Abstract
In this study we sought to characterize the relationship between several pharmacokinetic (PK) and pharmacodynamic (PD) parameters and virologic responses among HIV/HCV genotype-1 co-infected patients receiving pegylated interferon-alpha-2b (peg-IFN2b) and ribavirin. We also tried to establish the underlying mechanisms that lead to poor SVR rates observed with African Americans (AA) against Caucasians and compared their results with those observed in a cohort of HCV mono-infected patients.
Among our studied population, a viral decline of more than 1.0 log at day 3 combined with viral load of less than 5.0 log IU/ml at day 28 predicted SVR with NPV=100% and PPV=100%. AA had significantly (P<0.01) slower HCV VK as compared to Caucasians. However, peg-IFN2b concentrations and PK parameters, peg-IFN2b max and peg-IFN2b half-life, were similar in both groups and did not predict SVR. Nevertheless, the PD parameter Ec50, estimated from non-linear fitting of the viral kinetics together with peg-IFN2b concentration data, showed that HIV/HCV co-infected AA have lower sensitivity to interferon-alpha thus giving rise to slower viral decline. The combined PK/PD parameter IFNmax/Ec90 was excellent predictor of SVR, thus showing the importance to maintain peg-IFN2b levels above Ec90 to achieve successful treatment. Further studies are needed to evaluate whether these pharmacodynamical predictions are a result of differential host response to peg-IFN2b or other viral factors conferring relative resistance to peg-IFN2b.
INTRODUCTION
Infection by Hepatitis C virus (HCV) is commonly complicated by co-infection with HIV due to shared routes of transmission [1]. HCV co-infection also plays a major role in morbidity and mortality of HIV-infected individuals. HCV co-infection is seen in 15–30% of all HIV-infected individuals in the United States and Western Europe, with more than 250,000 individuals affected in the United States [1, 2]. The introduction of antiretroviral therapy (ART) has improved clinical outcomes in patients infected with HIV [3], however, liver disease has become a leading cause of morbidity and mortality in this population [4–7]. HIV infection is also associated with higher HCV viral levels in serum [8] and a rapid progression of liver disease among those who are also co-infected with HCV [9]. Several serious adverse events associated with ART are exacerbated in HCV/HIV co-infected individuals making it difficult to accomplish adequate virologic control of HIV [10–13]. Although combination therapy with pegylated interferon and ribavirin has resulted in higher cure rates among HCV mono-infected individuals[14, 15], HIV co-infected individuals have only modest responses to therapy [16–21].
Furthermore, several studies of HCV mono-infected individuals have shown that the overall cure rates observed with African Americans are much lower than Caucasians [15, 22]. Nevertheless, the reason for the poor response in African Americans is still not understood and its extent in co-infected patients is not clear. Characterizing the determinants of this decreased response among HIV-HCV co-infected patients, particularly African Americans may thus aid in optimizing therapeutic outcome.
Previously it has been shown that biphasic HCV viral kinetics have predictive ability for SVR among HCV mono-infected individuals [23, 24]. Recently, it has also been shown that monitoring HCV viral kinetics has similar predictive ability among HIV/HCV co-infected individuals as well.[25, 26]. Some studies have also shown the clinical significance of early virologic response (week 4 and week 8 viral responses) in predicting an SVR among HCV/HIV co-infected individuals treated with pegylated interferon and ribavirin [27–29]. Several factors including interferon-alpha pharmacokinetics have been shown to play a major role as the determinants of SVR among HCV monoinfected individuals treated with combination therapy (reviewed in [30]). Recently, a combination of factors including viral kinetics and interferon pharmacokinetic parameters, as defined as pharmacodynamic factors were found to be useful in predicting SVR [31]. Studies in HCV/HIV co-infected patients treated with interferon and ribavirin have demonstrated a relationship between the pharmacodynamics (a combination of viral kinetics and interferon pharmacokinetic parameters) of pegylated interferon-alpha and virologic response [31]. A recent study has also suggested possible differences in pharmacodynamics and viral kinetics between different racial groups [31]. However, these studies have not systematically addressed the nature of HCV viral kinetics and pharmacodynamics in predicting SVR among HIV/HCV co-infected individuals, particularly between African Americans and Caucasians. Furthermore, no comparison was yet attempted between the pharmacodynamics in HCV mono-infected versus co-infected patients
METHODS
Study Design
This was a pilot, prospective, open-label trial performed at the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) at Bethesda, Maryland from 2001 to 2004. Twenty-three HIV/HCV genotype-1 co-infected patients were treated with peginterferon alpha-2b at 1.5 µg/kg subcutaneously every week (Peg-Intron; Schering-Plough, Kenilworth, New Jersey, USA) and ribavirin daily (Rebetol, Schering-Plough, at 400mg Qam and 600mg Qpm for < 75kg, 600mg twice per day for > 75kg) for 48 weeks and followed up for 24 weeks after the end of treatment. All patients were receiving ART. All patients signed informed consent approved by the NIAID institutional review board prior to enrollment in the study.
Study Subjects
Patients were eligible for the study if they were > 18 years of age and had CD4+ T cell counts > 100 cells/mm3 absolute neutrophil counts > 1000 cells/mm3, HCV viral load > 2000 copies/mL, infected with HCV genotype-1, had no prior interferon-alpha based treatment and had histologic evidence of chronic hepatitis C.
Laboratory Studies
Liver chemistry and safety laboratory tests were performed prior to treatment and during each study visit. HCV RNA was measured during all study visits (days 0, 1, 3, 5, 7, 10, week 2, week 3, week 4, week 6, week 8, and then every 4 weeks until Week 48). HCV RNA concentration in plasma was measured by VERSANT HCV RNA 3.0 Assay (Bayer Diagnostics, Puteaux, France). The assay has a quantitation range of 615 to 7.7×106 HCV RNA IU/ml. Sustained virologic response was determined by a negative qualitative RNA PCR at 72 weeks.
Measurement of Serum Peg-interferon-alpha 2b concentrations
Peginterferon alfa-2b concentrations were determined by application of a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) method (Bender MedSystems Diagnostics GmbH, Vienna, Austria). The assay uses a murine anti human interferon-alpha monoclonal antibody adsorbed onto micro wells, which captures interferon alpha present in the samples or controls. A horseradish peroxidase-conjugated monoclonal anti-interferon-alpha antibody binds to captured interferon-alpha. After addition of substrate, IFN concentration is determined colorimetrically. Calibration curves were prepared by plotting the optical density versus the concentration of the standards. Standards from 78 pg/ml to 10000 pg/mL were prepared by diluting peginterferon alfa-2b in normal human serum (CLB, Amsterdam, The Netherlands). Plasma samples were diluted five times and tested on the same plates as the standards. All calibration standards, quality control samples and study samples were analyzed in duplicate. The detection limit of the assay was 100 pg/ml and the assay was linear up to a concentration of 5000 pg/mL. The inter assay coefficients of variation of standards and quality control samples did not exceed 20%. Peg-IFN concentrations were measured at days 0, 1, 3, 5, 7, 10, 14, 21, 28, 42, 56, and 84.
Modeling Viral Kinetics, Pharmacokinetics and Pharmacodynamics
HCV viral kinetics were calculated using a modification of a model previously described (Neumann et al. 1998):
| (Eq 1) |
| (Eq 2) |
| (Eq 3) |
Where T(t) represents target cells, I(t) represents productively infected cells and V(t) free virus. ST represents the source rate of target cells and dT their loss rate constant; β is the de-novo infection rate constant; p is the rate of virion release/production from each infected cell; δ is the infected cells loss rate constant with infected cells half-life of ln(2)/δ; c is the intrinsic clearance rate constant of free virus with free virions half-life of ln(2)/c.
According to Neumann et al. 1998, the anti-viral effect of interferon-alpha is mainly in blocking of viral production from infected cells (with effectiveness 0<ε≤1), thus we approximated that blocking de-novo infection is negligible (η=0) [23].
As a result of weekly peginterferon injections, interferon-alpha has non-constant levels during the week until the next injection, in contrast to the assumption of approximately constant drug level made in Neumann 1998 [23]. Therefore, we modified the model to allow the anti-viral effectiveness, ε, to vary with time as a Hill function of peginterferon concentration, (similar to [31–33]:
| (Eq. 4) |
Where εmax is a constant maximal individual efficacy that can be reached during peginterferon treatment, EC50 is the median individual effective concentration of peginterferon and n is the power of the Hill function representing the second order sensitivity to changes in IFN levels for blocking production. EC90, which better estimates the antiviral sensitivity than EC50, can be derived by:
| (Eq. 5) |
Peg-IFN concentrations were integrated into the model, using a standard pharmacokinetic model:
| (Eq. 6) |
Where the bio-available injected dose and volume of distribution are coupled into the parameter , ka is the absorption rate from the injection site and ke is the clearance rate from the serum circulation. Peg-IFN half-life in serum was calculated from the clearance rate:
| (Eq. 7) |
Note that since ε(t) is not constant in time, the analytical solution of Eqs 1–3 given in Neumann et al (1998) cannot be used for the integrated PK-PD model [23]. Thus, we used the ODE model (Eqs 1–3) together with the PD equation (Eq 4) and the PK model (Eq 6) for parameters' estimation.
Parameter estimates
First phase viral decline was estimated according to the maximal decline during first 3 days of the treatment, in log10 IU/ml. Second phase viral decline slope was calculated by viral decline during second and third weeks of the treatment, as log10 IU/ml per week. Before the treatment, the intrinsic clearance of virions is assumed to be in equilibrium with viral production in time scale of days, as well as equilibrium between infection rate and loss rate of infected cells, therefore giving rise to a steady state. The initial conditions can therefore be estimated. Viral load before the beginning of the treatment was measured, V0, and from the steady state assumptions we obtain I0= cV0/p and T0=s/(d+β*V0).
From the steady-state condition and using the V0 data we can also set β=dcδ/(sp-cδV0). In addition, we had set the next parameters to fixed values p=10 (virus/cell production rate), d=0.01 (day−1, target cells death rate), s=1*e9 (target cells/day production rate) according to the literature ([23, 31, 32]). We verified that changing these parameters over a number of orders of magnitude does not significantly change the results of our estimates.
Using nonlinear regression analysis (Madonna, Berkeley), we began by fitting Peg-IFN PK data for each patient, thus estimating the absorption rate, ka, clearance rate, ke, and absorbed dose, C0. Based on the PK profile, we fitted viral load kinetics by the combined viral dynamic/pharmacodynamic model, individually for each patient, estimating the viral kinetics parameters - viral clearance rate, c, infected cell death rate, δ, and the pharmacodynamic parameters Nhill co-efficient, n, and EC90. From these, we estimated IFNmax/EC90 and days IFN is over EC90.
Statistical Analysis
The non-parametric Mann-Whitney U test was used to test the significance of differences in distribution of continuous pharmacokinetic and pharmacodynamic variables between sub-groups of patients (e.g. race). The non-parametric Spearman test was used to test the significance of correlation between continuous variables. We compared parameters in 4 groups of therapeutic outcome (NR, VB, REL and SVR) by ANOVA test (SPSS 13.0). Significance was assumed at p<0.03. Results are presented as mean ± standard deviation. To evaluate pharmacodynamic and viral kinetic parameters (IFNmax/EC90, days IFN over EC90 and VL decline at day 3) as predictors of treatment outcome, we calculated the positive and negative predictive value.
RESULTS
HCV Viral Kinetics prediction of Therapeutic Response among HIV/HCV Co-infected Patients
When we examined the predictive ability of HCV viral kinetics for therapeutic response,, within each race group there wee differences in viral kinetics as function of the result of treatment. At all time points we examined, non-responders had significantly lower HCV viral load decline when compared to those who experience viral breakthrough, relapse or sustained virologic response (Figure 1B). In addition, absolute HCV viral load on days 3 and 28 was statistically significant lower for patients with SVR compared to patients with relapse (p=0.05). There was no significant difference in viral kinetics between the relapsers and viral breakthrough groups.
Figure 1.
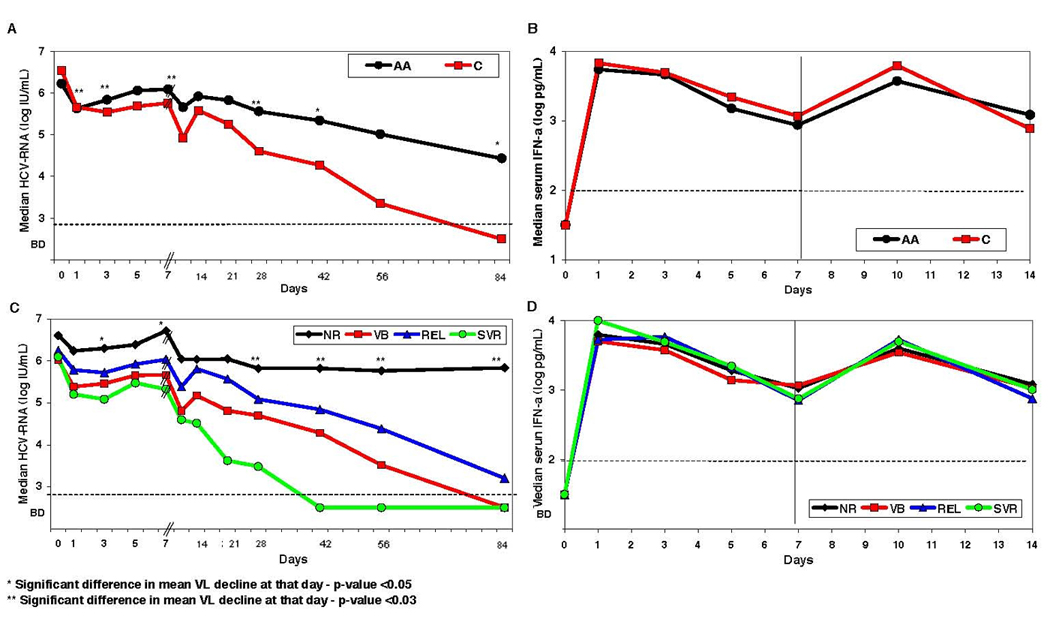
A–D: HCV Viral Kinetics (A and C) and pharmacokinetics (B and D) in HIV/HCV genotype 1 co-infected patients treated with peg-interferon alfa-2b (1.5 µg/kg/wk) and ribavirin (1–1.2 g/d). Median HCV RNA concentrations are plotted (A) as function of race (black circles for African Americans and red squares for Caucasians), and (C) according to the treatment outcome (green circles for SVR, blue triangles for REL, red squares for VB and black diamonds for NR) for the first 84 days of treatment. Note the different time scale in x-axis at days 0–7 versus days 7–84, in order to clarify the transient viral rebound at day 3–7. Interferon-alpha serum concentrations are plotted (B) as function of race (black circles for African Americans and red squares Caucasians), and (D) according to the treatment outcome (green circles for SVR, blue triangles for REL, red squares for VB and black diamonds for NR) for the first 14 days of treatment. Statistical significant differences in viral decline between the groups are marked with * (P<0.05) or ** (P<0.03). Dashed horizontal lines mark the level of HCV RNA and peg-IFN detection.
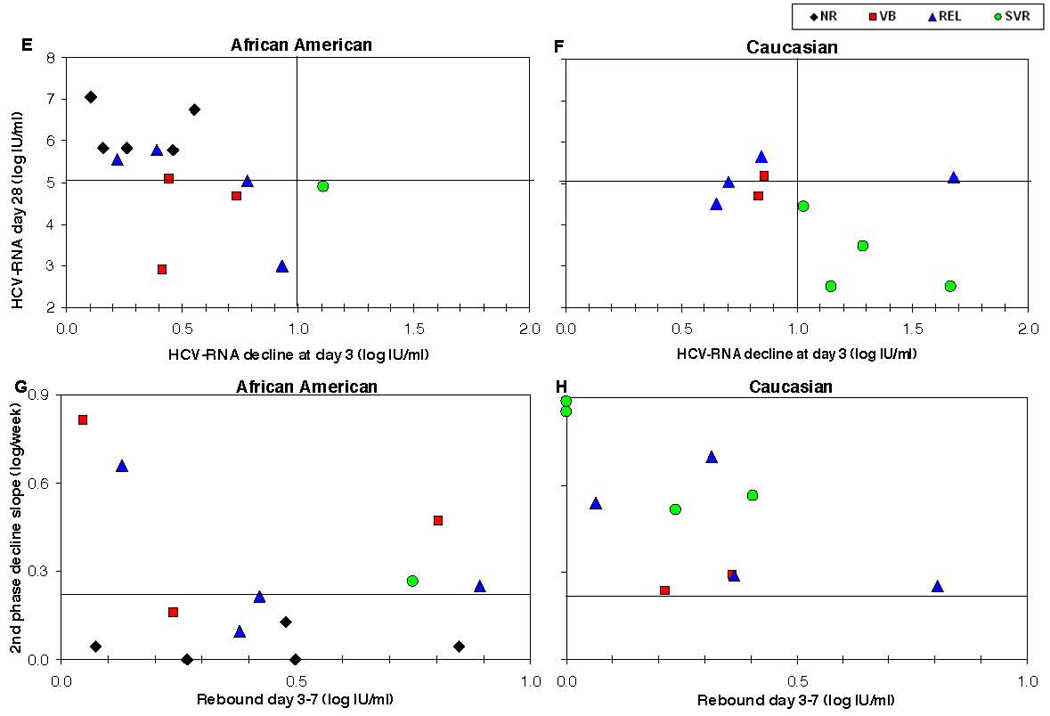
E–H: Correlation between early viral kinetic parameters and therapeutic response in African-American (A and C) and Caucasian (B and D) HIV/HCV co-infected patients. Sustained viral response (SVR, green circles) can be predicted in our study with NPV=100% and PPV=100% by the combination of HCV-RNA decline larger than 1.0 log IU/ml at day 3 (x-axis in A and B) and HCV-RNA load below 5.0 log IU/ml at day 28 (y-axis in A and B). Non-responders (NR, black diamonds) have lower decline in viral load than patients with viral breakthrough (VB, red squares) and patients with relapse (REL, blue triangles). Also, a second phase viral decline slope (y-axis in C and D) slower than 0.3 log/week is predictive of lack of SVR, but the transient rebound at days 3–7 (x-axis in C and D) is not correlated with therapeutic response). In addition, it is possible to observe the faster decline in Caucasians (B and D) as compared to African-Americans (A and C), in both first phase decline at day 3 and second phase decline, as well as viral load at day 28.
In order to better understand the relationship between early HCV kinetics and therapeutic response rates we analyzed the role of several HCV kinetic parameters as predictors of SVR (see Figure 1 E–G). All patients who achieved SVR (both African-Americans and Caucasians), experienced a larger than 1.0 log decline in HCV-RNA at day 3 (p=0.005), as well as an absolute HCV viral load of less than 5.0 log IU/ml at day 28 (p=0.002), suggesting that a rapid decline in viral load earlier during therapy may be predictive of SVR (Figure 1E–1F). The combination of a 1 log viral decline at day 3 with viral load less than 5 log IU/ml at day 28 gives NPV=100% and PPV=100% in our study.
When we examined the slope of the second phase decline (Figure 1G–1H), those patients who achieved an SVR, irrespective of their race, all had a second phase slope faster than 0.3 log/week (NPV=100%, p=0.005), but the PPV for the second phase slope was not as good. The rebound in viral load between days 3 and 7 was not predictive of the therapeutic response. Notably, serum interferon-alpha concentrations at all measured time points, as well as the pharmacokinetic parameters, maximum concentration of Interferon and IFN half-life, were comparable for all therapeutic end-point groups: SVR, REL, VB or NR (Figure 1D, Figure 3 and Table 2).
Figure 3.
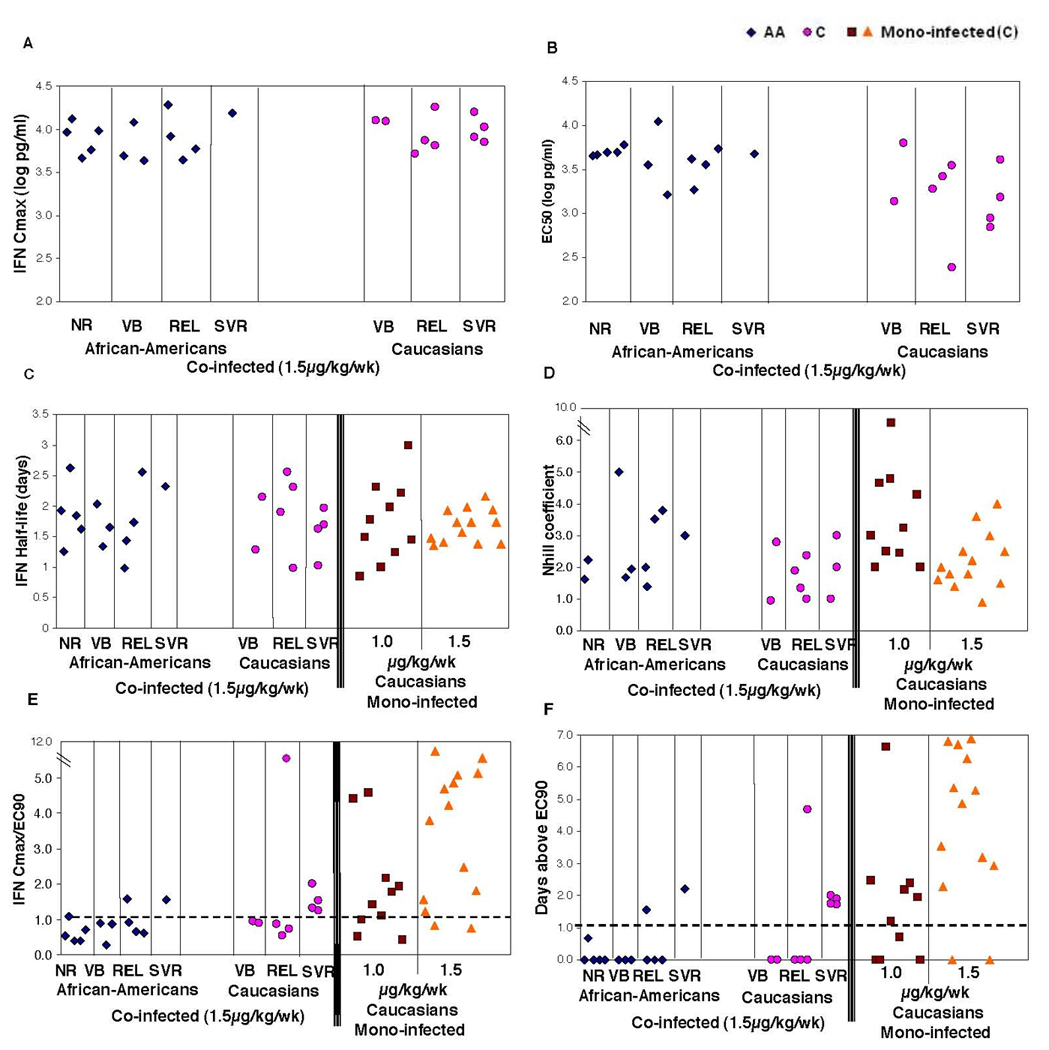
Pharmacokinetic (PK) and pharmacodynamic (PD) parameters for individual patients given as function of race and therapeutic response. No difference in the IFN max concentration (Cmax, A) or in the IFN half-life (B) is observed between any of the groups. Ec50 (B) is significantly (P<0.03) higher for African-Americans as compared to Caucasians but is not predictive of therapeutic response. Nhill (F) is slightly but not significantly higher for African-American vs Caucasians. The combined PK/PD measures – Cmax/Ec90 (E) and days IFN level is above Ec90 during the first week (F) – are higher for Caucasians relative to African-Americans and are predictive of SVR. For comparison sake we also show here the parameters values obtained from our analysis of the data obtained for HCV mono-infected Caucasians treated with pegylated-interferon-a 2b 1.0 mg/kg [35]. Mono-infected patients show better Cmax/Ec90 properties than co-infected patients.
Table 2.
HIV/HCV co-infected patients - summary of viral kinetics, pharmacokinetics and pharmacodynamics parameters per patient.
| Patient No | Response | Initial Viral Load (log IU/ml) |
First phase viral decline (log IU/ml) |
Viral decline at day 7 (log IU/ml) |
Viral decline at day 28 (log IU/ml) |
2nd phase viral decline slope (log/wk) |
Infected cell loss rate (Delta) |
IFN Cmax (log pg/ml) |
IFN half-life (days) |
Ec50 (log pg/ml) |
Nhill co- efficient |
IFN Cmax / EC90 |
Days above EC90 during 1st week (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African-Americans | |||||||||||||
| 4 | NR | 6.80 | 0.55 | 0.05 | 0.10 | 0.00 | 0.01 | 3.99 | 1.63 | 3.78a | ND b | 0.65a | 0.00 |
| 20 | NR | 6.44 | 1.11 | 0.26 | 0.63 | 0.05 | 0.10 | 4.12 | 1.26 | 3.66 | 2.24 | 1.08 | 0.67 |
| 11 | NR | 6.81 | 0.58 | 0.10 | 1.04 | 0.13 | 0.18 | 3.97 | 1.93 | 3.65 | 1.64 | 0.54 | 0.00 |
| 14 | NR | 6.62 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 3.76 | 1.84 | 3.70a | ND b | 0.54a | 0.00 |
| 3 | NR | 6.20 | 0.26 | 0.19 | 0.40 | 0.04 | 0.04 | 3.66 | 2.62 | 3.70a | ND b | 0.31a | 0.00 |
| 7 | VB | 5.80 | 0.42 | 0.37 | 2.88 | 0.81 | 0.66 | 4.08 | 1.34 | 4.05 | 1.68 | 0.29 | 0.00 |
| 15 | VB | 6.00 | 0.72 | 0.00 | 0.92 | 0.47 | 0.19 | 3.70 | 2.04 | 3.56 | 5.00 | 0.89 | 0.00 |
| 9 | VB | 6.05 | 0.74 | 0.50 | 1.36 | 0.16 | 0.26 | 3.64 | 1.65 | 3.21 | 1.95 | 0.87 | 0.00 |
| 17 | REL | 5.90 | 1.04 | 0.15 | 0.85 | 0.25 | 0.13 | 4.29 | 0.99 | 3.62 | 2.00 | 1.57 | 1.56 |
| 23 | REL | 5.82 | 0.93 | 0.80 | 2.81 | 0.66 | 0.42 | 3.92 | 1.44 | 3.27 | 1.40 | 0.92 | 0.00 |
| 1 | REL | 6.22 | 0.44 | 0.01 | 0.68 | 0.21 | 0.13 | 3.64 | 1.73 | 3.55 | 3.53 | 0.67 | 0.00 |
| 6 | REL | 6.23 | 0.39 | 0.01 | 0.44 | 0.09 | 0.07 | 3.77 | 2.55 | 3.73 | 3.80 | 0.62 | 0.00 |
| 13 | SVR | 6.10 | 1.11 | 0.26 | 1.16 | 0.27 | 0.11 | 4.19 | 2.33 | 3.68 | 3.00 | 1.54 | 2.22 |
|
Mean (± SD) |
SVR 8% |
6.23 (0.34) |
0.64 (0.33) |
0.21 (0.24) |
1.02 (0.9) |
0.24 (0.26) |
0.18 (0.18) |
3.90 (0.22) |
1.80 (0.49) |
3.63 (0.21) |
2.62 (1.17) |
0.81 (0.4) |
0.34 (0.72) |
| Caucasians | |||||||||||||
| 22 | VB | 6.29 | 0.85 | 0.64 | 1.60 | 0.24 | 0.22 | 4.10 | 1.28 | 3.14 | 0.96 | 0.93 | 0.00 |
| 16 | VB | 6.68 | 0.86 | 0.50 | 1.53 | 0.29 | 0.30 | 4.10 | 2.15 | 3.80 | 2.80 | 0.90 | 0.00 |
| 18 | REL | 6.25 | 0.65 | 0.59 | 1.75 | 0.54 | 0.16 | 3.81 | 2.31 | 3.54 | 2.36 | 0.74 | 0.00 |
| 12 | REL | 7.10 | 0.94 | 0.57 | 1.44 | 0.29 | 0.18 | 3.72 | 1.90 | 3.28 | 1.90 | 0.87 | 0.00 |
| 2 | REL | 6.90 | 0.71 | 0.39 | 1.91 | 0.70 | 0.05 | 3.87 | 2.56 | 3.42 | 1.35 | 0.54 | 0.00 |
| 10 | REL | 6.70 | 1.68 | 0.87 | 1.58 | 0.25 | 0.01 | 4.26 | 0.99 | 2.39 | 1.00 | 8.28 | 4.69 |
| 19 | SVR | 3.35 | 1.67 | 1.35 | 1.35 | 0.89 | ND c | 4.20 | 1.03 | 2.95 | ND c | 2.00 | 2.00 |
| 5 | SVR | 6.40 | 1.29 | 1.05 | 2.90 | 0.52 | 0.50 | 4.02 | 1.97 | 3.61 | 3.00 | 1.25 | 1.91 |
| 8 | SVR | 5.50 | 1.38 | 1.33 | 3.50 | 0.89 | 0.90 | 3.85 | 1.69 | 3.19 | 2.00 | 1.53 | 1.71 |
| 21 | SVR | 6.84 | 1.03 | 0.63 | 2.38 | 0.56 | 0.16 | 3.91 | 1.63 | 2.84 | 1.00 | 1.31 | 1.75 |
|
Mean (± SD) Difference |
SVR 40% N.S. |
6.20 (1.1) N.S. |
1.10 (0.38) P<0.02 |
0.79 (0.34) P<0.001 |
1.99 (0.71) P<0.01 |
0.52 (0.25) P<0.01 |
0.28 (0.27) N.S. |
3.98 (0.18) N.S. |
1.75 (0.53) N.S. |
3.21 (0.42) p<0.03 |
1.82 (0.79) N.S. |
1.84 (2.3) N.S. |
1.21 (1.53) N.S. |
Minimal estimate due to negligible viral decline.
Cannot be estimated due to negligible viral decline.
Cannot be estimated due to rapid viral decline below detection level.
Pharmacodynamic Parameters as Combination Predictors of Therapeutic Response
In order to better understand the relationship between HCV viral decline and interferon-alpha pharmacokinetics, we have fit the viral decline as function of the drug concentration data. This was achieved by an ODE mathematical model of pharmacokinetics combined with viral dynamics with a linking pharmacodynamical function as described in the Methods. The fit of both the viral kinetics and the pharmacokinetics was good as can be seen in Figure 2 for 4 representative examples from each of the non responders, relapsers, viral breakthrough and SVR groups.
Figure 2.
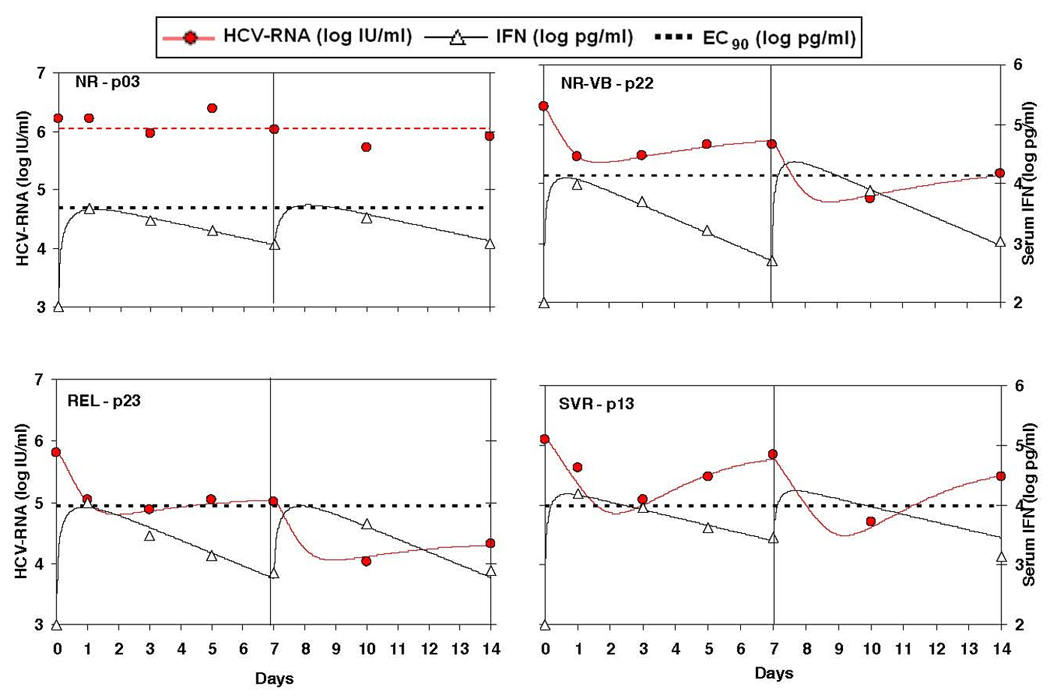
Non-linear fitting of the viral load data (red circles) and the IFN concentration data (white triangles) by a pharmacodynamical model (solid lines, Eqs 1–6) for each individual patient (given here for 4 representative cases each for a different therapeutic response group). Note that the transient rebound in viremia at days 3–7 (and 10–14) are due to the decline in the IFN levels below the Ec90 threshold (dashed horizontal line). This non-linear fitting allowed us to obtain individual estimates for the pharmacodynamical parameters Ec90 (or Ec50) and Nhill.
Consequently, from the non-linear fits, we obtained estimates of the pharmacodynamic parameters Ec50 (or Ec90), describing the sensitivity of the virus to IFN, and the Nhill coefficient describing the second order sensitivity to changes in IFN concentration. Although, as mentioned above, the IFN pharmacokinetics were similar among all different groups of patients (Figures 3A and 3C), we nevertheless find that Ec50 is significantly (P<0.03) lower for Caucasians (3.2 log pg/ml) as compared to African-Americans (3.6), see Figure 3B and Table 2. Thus suggesting that the differences in viral kinetics between the races is not due to abnormal pharmacokinetics of interferon-alpha but due to lower sensitivity of the virus, or most probably virus related host factors, to interferon-alfa in African-Americans. There was no statistically significant difference in the Nhill coefficient between Caucasians and African-Americans (see Table 2 and Figure 3F), although there was a trend for lower Nhill in Caucasians (1.8) as compared to AA (2.6).
As to the effect of pharmacodynamics on therapeutic response, there were no statistical differences in relation to either the Ec50 (or Ec90) or Nhill coefficients among the different therapeutic response groups, non-responders, viral breakthroughs, relapsers. or SVR. Nevertheless, when we calculated the number of days for which the predicted concentration of interferon-alpha (see Figure 2) remained above the Ec90 level, 80% of Caucasians (4/5) and 50% of African Americans (1/2) who had maintained interferon-alpha concentration above EC90 for at least 1 day during the first week achieved SVR (Figure 3D). Moreover, none of the AA (10) or Caucasians (5) patients who did not achieve an IFN Cmax concentration above their own Ec90 level had achieved SVR (NPV=100%), see Figure 3E.
HIV/HCV Co-infected African Americans have Slower Viral Kinetics but Similar Pharmacokinetics when Compared to Caucasians
The baseline characteristics for study subjects enrolled are shown in Table 1. There were 4 patients on abacavir; of these two were non-responders, one was a relapser and the other was SVR. All patients received similar doses of Ribavirin throughout the study and no one was dose-reduced for toxicity. The average liver fibrosis score determined by Ishak scale was 2.3 for African Americans and 2.1 for Caucasians. Moreover, of the three patients who were infected with mixed genotype 1, one each achieved non-response, relapse and SVR. The study subjects were predominantly male and comprised of 10 Caucasians (43%) 12 African-Americans (AA, 52%) and one black Hispanic (5%), who was grouped with the AA for this analysis. Baseline HCV and HIV viral levels and baseline CD4+ T cell counts were similar between the groups. Most of the patients had undetectable HIV viral load. We found no correlation between HIV viral load (only 9 patients had detectable HIV VL at baseline, of which 3 were relapsers and 4 were SVR and only 2 were non-responders) and HCV therapeutic outcome. All patients had a similar HCV viral kinetic pattern although with a different behavior (see Figure 1), with a rapid first phase decline in days 0–3, followed by a transient viral rebound at days 3–7 after each injection (in conjugation with the decline in IFN serum levels at same time), as observed here in the first week as well as in the second week when mid-week samples were available. Consequently, a slower second phase viral decline slope is observed from one week to the next that is consistent as long as can be observed above the limit of detection.
Table 1.
Profile of Patients participated in the study
| Age | Gender | Race | Risk | Base line CD4 (%) | log HIV VL | log HCV VL | HCV Genotype | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | Male | AA | MSM/SN | 980 (31) | 3.42 | 6.38 | 1B | Relapser |
| 2 | 42 | Male | C | MSM | 747 (30) | 1.69 | 6.82 | 1A | Relapser |
| 3 | 48 | Male | AA | SN | 1273 (51) | 1.69 | 6.86 | 1 | Relapser |
| 4 | 39 | Male | C | MSM/SN | 873 (35) | 1.69 | 6.68 | 1 | Relapser |
| 5 | 38 | Male | C | MSM/SN | 697 (38) | 1.69 | 7.35 | 1A | Relapser |
| 6 | 51 | Female | AA | SN | 512 (22) | 1.69 | 5.87 | 1 | Relapser |
| 7 | 62 | Male | C | MSM | 541 (28) | 2.69 | 6.26 | 1 | Relapser |
| 8 | 52 | Male | AA | MSM | 566 (16) | 3.74 | 5.81 | 1A | Relapser |
| 9 | 51 | Male | AA | MSM/SN | 710 (42) | 2.10 | 6.50 | 1A | Non responder |
| 10 | 44 | Female | AA | Heterosexual | 108 (6) | 1.69 | 6.55 | 1A | Non responder |
| 11 | 49 | Male | AA | SN | 392 (19) | 1.69 | 6.18 | 1B | Non responder |
| 12 | 48 | Male | C | SN | 610 (33) | 1.69 | 6.69 | 1 | Non responder |
| 13 | 49 | Male | AA | SN | 394 (22) | 1.69 | 6.66 | 1 | Non responder |
| 14 | 43 | Male | AA | MSM | 907 (31) | 1.69 | 5.92 | 1A | Non responder |
| 15 | 33 | Male | AA | MSM | 409 (22) | 1.69 | 6.72 | 1A | Non responder |
| 16 | 49 | Male | AA | SN | 516 (25) | 1.69 | 5.99 | 1 | Non responder |
| 17 | 35 | Male | C | MSM | 301 (20) | 2.84 | 6.30 | 1 | Non responder |
| 18 | 51 | Male | AA | SN | 301 (24) | 1.69 | 6.45 | 1 | Non responder |
| 19 | 42 | Male | C | MSM | 797 (27) | 3.76 | 6.89 | 1 | SVR |
| 20 | 48 | Male | AA | MSM | 553 (34) | 3.17 | 6.21 | 1A | SVR |
| 21 | 46 | Male | C | MSM | 519 (36) | 1.85 | 3.32 | 1A | SVR |
| 22 | 38 | Male | C | MSM/SN | 602 (31) | 4.52 | 6.40 | 1A | SVR |
| 23 | 48 | Male | C | MSM | 778 (33) | 1.69 | 5.80 | 1A | SVR |
When we examined the HCV viral kinetics among African Americans and Caucasians, the mean decline in HCV viral load at 4 weeks was significantly (p=0.012) smaller (1.01 ± 0.99 log) among African-Americans compared to a the decline seen among Caucasians (1.99 ± 0.71 log) (Figure 1 and Table 2). Also, after 12 weeks of treatment, African-Americans had a mean HCV decline of 1.92 ± 1.2 log which was significantly (p=0.026) smaller than a mean HCV decline of 3.23 ± 0.78 log observed among Caucasians. This translated to a lower SVR rate in AA (1/13, 7.6%) as compared to Caucasians (4/10, 40%).
The significant difference in HCV-RNA decline between Caucasians and African-Americans starts already as early as days 1, 3 and 7 as seen in Figures 1 and 2. First phase HCV viral decline was significantly smaller among African Americans (mean 0.64 ± 0.33 log IU/ml) than Caucasians (mean 1.104 ± 0.377; p=0.015). African Americans also had slower second phase viral kinetics (mean 0.245 ± 0.267 log/week) than Caucasians (mean 0.52± 0.25; p= 0. 006) (Table 2 and Figure 1 E–H). With respect to end of week rebound (days 3–7), there was no significant difference in the magnitude of the rebound or the proportion of patients who experienced a viral rebound between the two groups (P>0.05). However, when we investigated the role of interferon-alpha to explain the differences in HCV viral kinetics, we found that the pharmacokinetics of interferon-alpha, in terms of maximal drug concentration and drug half-life, were comparable between African Americans and Caucasians (Figure 1C, Figure 3 and Table 2). With respect to baseline CD4+ T cell counts between the two groups, African Americans had similar levels compared to that of Caucasians (538 ± 313 cells/ uL vs. 646 ± 168 cells/uL; p=0.27)
Comparison of pharmacokinetics and viral kinetics in HIV/HCV Co-infected patients versus HCV mono-infected patients
The mean reduction from baseline in HCV-RNA after 4 weeks and 12 weeks of treatment for all the HIV/HCV co-infected patients in our study was 1.38 ± 0.92 log IU/ml and 2.65 ± 1.24 log IU/ml respectively. In a similar study treating mono-infected HCV patients with the same treatment regimen [34] the mean decline in HCV viral load at 4 weeks was 1.89 ± 0.577 and a 3 log decline after 8 weeks of treatment, significantly higher than observed with our HCV/HIV co-infected patients. However, the comparison of viral kinetics and treatment response rates between HIV/HCV co-infected patients and HCV mono-infected patients is questionable due to potential differences in the pharmacokinetics that could differ between the populations.
On the other hand, the combined pharmacodynamical parameters, e.g. Cmax/Ec90, should allow a more reliable comparison between the 2 populations, since they normalize the potential differences in pharmacokinetics. In fact, a similar pharmacodynamics analysis was performed by us on HCV mono-infected patients treated with pegylated-interfron-a2b 1.0 mg/kg qw in a study by Bruno et al [35]. Unfortunately, the IFN Cmax concentration and Ec90 coefficient cannot be compared between the studies due to the different dose used. We have nevertheless compared the IFN half-life observed in our patients to that found for HCV mono-infected patients and found no significant difference (see Figure 3C). The Nhill coefficient was higher in mono-infected Caucasian patients (3.7) as compared to the HIV/HCV Caucasian patients in our study (1.8), see Figure 3F. Furthermore, we found a higher proportion (80%) of HCV mono-infected Caucasian patients were able to achieve Cmax levels of interferon-alpha over their individual EC90 as compared to HIV/HCV co-infected Caucasian individuals in our study (50%) (Figure 3E).
DISCUSSION
Our study suggests that African American HIV/HCV co-infected individuals treated with Peg-interferon-alpha-2b and ribavirin have slower HCV viral kinetics compared to co-infected Caucasians due to a lower pharmacodynamical sensitivity, Ec50, to interferon-alpha. Our results also imply that the best predictors of SVR in HIV/HCV co-infected individuals were viral kinetics, as early as the viral decline at day 3 of treatment, and pharmacodynamic characteristics of the patients, in particular the ability to maintain levels of IFN over the EC90 levels for a longer period of time. As a result, African-American individuals experienced a lower rate of SVR when compared to that of Caucasians.
While this depressed response to therapy among African-Americans has been described among HCV mono-infected individuals, the factors, which may contribute to this adverse outcome, are not well characterized. Since HIV/HCV co-infected patient population in the US consist of a higher proportion of African American origin, understanding the underlying mechanisms involved in lower response rates to IFN-based combination therapy for HCV is important to optimize therapy.
In our study, a rapid decline in HCV viral load was seen during first phase (days 1–3) viral kinetics among those who achieved sustained virologic response (in both African-Americans and Caucasians). Additionally, all SVR experienced a significantly faster second phase slope of viral decline compared to NR. These results are consistent with those obtained when HCV monoinfected individuals were treated with Peginterferon-alpha 2b and ribavirin [34]. Furthermore, these results suggest that the mechanisms that determine the rate of HCV viral decline may play a role in determining sustained virologic response in HIV/HCV co-infected individuals. Our results demonstrate the clinical significance of monitoring early viral kinetics in predicting virologic response to therapy with pegylated interferon-apha-2b and ribavirin among HIVHCV co-infected individuals. However, the mechanisms involved in a lack of early virologic response among these individuals were not clearly understood. Although one-log viral decline and day 28 HCV VL offers a 100% NPV and PPV in our study, these results need to be validated in larger clinical trials for clinical utility.
In order to understand the role of achievable levels of interferon alpha in the serum of these individuals on HCV viral decline, we measured the levels of interferon-alpha in serum at frequent intervals after administration of peginterferon alpha 2b and ribavirin. While the pharmacokinetics of interferon-alpha were comparable in all groups, differences were observed in pharmacodynamic properties among those who achieved SVR. Notably, all patients who achieved SVR maintained a serum concentration of interferon-alpha above the EC-90 levels for a longer period of time compared with the non-responder groups, suggesting that maintaining levels above EC90 is essential in achieving sustained virologic response. This finding is further corroborated by the observation that among the HCV mono-infected patients that we examined, a higher proportion was able to maintain levels of interferon-alpha over the EC90 levels consistent with the higher rate of SVR in this group. When different pharmacodynamic parameters were examined among HIV/HCV co-infected and mono-infected individuals, a higher ratio of interferon-alpha maximum concentration to EC90 levels was noted among HCV mono-infected patients and those co-infected patients who achieved an SVR. These results suggest that maintaining levels of interferon alpha concentrations above the levels that is required for maximal antiviral effect is vital in clearance of HCV. Since the maximal antiviral effect of interferon alpha depends on both viral and host factors, the clearance of HCV depends on the sensitivity of virus to respond to interferon and the ability of interferon to induce host response to clear the virus. This study illustrates the fact that antiviral effect in vivo is a collective function of viral sensitivity to interferon-alpha and the ability of interferon-alpha to induce host factors that result in clearance of HCV. Our data suggest that the pharmacodynamic parameters can accurately estimate the antiviral efficacy of interferon-alpha and possibly shed light into the mechanisms for lack of HCV clearance among HIV/HCV co-infected African American individuals.
Our study highlights possible parameters related to viral kinetics and pharmacodynamics, which may explain the difference in treatment efficacy among HIV-HCV co-infected patients and in African-Americans compared with Caucasians. This study extends our understanding about the mechanisms of non-response to IFN based therapy among HIV/HCV co-infected individuals. Based on our findings, it is highly probable that HIV/HCV co-infected African Americans have a higher threshold for maximal antiviral response including which results in lower rates of SVR. Further studies will evaluate whether this higher threshold is as a result of differential host response to IFN or other antiviral factors conferring resistance to IFN. Although one study has used a unique approach based on gene expression profiles of peripheral blood mononuclear cells (PBMC) of HCV mono-infected AA and Caucasians suggesting differential mechanisms underlying lack of SVR, further studies are required among HCV/HIV co-infected individuals to delineate the mechanisms for non-response to IFN [36].
While our small sample size may limit the conclusions that can be drawn, our findings do suggest that rapid viral decline as well as pharmacodynamic parameters should be considered as valuable factors in predicting therapeutic response. Given these findings, future studies investigating the effects of intensification of treatment by utilizing an increased dosing regimen or a conjugated vehicle to maximize the achievable levels of IFN over EC90 levels, may have a significant impact on improving the rates of therapeutic response among co-infected HIV/HCV affected African American individuals.
Acknowledgment
This research was supported by the Intramural Research Program of the NIH, [National Institute of Allergy and Infectious Diseases and National Institute of Digestive Diseases and Kidney]. LR, AUN, and RLD did mathematical modeling and viral kinetic analysis, MM was the nurse specialist for the study, RD performed HCV viral load assays, BL performed pharmacokinetic assays, GC organized data and helped with manuscript, MAP, HM helped with study design and PF, MS and SK were principal investigators for the studies.
Abbreviations
- VB
Viral Breakthrough
- ETR
End of Treatment Responders
- SVR
Sustained Virologic Responders
- NPV
Negative Predictive Value
- PPV
Positive Predictive Value
- ART
Antiretroviral therapy
- MSM
men having sex with men
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organization imply endorsement by the U.S. Government.
Conflict of Interest Statement
None of the other authors have any conflicts of interest to report.
References
- 1.Alter MJ. Hepatitis C virus infection in the United States. Journal of hepatology. 1999;31 Suppl 1:88–91. doi: 10.1016/s0168-8278(99)80381-x. [DOI] [PubMed] [Google Scholar]
- 2.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 3.1997 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus: disease-specific recommendations. USPHS/IDSA Prevention of Opportunistic Infections Working Group. US Public Health Services/Infectious Diseases Society of America. Clin Infect Dis. 1997;25 Suppl 3:S313–S335. [PubMed] [Google Scholar]
- 4.Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550–552. doi: 10.1016/j.jhep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Jain MK, Skiest DJ, Cloud JW, Jain CL, Burns D, Berggren RE. Changes in mortality related to human immunodeficiency virus infection: comparative analysis of inpatient deaths in 1995 and in 1999–2000. Clin Infect Dis. 2003;36:1030–1038. doi: 10.1086/368186. [DOI] [PubMed] [Google Scholar]
- 6.Selik RM, Byers RH, Jr., Dworkin MS. Trends in diseases reported on U.S. death certificates that mentioned HIV infection, 1987–1999. J Acquir Immune Defic Syndr. 2002;29:378–387. doi: 10.1097/00126334-200204010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-Infected Person. Annals of internal medicine. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 8.Eyster ME, Fried MW, Di Bisceglie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994;84:1020–1023. [PubMed] [Google Scholar]
- 9.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 10.Aceti A, Pasquazzi C, Zechini B, De Bac C. Hepatotoxicity development during antiretroviral therapy containing protease inhibitors in patients with HIV: the role of hepatitis B and C virus infection. Journal of acquired immune deficiency syndromes (1999) 2002;29:41–48. doi: 10.1097/00042560-200201010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Sulkowski MS. Hepatotoxicity associated with antiretroviral therapy containing HIV-1 protease inhibitors. Seminars in liver disease. 2003;23:183–194. doi: 10.1055/s-2003-39949. [DOI] [PubMed] [Google Scholar]
- 12.Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS (London, England) 2004;18:2277–2284. doi: 10.1097/00002030-200411190-00008. [DOI] [PubMed] [Google Scholar]
- 13.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology (Baltimore, Md. 2002;35:182–189. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 14.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr., et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 15.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 16.Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. Jama. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 17.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. The New England journal of medicine. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laguno M, Murillas J, Blanco JL, Martinez E, Miquel R, Sanchez-Tapias JM, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS (London, England) 2004;18:F27–F36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Olmeda M, Nunez M, Romero M, Gonzalez J, Castro A, Arribas JR, et al. Pegylated IFN-alpha2b plus ribavirin as therapy for chronic hepatitis C in HIV-infected patients. AIDS (London, England) 2003;17:1023–1028. doi: 10.1097/00002030-200305020-00011. [DOI] [PubMed] [Google Scholar]
- 20.Torriani FJ, Ribeiro RM, Gilbert TL, Schrenk UM, Clauson M, Pacheco DM, Perelson AS. Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) dynamics during HCV treatment in HCV/HIV coinfection. J Infect Dis. 2003;188:1498–1507. doi: 10.1086/379255. [DOI] [PubMed] [Google Scholar]
- 21.Voigt E, Schulz C, Klausen G, Goelz J, Mauss S, Schmutz G, et al. Pegylated interferon alpha-2b plus ribavirin for the treatment of chronic hepatitis C in HIV-coinfected patients. The Journal of infection. 2006;53:36–42. doi: 10.1016/j.jinf.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 24.Zeuzem S, Herrmann E, Lee JH, Fricke J, Neumann AU, Modi M, et al. Viral kinetics in patients with chronic hepatitis C treated with standard or peginterferon alpha2a. Gastroenterology. 2001;120:1438–1447. doi: 10.1053/gast.2001.24006. [DOI] [PubMed] [Google Scholar]
- 25.Shire NJ, Horn PS, Rouster SD, Stanford S, Eyster ME, Sherman KE. HCV kinetics, quasispecies, and clearance in treated HCV-infected and HCV/HIV-1-coinfected patients with hemophilia. Hepatology. 2006;44:1146–1157. doi: 10.1002/hep.21374. [DOI] [PubMed] [Google Scholar]
- 26.Talal AH, Shata MT, Markatou M, Dorante G, Chadburn A, Koch R, et al. Virus dynamics and immune responses during treatment in patients coinfected with hepatitis C and HIV. J Acquir Immune Defic Syndr. 2004;35:103–113. doi: 10.1097/00126334-200402010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Laguno M, Larrousse M, Murillas J, Blanco JL, Leon A, Milinkovic A, et al. Predictive value of early virologic response in HIV/hepatitis C virus-coinfected patients treated with an interferon-based regimen plus ribavirin. Journal of acquired immune deficiency syndromes (1999) 2007;44:174–178. doi: 10.1097/QAI.0b013e31802b812d. [DOI] [PubMed] [Google Scholar]
- 28.Moreno A, Barcena R, Garcia-Garzon S, Muriel A, Quereda C, Moreno L, et al. HCV clearance and treatment outcome in genotype 1 HCV-monoinfected, HIV-coinfected and liver transplanted patients on peg-IFN-alpha-2b/ribavirin. Journal of hepatology. 2005;43:783–790. doi: 10.1016/j.jhep.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Soriano V, Nunez M, Camino N, Maida I, Barreiro P, Romero M, et al. Hepatitis C virus-RNA clearance in HIV-coinfected patients with chronic hepatitis C treated with pegylated interferon plus ribavirin. Antiviral therapy. 2004;9:505–509. [PubMed] [Google Scholar]
- 30.Zeuzem S, Welsch C, Herrmann E. Pharmacokinetics of peginterferons. Semin Liver Dis. 2003;23 Suppl 1:23–28. doi: 10.1055/s-2003-41631. [DOI] [PubMed] [Google Scholar]
- 31.Talal AH, Ribeiro RM, Powers KA, Grace M, Cullen C, Hussain M, et al. Pharmacodynamics of PEG-IFN alpha differentiate HIV/HCV coinfected sustained virological responders from nonresponders. Hepatology. 2006;43:943–953. doi: 10.1002/hep.21136. [DOI] [PubMed] [Google Scholar]
- 32.Perelson AS, Herrmann E, Micol F, Zeuzem S. New kinetic models for the hepatitis C virus. Hepatology (Baltimore, Md. 2005;42:749–754. doi: 10.1002/hep.20882. [DOI] [PubMed] [Google Scholar]
- 33.Powers KA, Dixit NM, Ribeiro RM, Golia P, Talal AH, Perelson AS. Modeling viral and drug kinetics: hepatitis C virus treatment with pegylated interferon alfa-2b. Seminars in liver disease. 2003;23 Suppl 1:13–18. doi: 10.1055/s-2003-41630. [DOI] [PubMed] [Google Scholar]
- 34.Silva M, Poo J, Wagner F, Jackson M, Cutler D, Grace M, et al. A randomised trial to compare the pharmacokinetic, pharmacodynamic, and antiviral effects of peginterferon alfa-2b and peginterferon alfa-2a in patients with chronic hepatitis C (COMPARE) Journal of hepatology. 2006;45:204–213. doi: 10.1016/j.jhep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Bruno R, Sacchi P, Ciappina V, Zochetti C, Patruno S, Maiocchi L, Filice G. Viral dynamics and pharmacokinetics of peginterferon alpha-2a and peginterferon alpha-2b in naive patients with chronic hepatitis c: a randomized, controlled study. Antiviral therapy. 2004;9:491–497. [PubMed] [Google Scholar]
- 36.Luo S, Cassidy W, Jeffers L, Reddy KR, Bruno C, Howell CD. Interferon-stimulated gene expression in black and white hepatitis C patients during peginterferon alfa-2a combination therapy. Clinical gastroenterology and hepatology. 2005;3:499–506. doi: 10.1016/s1542-3565(04)00615-9. [DOI] [PubMed] [Google Scholar]


