinflation [142] and PEEP is likely to increase hyperinflation in some patients [143]. Thus, the need to customize lung-protective ventilation according to individual patient mechanics became obvious. Mercat et al. titrated PEEP to reach a Pplat of 28–30 cm H2O and failed to show benefit [144]. By study design, patients with more severe lung injury and lower respiratory system compliance received lower levels of PEEP. Grasso et al. used the stress index, which is a monitoring tool that calculates hyperinflation over recruitment/derecruitment based on the curvature of the pressure–time tracing during constant flow ventilation on a breath-by-breath basis [145]. The stress index revealed hyperinflation in all patients during the ARDS network strategy and PEEP was decreased. Plasma concentrations of inflammatory markers were significantly lower during the ‘stress index’ ventilation. Talmor et al. used Pes measurements to calculate Ptp and found a great percentage of patients with estimated negative Ptp at end-expiration [75]. They considered those patients to be at risk for tidal derecruitment [75,89]. In a subsequent study, they used Pes to increase PEEP to a level that corresponded to positive end-expiratory Ptp, sometimes at the cost of high Pplat [77]. This strategy was associated with significant improvements in oxygenation and respiratory system compliance, paralleled by a strong trend towards increased survival and shorter durations of MV.
In aggregate, to date, all trials aimed at defining ‘best PEEP’ in patients with ALI are limited by uncertainty about the topographical distribution of stress and the corresponding susceptibility of different lung structures to stress. Those who believe in ‘opening the lung’ have pursued this quest with relative disregard for high inflation pressures, while those concerned with hyperinflation keep pushing for lower Pplat targets, thereby accepting lower PEEP, tidal volume, oxygenation and alveolar ventilation targets.
Expert commentary
Ventilator-induced lung injury is an insidious process, the clinical expressions of which may lag causal management choices by hours or even days. Clinicians, who must choose a ventilator setting, are understandably influenced by physiologic response variables, which can be easily measured and which provide immediate feedback to a change in therapy, namely, airway pressure and arterial oxygen tension. However, it is not always clear whether these are appropriate surrogates of efficacy. Therefore, advances in lung protective mechanical ventilation await a refined means of being able to measure the topographical distribution of parenchymal stress and strain and the consequences of management decisions on them. In their absence, the debate, regarding whether it is more important to recruit as opposed to worry about injury from hyperinflation, will probably continue.
Five-year view
In the coming 5 years we will hopefully learn if lung stress-guided ventilator management is superior to airway pressure-guided management. Esophageal manometry and the less-established elastography techniques should make this possible. The lessons would undoubtedly inform the debate about specific biophysical determinants of injury and their relative impact on patient outcomes. They will have to be integrated with experimental and clinical research on topics such as permissive hypoxemia, therapeutic hypercapnia and on molecular mechanotransduction mechanisms.
Key issues
Ventilator-induced lung injury can result from overdistension of nondependent and already open lung units or from under-recruitment of dependent nonaerated units. Both regions coexist in the heterogeneously injured acute respiratory distress syndrome-lung to varying degrees.
Ventilatory strategies that aim to maximize recruitment of dependent regions through the application of high positive end-expiratory pressure put nondependent regions at risk of injury from overdistension.
Ventilatory strategies that aim to minimize the risk of injuring nondependent regions by overdistension implicitly accept the risk of low-volume trauma from under-recruitment.
Thoracic imaging approaches and measurements of respiratory system mechanics have been used to balance these risks, but have yet to establish clinical efficacy.
Footnotes
Financial & competing interests disclosure
This study was supported by NIH grant HL63178. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Bernard GR, Artigas A, Brigham KL, et al. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. • The first description of 12 adult patients with acute respiratory distress syndrome (ARDS).
- 3. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N. Engl. J. Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. •• ARDS Network study demonstrating benefit by comparing mortality with a tidal volume of 6 versus 12 ml/kg predicted bodyweight in patients with ARDS.
- 4.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 6.Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am. J. Respir. Crit. Care Med. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 7.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 8.Greenfield LJ, Ebert PA, Benson DW, et al. Effect of positive pressure ventilation on surface tension properties of lung extracts. Anesthesiology. 1964;25:312–316. doi: 10.1097/00000542-196405000-00009. [DOI] [PubMed] [Google Scholar]
- 9. Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am. J. Respir. Crit. Care Med. 1998;157(1):294–323. doi: 10.1164/ajrccm.157.1.9604014. •• Comprehensive review of findings from experimental studies on the mechanisms of ventilator-induced lung injury (VILI).
- 10.Vlahakis NE, Hubmayr RD. Response of alveolar cells to mechanical stress. Curr. Opin. Crit. Care. 2003;9(1):2–8. doi: 10.1097/00075198-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 11. Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am. J. Respir. Crit. Care Med. 2005;171(12):1328–1342. doi: 10.1164/rccm.200408-1036SO. •• Excellent review of the mechanisms through which lung cells respond to deformation and discussion of their relevance in the context of VILI.
- 12.Gattinoni L, Pesenti A, Torresin A, et al. Adult respiratory distress syndrome profiles by computed tomography. J. Thorac. Imag. 1986;3:25–30. doi: 10.1097/00005382-198607000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Maunder RJ, Shuman WP, McHugh JW, Marglin SI, Butler J. Preservation of normal lung regions in the adult respiratory distress syndrome. Analysis by computed tomography. JAMA. 1986;255(18):2463–2465. [PubMed] [Google Scholar]
- 14.Gattinoni L, Pesenti A, et al. ARDS: the non-homogeneous lung; facts and hypothesis. Intensive Crit. Care Dig. 1987;6:1–4. [Google Scholar]
- 15. Gattinoni L, Pesenti A. The concept of ‘baby lung’. Intensive Care Med. 2005;31(6):776–784. doi: 10.1007/s00134-005-2627-z. • Review of the findings that led the author to formulate the idea of the ‘baby lung’ and its subsequent implications.
- 16.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure–volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am. Rev. Resp. Dis. 1987;136(3):730–736. doi: 10.1164/ajrccm/136.3.730. [DOI] [PubMed] [Google Scholar]
- 17.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am. Rev. Resp. Dis. 1988;137(5):1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am. J. Respir. Crit. Care Med. 2001;164(9):1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 19.Bone RC. The ARDS lung. New insights from computed tomography. JAMA. 1993;269(16):2134–2135. doi: 10.1001/jama.269.16.2134. [DOI] [PubMed] [Google Scholar]
- 20. Hubmayr RD. Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am. J. Respir. Crit. Care Med. 2002;165(12):1647–1653. doi: 10.1164/rccm.2001080-01CP. •• Insightful review that challenges the traditional concept of the injured dependent ARDS lung as atelectatic versus flooded.
- 21.Martynowicz MA, Minor TA, Walters BJ, Hubmayr RD. Regional expansion of oleic acid-injured lungs. Am. J. Respir. Crit. Care Med. 1999;160(1):250–258. doi: 10.1164/ajrccm.160.1.9808101. [DOI] [PubMed] [Google Scholar]
- 22.Smith JC, Stamenovic D. Surface forces in lungs. I. Alveolar surface tension-lung volume relationships. J. Appl. Physiol. 1986;60(4):1341–1350. doi: 10.1152/jappl.1986.60.4.1341. [DOI] [PubMed] [Google Scholar]
- 23.Stamenovic D, Smith JC. Surface forces in lungs. III. Alveolar surface tension and elastic properties of lung parenchyma. J. Appl. Physiol. 1986;60(4):1358–1362. doi: 10.1152/jappl.1986.60.4.1358. [DOI] [PubMed] [Google Scholar]
- 24.Stamenovic D, Smith JC. Surface forces in lungs. II. Microstructural mechanics and lung stability. J. Appl. Physiol. 1986;60(4):1351–1357. doi: 10.1152/jappl.1986.60.4.1351. [DOI] [PubMed] [Google Scholar]
- 25.Wilson TA, Anafi RC, Hubmayr RD. Mechanics of edematous lungs. J. Appl. Physiol. 2001;90(6):2088–2093. doi: 10.1152/jappl.2001.90.6.2088. [DOI] [PubMed] [Google Scholar]
- 26.Hubmayr RD. Another look at the opening and collapse story. Crit. Care Med. 2009;37(9):2667–2668. doi: 10.1097/CCM.0b013e3181ac47e6. [DOI] [PubMed] [Google Scholar]
- 27.Mertens M, Tabuchi A, Meissner S, et al. Alveolar dynamics in acute lung injury: heterogeneous distension rather than cyclic opening and collapse. Crit. Care Med. 2009;37(9):2604–2611. doi: 10.1097/CCM.0b013e3181a5544d. [DOI] [PubMed] [Google Scholar]
- 28.Artigas A, Bernard GR, Carlet J, et al. The American–European Consensus Conference on ARDS, part 2: Ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1998;157(4 Pt 1):1332–1347. doi: 10.1164/ajrccm.157.4.ats2-98. [DOI] [PubMed] [Google Scholar]
- 29.Spragg RG, Lewis JF. Pathology of the surfactant system of the mature lung: second San Diego conference. Am. J. Respir. Crit. Care Med. 2001;163(1):280–282. doi: 10.1164/ajrccm.163.1.2004028. [DOI] [PubMed] [Google Scholar]
- 30.Cook CD, Mead J, Schreiner GL, Frank NR, Craig JM. Pulmonary mechanics during induced pulmonary edema in anesthetized dogs. J. Appl. Physiol. 1959;14(2):177–186. doi: 10.1152/jappl.1959.14.2.177. [DOI] [PubMed] [Google Scholar]
- 31.Delaunois L, Sergysels R, Martin RR. Acute effects on airways mechanics of pulmonary edema induced by intravenous oleic acid in dogs. Bull. Eur. Physiopathol. Respir. 1980;16(1):47–55. [PubMed] [Google Scholar]
- 32.Frazer DG, Stengel PW, Weber KC. The effect of pulmonary edema on gas trapping in excised rat lungs. Resp. Physiol. 1979;38(3):325–333. doi: 10.1016/0034-5687(79)90058-6. [DOI] [PubMed] [Google Scholar]
- 33.Chung KF, Keyes SJ, Morgan BM, Jones PW, Snashall PD. Mechanisms of airway narrowing in acute pulmonary oedema in dogs: influence of the vagus and lung volume. Clin. Sci. (Lond.) 1983;65(3):289–296. doi: 10.1042/cs0650289. [DOI] [PubMed] [Google Scholar]
- 34.Derks CM, D’Hollander AA, Jacobovitz-Derks D. Gas exchange and respiratory mechanics in moderate and severe pulmonary oedema in dogs. Bull. Eur. Physiopathol. Respir. 1981;17(2):163–177. [PubMed] [Google Scholar]
- 35.Esbenshade AM, Newman JH, Lams PM, Jolles H, Brigham KL. Respiratory failure after endotoxin infusion in sheep: lung mechanics and lung fluid balance. J. Appl. Physiol. 1982;53(4):967–976. doi: 10.1152/jappl.1982.53.4.967. [DOI] [PubMed] [Google Scholar]
- 36. Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J. Appl. Physiol. 1970;28(5):596–608. doi: 10.1152/jappl.1970.28.5.596. •• Milestone study on lung architectural interdependence.
- 37.Bachofen H, Schurch S, Michel RP, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs. Morphology. Am. Rev. Resp. Dis. 1993;147(4):989–996. doi: 10.1164/ajrccm/147.4.989. [DOI] [PubMed] [Google Scholar]
- 38.Bilek AM, Dee KC, Gaver DP., 3rd Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J. Appl. Physiol. 2003;94(2):770–783. doi: 10.1152/japplphysiol.00764.2002. [DOI] [PubMed] [Google Scholar]
- 39.Gaver DP, 3rd, Kute SM. A theoretical model study of the influence of fluid stresses on a cell adhering to a microchannel wall. Biophys. J. 1998;75(2):721–733. doi: 10.1016/S0006-3495(98)77562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh D, Fujioka H, Tung YC, et al. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc. Natl Acad. Sci. USA. 2007;104(48):18886–18891. doi: 10.1073/pnas.0610868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fung Y, et al. Biomechanics: Mechanical Properties of Living Tissues. New York, USA: Springer-Verlag; 1981. [Google Scholar]
- 42.Rodarte JR, Hubmayr RD, Stamenovic D, Walters BJ. Regional lung strain in dogs during deflation from total lung capacity. J. Appl. Physiol. 1985;58(1):164–172. doi: 10.1152/jappl.1985.58.1.164. [DOI] [PubMed] [Google Scholar]
- 43.Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2008;178(4):346–355. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 44.Waters CM, Sporn PH, Liu M, Fredberg JJ. Cellular biomechanics in the lung. Am. J. Physiol. 2002;283(3):L503–L509. doi: 10.1152/ajplung.00141.2002. [DOI] [PubMed] [Google Scholar]
- 45.Agostoni E, et al. Mechanics of the pleural space. In: Geiger S, editor. Handbook of Physiology. MD, USA: American Physiological Society; 1986. pp. 531–559. [Google Scholar]
- 46.D’Angelo E, Michelini S, Agostoni E. Partition of factors contributing to the vertical gradient of transpulmonary pressure. Respir. Physiol. 1971;12(1):90–101. doi: 10.1016/0034-5687(71)90104-6. [DOI] [PubMed] [Google Scholar]
- 47.Rodarte J, Fung Y, et al. Distribution of stresses within the lung. In: Fishman A, editor. Handbook of Physiology. Section 3: Respiratory System. MD, USA: Williams and Wilkins Co.; 1986. pp. 233–246. [Google Scholar]
- 48.Wilson TA, et al. Solid mechanics. In: Fishman A, et al., editors. Handbook of Physiology. Section 3: Respiratory System. MD, USA: Williams and Wilkins Co.; 1986. pp. 35–40. [Google Scholar]
- 49.Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. Am. J. Respir. Crit. Care Med. 2000;161(5):1660–1665. doi: 10.1164/ajrccm.161.5.9901037. [DOI] [PubMed] [Google Scholar]
- 50.Bar-Yishay E, Hyatt RE, Rodarte JR. Effect of heart weight on distribution of lung surface pressures in vertical dogs. J. Appl. Physiol. 1986;61(2):712–718. doi: 10.1152/jappl.1986.61.2.712. [DOI] [PubMed] [Google Scholar]
- 51.Agostoni E, D’Angelo E, Bonanni MV. The effect of the abdomen on the vertical gradient of pleural surface pressure. Respir. Physiol. 1970;8(3):332–346. doi: 10.1016/0034-5687(70)90040-x. [DOI] [PubMed] [Google Scholar]
- 52.Chang H, Lai-Fook SJ, Domino KB, et al. Spatial distribution of ventilation and perfusion in anesthetized dogs in lateral postures. J. Appl. Physiol. 2002;92(2):745–762. doi: 10.1152/japplphysiol.00377.2001. [DOI] [PubMed] [Google Scholar]
- 53.Hubmayr RD, Rodarte JR, Walters BJ, Tonelli FM. Regional ventilation during spontaneous breathing and mechanical ventilation in dogs. J. Appl. Physiol. 1987;63(6):2467–2475. doi: 10.1152/jappl.1987.63.6.2467. [DOI] [PubMed] [Google Scholar]
- 54.Weibel ER, Gil J, et al. Structure–function relationships at the alveolar level. In: West JB, et al., editors. Bioengineering Aspects of the Lung. NY, USA: Dekker; 1977. pp. 1–81. [Google Scholar]
- 55.Wilson TA, Bachofen H. A model for mechanical structure of the alveolar duct. J. Appl. Physiol. 1982;52(4):1064–1070. doi: 10.1152/jappl.1982.52.4.1064. [DOI] [PubMed] [Google Scholar]
- 56. Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J. Appl. Physiol. 2005;98(5):1892–1899. doi: 10.1152/japplphysiol.01087.2004. • An excellent review on the structure and micromechanics of the lung parenchyma.
- 57.Ingber D. Integrins as mechanochemical transducers. Curr. Opin. Cell Biol. 1991;3(5):841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 58.Misof K, Rapp G, Fratzl P. A new molecular model for collagen elasticity based on synchrotron X-ray scattering evidence. Biophys. J. 1997;72(3):1376–1381. doi: 10.1016/S0006-3495(97)78783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachofen H, Schurch S. Alveolar surface forces and lung architecture. Comp. Biochem. Physiol. 2001;129(1):183–193. doi: 10.1016/s1095-6433(01)00315-4. [DOI] [PubMed] [Google Scholar]
- 60.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area–volume relationship in isolated rat lungs. J. Appl. Physiol. 1999;86(6):2026–2033. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- 61.Puybasset L, Cluzel P, Gusman P, et al. Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. CT Scan ARDS Study Group. Intensive Care Med. 2000;26(7):857–869. doi: 10.1007/s001340051274. [DOI] [PubMed] [Google Scholar]
- 62.Puybasset L, Gusman P, Muller JC, et al. Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. CT Scan ARDS Study Group. Adult Respiratory Distress Syndrome. Intensive Care Med. 2000;26(9):1215–1227. doi: 10.1007/s001340051340. [DOI] [PubMed] [Google Scholar]
- 63.Caironi P, Cressoni M, Chiumello D, et al. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2010;181:578–586. doi: 10.1164/rccm.200905-0787OC. [DOI] [PubMed] [Google Scholar]
- 64.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006;354(17):1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 65.Nieszkowska A, Lu Q, Vieira S, et al. Incidence and regional distribution of lung overinflation during mechanical ventilation with positive end-expiratory pressure. Crit. Care Med. 2004;32(7):1496–1503. doi: 10.1097/01.ccm.0000130170.88512.07. [DOI] [PubMed] [Google Scholar]
- 66.Lichtenstein D, Meziere G, Biderman P, Gepner A, Barre O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997;156(5):1640–1646. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 67.Bouhemad B, Liu ZH, Arbelot C, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit. Care Med. 2010;38(1):84–92. doi: 10.1097/CCM.0b013e3181b08cdb. [DOI] [PubMed] [Google Scholar]
- 68.Deans KJ, Minneci PC, Cui X, et al. Mechanical ventilation in ARDS: one size does not fit all. Crit. Care Med. 2005;33(5):1141–1143. doi: 10.1097/01.ccm.0000162384.71993.a3. [DOI] [PubMed] [Google Scholar]
- 69.Brochard L, et al. Respiratory pressure–volume curves. In: Tobin M, et al., editors. Principles and Practice of Intensive Care Monitoring. NY, USA: McGraw-Hill; 1997. pp. 597–616. [Google Scholar]
- 70.Crotti S, Mascheroni D, Caironi P, et al. Recruitment and derecruitment during acute respiratory failure: a clinical study. Am. J. Respir. Crit. Care Med. 2001;164(1):131–140. doi: 10.1164/ajrccm.164.1.2007011. [DOI] [PubMed] [Google Scholar]
- 71.Pelosi P, Goldner M, McKibben A, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am. J. Respir. Crit. Care Med. 2001;164(1):122–130. doi: 10.1164/ajrccm.164.1.2007010. [DOI] [PubMed] [Google Scholar]
- 72.Slutsky AS. Mechanical ventilation. American College of Chest Physicians’ Consensus Conference; Chest; 1993. pp. 1833–1859. [DOI] [PubMed] [Google Scholar]
- 73.Gattinoni L, Chiumello D, Carlesso E, Valenza F. Bench-to-bedside review: chest wall elastance in acute lung injury/acute respiratory distress syndrome patients. Crit. Care. 2004;8(5):350–355. doi: 10.1186/cc2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hess DR, Bigatello LM. The chest wall in acute lung injury/acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2008;14(1):94–102. doi: 10.1097/MCC.0b013e3282f40952. [DOI] [PubMed] [Google Scholar]
- 75.Talmor D, Sarge T, O’Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit. Care Med. 2006;34(5):1389–1394. doi: 10.1097/01.CCM.0000215515.49001.A2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loring SH, O’Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury-do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J. Appl. Physiol. 2010;108(3):515–522. doi: 10.1152/japplphysiol.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N. Engl. J. Med. 2008;359(20):2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hager DN, Krishnan JA, Hayden DL, Brower RG. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am. J. Respir. Crit. Care Med. 2005;172(10):1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brower RG, Hubmayr RD, Slutsky AS. Lung stress and strain in acute respiratory distress syndrome: good ideas for clinical management? Am. J. Respir. Crit. Care Med. 2008;178(4):323–324. doi: 10.1164/rccm.200805-733ED. [DOI] [PubMed] [Google Scholar]
- 80.Maisch S, Boehm SH, Weismann D, et al. Determination of functional residual capacity by oxygen washin-washout: a validation study. Intensive Care Med. 2007;33(5):912–916. doi: 10.1007/s00134-007-0578-2. [DOI] [PubMed] [Google Scholar]
- 81.Olegard C, Sondergaard S, Palsson J, Lundin S, Stenqvist O. Validation and clinical feasibility of nitrogen washin/washout functional residual capacity measurements in children. Acta Anaesthesiol. Scand. 2010;54(3):370–376. doi: 10.1111/j.1399-6576.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 82.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am. Rev. Resp. Dis. 1974;110(5):556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 83.Gibson GJ, Pride NB. Lung distensibility. The static pressure–volume curve of the lungs and its use in clinical assessment. Br. J. Dis. Chest. 1976;70(3):143–184. doi: 10.1016/0007-0971(76)90027-9. [DOI] [PubMed] [Google Scholar]
- 84.Hager DN, Brower RG. Customizing lung-protective mechanical ventilation strategies. Crit. Care Med. 2006;34(5):1554–1555. doi: 10.1097/01.CCM.0000216183.25478.03. [DOI] [PubMed] [Google Scholar]
- 85.Milic-Emili J, Mead J, Turner JM, et al. Topography of esophageal pressure as a function of posture in man. J. Appl. Physiol. 1964;19:212–216. doi: 10.1152/jappl.1964.19.2.212. [DOI] [PubMed] [Google Scholar]
- 86.Milic-Emili J, Mead J, Turner JM, Glauser EM, et al. Improved technique for estimating pleural pressure from esophageal balloons. J. Appl. Physiol. 1964;19:207–211. doi: 10.1152/jappl.1964.19.2.207. [DOI] [PubMed] [Google Scholar]
- 87.Washko GR, O’Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J. Appl. Physiol. 2006;100(3):753–758. doi: 10.1152/japplphysiol.00697.2005. [DOI] [PubMed] [Google Scholar]
- 88.Hubmayr RD, et al. Is there a place for esophageal manometry in the care of patients with injured lungs? J. Appl. Physiol. 2010;108:481–482. doi: 10.1152/japplphysiol.00027.2010. [DOI] [PubMed] [Google Scholar]
- 89.Sarge T, Talmor D. Targeting transpulmonary pressure to prevent ventilator induced lung injury. Minerva Anestesiol. 2009;75(5):293–299. [PubMed] [Google Scholar]
- 90.McGee KP, Hubmayr RD, Levin D, Ehman RL. Feasibility of quantifying the mechanical properties of lung parenchyma in a small-animal model using 1H magnetic resonance elastography (MRE) J. Magn. Reson. Imaging. 2009;29(4):838–845. doi: 10.1002/jmri.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGee KP, Hubmayr RD, Ehman RL. MR elastography of the lung with hyperpolarized 3He. Magn. Reson. Med. 2008;59(1):14–18. doi: 10.1002/mrm.21465. [DOI] [PubMed] [Google Scholar]
- 92.Goss BC, McGee KP, Ehman EC, Manduca A, Ehman RL. Magnetic resonance elastography of the lung: technical feasibility. Magn. Reson. Med. 2006;56(5):1060–1066. doi: 10.1002/mrm.21053. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X, Qiang B, Hubmayr R, et al. Preliminary study of human lung elasticity with the noninvasive surface wave technique; Presented at: UFFC Meeting 2010; 2–4 June 2010; CA, USA. [Google Scholar]
- 94.Bachofen H, Gerber U, Schurch S. Effects of fixatives on function of pulmonary surfactant. J. Appl. Physiol. 2002;93(3):911–916. doi: 10.1152/japplphysiol.00227.2002. [DOI] [PubMed] [Google Scholar]
- 95.Oldmixon EH, Hoppin FG., Jr Alveolar septal folding and lung inflation history. J. Appl. Physiol. 1991;71(6):2369–2379. doi: 10.1152/jappl.1991.71.6.2369. [DOI] [PubMed] [Google Scholar]
- 96.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir. Physiol. 2000;119(1):1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 97.Perlman CE, Bhattacharya J, et al. Alveolar expansion imaged by optical sectioning microscopy. J. Appl. Physiol. 2007;103(3):1037–1044. doi: 10.1152/japplphysiol.00160.2007. [DOI] [PubMed] [Google Scholar]
- 98.Carney DE, Bredenberg CE, Schiller HJ, et al. The mechanism of lung volume change during mechanical ventilation. Am. J. Respir. Crit. Care Med. 1999;160(5 Pt 1):1697–1702. [PubMed] [Google Scholar]
- 99.Schiller HJ, Steinberg J, Halter J, et al. Alveolar inflation during generation of a quasi-static pressure/volume curve in the acutely injured lung. Crit. Care Med. 2003;31(4):1126–1133. doi: 10.1097/01.CCM.0000059997.90832.29. [DOI] [PubMed] [Google Scholar]
- 100.Halter JM, Steinberg JM, Schiller HJ, et al. Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/ derecruitment. Am. J. Respir. Crit. Care Med. 2003;167(12):1620–1626. doi: 10.1164/rccm.200205-435OC. [DOI] [PubMed] [Google Scholar]
- 101.Steinberg J, Schiller HJ, Halter JM, et al. Tidal volume increases do not affect alveolar mechanics in normal lung but cause alveolar overdistension and exacerbate alveolar instability after sur factant deactivation. Crit. Care Med. 2002;30(12):2675–2683. doi: 10.1097/00003246-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 102.Schiller HJ, McCann UG, 2nd, Carney DE, et al. Altered alveolar mechanics in the acutely injured lung. Crit. Care Med. 2001;29(5):1049–1055. doi: 10.1097/00003246-200105000-00036. [DOI] [PubMed] [Google Scholar]
- 103.Pavone LA, Albert S, Carney D, et al. Injurious mechanical ventilation in the normal lung causes a progressive pathologic change in dynamic alveolar mechanics. Crit. Care. 2007;11(3):R64. doi: 10.1186/cc5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Azeloglu EU, Bhattacharya J, Costa KD. Atomic force microscope elastography reveals phenotypic differences in alveolar cell stiffness. J. Appl. Physiol. 2008;105(2):652–661. doi: 10.1152/japplphysiol.00958.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berrios JC, Schroeder MA, Hubmayr RD. Mechanical properties of alveolar epithelial cells in culture. J. Appl. Physiol. 2001;91(1):65–73. doi: 10.1152/jappl.2001.91.1.65. [DOI] [PubMed] [Google Scholar]
- 106.Banes AJ, Tsuzaki M, Yamamoto J, et al. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem. Cell. Biol. 1995;73(7–8):349–365. doi: 10.1139/o95-043. [DOI] [PubMed] [Google Scholar]
- 107.Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am. Rev. Resp. Dis. 1993;148(5):1194–1203. doi: 10.1164/ajrccm/148.5.1194. [DOI] [PubMed] [Google Scholar]
- 108.Egan EA. Lung inflation, lung solute permeability, and alveolar edema. J. Appl. Physiol. 1982;53(1):121–125. doi: 10.1152/jappl.1982.53.1.121. [DOI] [PubMed] [Google Scholar]
- 109.Gajic O, Lee J, Doerr CH, et al. Ventilator-induced cell wounding and repair in the intact lung. Am. J. Respir. Crit. Care Med. 2003;167(8):1057–1063. doi: 10.1164/rccm.200208-889OC. [DOI] [PubMed] [Google Scholar]
- 110.Yoshigi M, Clark EB, Yost HJ. Quantification of stretch-induced cytoskeletal remodeling in vascular endothelial cells by image processing. Cytometry A. 2003;55(2):109–118. doi: 10.1002/cyto.a.10076. [DOI] [PubMed] [Google Scholar]
- 111.Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys. J. 1994;66(6):2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heidemann SR, Wirtz D. Towards a regional approach to cell mechanics. Trends Cell Biol. 2004;14(4):160–166. doi: 10.1016/j.tcb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Ko KS, McCulloch CA. Partners in protection: interdependence of cytoskeleton and plasma membrane in adaptations to applied forces. J. Membr. Biol. 2000;174(2):85–95. doi: 10.1007/s002320001034. [DOI] [PubMed] [Google Scholar]
- 114.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am. J. Respir. Crit. Care Med. 2002;166(9):1282–1289. doi: 10.1164/rccm.200203-207OC. [DOI] [PubMed] [Google Scholar]
- 115.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Deformation-induced lipid trafficking in alveolar epithelial cells. Am. J. Physiol. 2001;280(5):L938–L946. doi: 10.1152/ajplung.2001.280.5.L938. [DOI] [PubMed] [Google Scholar]
- 116.Oeckler RA, Hubmayr RD, et al. Cell wounding and repair in ventilator injured lungs. Respir. Physiol. Neurobiol. 2008;163(1–3):44–53. doi: 10.1016/j.resp.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chakrabarti S, Kobayashi KS, Flavell RA, et al. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J. Cell Biol. 2003;162(4):543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J. Clin. Invest. 1997;99(5):944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pugin J. Molecular mechanisms of lung cell activation induced by cyclic stretch. Crit. Care Med. 2003;31(4 Suppl.):S200–S206. doi: 10.1097/01.CCM.0000057844.31307.ED. [DOI] [PubMed] [Google Scholar]
- 120.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J. Appl. Physiol. 2000;89(4):1645–1655. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 121.West JB, Tsukimoto K, Mathieu-Costello O, Prediletto R. Stress failure in pulmonary capillaries. J. Appl. Physiol. 1991;70(4):1731–1742. doi: 10.1152/jappl.1991.70.4.1731. [DOI] [PubMed] [Google Scholar]
- 122.Tsukimoto K, Mathieu-Costello O, Prediletto R, Elliott AR, West JB. Ultrastructural appearances of pulmonary capillaries at high transmural pressures. J. Appl. Physiol. 1991;71(2):573–582. doi: 10.1152/jappl.1991.71.2.573. [DOI] [PubMed] [Google Scholar]
- 123.Berg JT, Fu Z, Breen EC, et al. High lung inflation increases mRNA levels of ECM components and growth factors in lung parenchyma. J. Appl. Physiol. 1997;83(1):120–128. doi: 10.1152/jappl.1997.83.1.120. [DOI] [PubMed] [Google Scholar]
- 124.Pugin J, Dunn I, Jolliet P, et al. Activation of human macrophages by mechanical ventilation in vitro. Am. J. Physiol. 1998;275(6 Pt 1):L1040–L1050. doi: 10.1152/ajplung.1998.275.6.L1040. [DOI] [PubMed] [Google Scholar]
- 125.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am. J. Physiol. 1999;277(1 Pt 1):L167–L173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 126.Li LF, Ouyang B, Choukroun G, et al. Stretch-induced IL-8 depends on c-Jun NH2-terminal and nuclear factor-κB-inducing kinases. Am. J. Physiol. 2003;285(2):L464–L475. doi: 10.1152/ajplung.00031.2003. [DOI] [PubMed] [Google Scholar]
- 127.D’Angelo E, Pecchiari M, Della Valle P, Koutsoukou A, Milic-Emili J. Effects of mechanical ventilation at low lung volume on respiratory mechanics and nitric oxide exhalation in normal rabbits. J. Appl. Physiol. 2005;99(2):433–444. doi: 10.1152/japplphysiol.01368.2004. [DOI] [PubMed] [Google Scholar]
- 128.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am. J. Physiol. 2008;295(3):L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hubmayr RD. Ventilator-induced lung injury without biotrauma? J. Appl. Physiol. 2005;99(2):384–385. doi: 10.1152/japplphysiol.00357.2005. [DOI] [PubMed] [Google Scholar]
- 130.Brower RG, Shanholtz CB, Fessler HE, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit. Care Med. 1999;27(8):1492–1498. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 131.Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N. Engl. J. Med. 1998;338(6):355–361. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 132.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 133.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit. Care Med. 2004;32(9):1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 134.Wolthuis EK, Choi G, Dessing MC, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2008;108(1):46–54. doi: 10.1097/01.anes.0000296068.80921.10. [DOI] [PubMed] [Google Scholar]
- 135.Determann RM, Wolthuis EK, Choi G, et al. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. Am. J. Physiol. 2008;294(2):L344–L350. doi: 10.1152/ajplung.00268.2007. [DOI] [PubMed] [Google Scholar]
- 136.Schultz MJ, Haitsma JJ, Slutsky AS, Gajic O, et al. What tidal volumes should be used in patients without acute lung injury? Anesthesiology. 2007;106(6):1226–1231. doi: 10.1097/01.anes.0000267607.25011.e8. [DOI] [PubMed] [Google Scholar]
- 137.Kavanagh BP, Laffey JG. Hypercapnia: permissive and therapeutic. Minerva Anestesiol. 2006;72(6):567–576. [PubMed] [Google Scholar]
- 138.Laffey JG, Engelberts D, Kavanagh BP. Buffering hypercapnic acidosis worsens acute lung injury. Am. J. Respir. Crit. Care Med. 2000;161(1):141–146. doi: 10.1164/ajrccm.161.1.9905080. [DOI] [PubMed] [Google Scholar]
- 139.Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill – too little of a good thing? Lancet. 1999;354(9186):1283–1286. doi: 10.1016/S0140-6736(99)02388-0. [DOI] [PubMed] [Google Scholar]
- 140.Mascheroni D, Kolobow T, Fumagalli R, et al. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med. 1988;15(1):8–14. doi: 10.1007/BF00255628. [DOI] [PubMed] [Google Scholar]
- 141.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 142.Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2007;175(2):160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 143.Grasso S, Fanelli V, Cafarelli A, et al. Effects of high versus low positive end-expiratory pressures in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2005;171(9):1002–1008. doi: 10.1164/rccm.200407-940OC. [DOI] [PubMed] [Google Scholar]
- 144.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 145.Grasso S, Stripoli T, De Michele M, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end-expiratory pressure. Am. J. Respir. Crit. Care Med. 2007;176(8):761–767. doi: 10.1164/rccm.200702-193OC. [DOI] [PubMed] [Google Scholar]