Abstract
Chromosome 22q11.2 deletion syndrome (22q11.2DS) is a result of a hemizygotic microdeletion that results in a variety of impairments in children including greater risk for psychiatric ailments in adulthood. We used high-resolution magnetic resonance imaging to accurately quantify the length and, for the first time, volume, of cavum septum pellucidum (CSP) in children aged 7 to 14 years with 22q11.2DS and typically developing (TD) controls. Significantly greater anteroposterior length and greater CSP volumes were found in children with 22q11.2DS compared to controls. Furthermore, the largest CSP were found only in the 22q11.2DS group and with a much higher incidence than previously reported in the literature. Given the significant midline anomalies in the brains of those affected by 22q11.2DS, large CSP may be a biomarker of atypical brain development. The implication of these larger CSP for cognitive and behavioral development is a topic in need of further investigation.
Keywords: genetic disorder, pediatric neuroimaging, brain volumetric methods, velocardiofacial syndrome (VCFS), DiGeorge syndrome
1. Introduction
One of the earliest developmental processes in brain is the formation of the ventricular system and the associated septum separating the two chambers (Galarza et al., 2004). Typically, from the second to the fifth months of embryonic and fetal development, the lateral ventricles elongate and expand laterally away from the midline along with the expansion of the cerebral cortex. The anterior, posterior, and inferior horns as well as the bodies of the lateral ventricles become delineated and larger through the seventh month of development. The septum pellucidum forms a medial wall between the body and the anterior horn of the lateral ventricles. Incomplete fusion of the laminae can manifest as one or two separate cavities: a cavum septum pellucidum (CSP) and a cavum vergae (CV).
Anatomically, the CSP is defined anteriorly by the genu of the corpus callosum, superiorly by the body of the corpus callosum, posteriorly by the anterior limb and pillars of the fornix, and inferiorly by the rostrum of the corpus callosum and the anterior commissure. In prenatal month five, anterior to posterior and superior to inferior consolidation of the corpus callosum begins. The rostrum of the corpus callosum links the genu and the lamina terminalis while the fornix remains relatively stationary and the forceps minor grow to the frontal lobes by month seven. As callosal consolidation occurs, the leaflets of the septum pellucidum are drawn together and towards the lamina terminalis, closing the CSP from rostrum to fornix. Typically, the more anterior CSP is separated from the posterior CV by the anterior columns of the fornix. If the fornix is insufficiently fused with the corpus callosum, the CSP and CV will form into one continuous cavity (Born et al., 2004). In 15% of typically developing (TD) infants, the laminae fuse within one month post-partum, with the majority (85%) showing laminae fusion within six months.
The mechanisms by which the septum pellucidum closes and by which a CSP is maintained are still not completely understood (Shashi et al., 2004) but fusion of the laminae depends on the normal development of surrounding structures, particularly the hippocampus and the corpus callosum (Sarwar, 1989). Galarza and colleagues (2004) suggest several possible modulators of CSP maintenance, including primary atrophy in the form of reduced frontal and temporal lobe volumes and overall hemispheric volume reduction with ventricular enlargement. Support for this mechanism includes the common clinical neuroimaging correlation of CSP with brain anomalies characterized by global decrease in cerebral mass, such as in the pachygyria-lissencephaly spectrum and non-specific microcephaly (personal observation, JP). The proposal that laterally applied pressure, such as would be expected from increasing lobar volumes in normal brain development, can close the CSP is suggested by the observation of transient closure in a premature baby with hydrocephalus resulting from an intraventricular bleed (Needelman et al., 2006). Reduced connectivity (with resultant decreased tractional force exerted in an anterolateral direction) between the genu of the corpus callosum (the anterior border of the septum pellucidum) and the frontal lobes might also result in the maintenance of a CSP anteriorly. Since the corpus callosum also provides the superior attachment of the septum pellucidum, aberrant development leading to more lateral displacement could also result in less transmission of mass from superolateral cortical structures through the midline structures, which could in turn lead to maintenance of a CSP as well. CSP has also been noted with increased frequency in developmentally delayed individuals, suggesting it is commonly related to cerebral dysgenesis of many types (Bodensteiner and Schaefer, 1997). Indeed, it has been suggested that a wide CSP may be a non-specific marker for disturbed development (Bodensteiner and Schaefer, 1990).
We have informally observed enlarged CSP in a large number of children with chromosome 22q11.2 deletion syndrome (22q11.2DS) in the course of collecting and reviewing structural brain imaging data over the last several years. Van Amelsvoort and others (2001) report a CSP/CV incidence rate of 40% in adults with 22q11.2DS versus a matched control group. Consistent with this, Campbell and colleagues (2006) reported a 69% incidence rate of midline anomalies, in particular those of CSP/CV, in children with 22q11.2DS versus 35% of sibling controls. Shashi and others (2004) reported the presence of CSP in 4 of 13 children with 22q11.2DS making it the most common midline brain anomaly in their sample of non-psychotic children with the chromosomal deletion. 22q11.2DS encompasses the phenotypes of DiGeorge (DiGeorge, 1965), velocardiofacial (Shprintzen et al., 1978), and several other syndromes and is caused by hemizygous 1.5-3.0 Mb interstitial deletions on the q11 band of chromosome 22 (Driscoll et al., 1992). 22q11.2DS prevalence is between 1:2000 and 1:5000 live births (Oskarsdottir et al., 2004; Shprintzen, 2008) and is characterized by T-cell abnormalities, cleft palate, heart defects, facial dysmorphisms, and neonatal hypocalcemia (McDonald-McGinn et al., 1999; Antshel et al., 2005). Impairments or delays in language production and comprehension, visuospatial and numerical processing (Moss et al., 1999; Swillen et al., 1999; Bearden et al., 2001; Woodin et al., 2001; Simon et al., 2005a) and executive function (Sobin et al., 2004; Bish et al., 2005) are common, with mean IQ typically ranging from 70 to 85 (Woodin et al., 2001).
Given our recently stated position that midline anomalies of the brain may be at the root of many of the cognitive impairments experienced by those with 22q11.2DS (Simon, 2008), it may be that further understanding of this phenomenon could highlight an important early detectable biomarker for later neurocognitive dysfunction or even help to explain the mechanism by which the later impairments are created. However, to the best of our knowledge, specific quantification of CSP volumetric variability in children with 22q11.2DS has not been undertaken. Furthermore, most analytical studies of CSP have reported measurements of length and width (Born et al., 2004; Filipović et al., 2004), but few groups have examined CSP in volumetric terms (Crippa et al., 2006; Brisch et al., 2007). Since CSP is an anomaly that consists of a complex three-dimensional volume, strictly linear parameters such as length and width are unable to fully characterize its extent. Thus, volumetric measurements provide more accurate information than linear measurements about the true size of the cavum (Crippa et al., 2006).
The purpose of the current study was to both replicate and extend the existing literature on brain dysmorphia with a specific focus on CSP in children with 22q11.2DS. To this end, we used high-resolution magnetic resonance imaging (MRI) to specifically quantify and compare CSP volumes in children with chromosome 22q11.2DS and TD controls of a similar age. We hypothesized that CSP would be present more often in children with 22q11.2DS versus TD children. We also hypothesized that CSP lengths and volumes would be larger in children with 22q11.2DS versus typical controls.
2. Methods
2.1 Participants
Participants were 45 children with 22q11.2DS as confirmed by fluorescence in-situ hybridization (20 male, 25 female; mean age = 10 yr., 5 mo., SD = 1 yr., 11 mo.) and 35 TD children (22 males, 13 females; mean age = 10 yr. 10 months, SD = 2 yr., 2 mo.) recruited at the Children’s Hospital of Philadelphia (CHOP), the Hospital of the University of Pennsylvania (HUP), and at the University of California, Davis (UCD). After describing the study, assent was obtained from the child participants and written consent was obtained from guardians as approved by the Institutional Review Board of CHOP, HUP or UCD., The mean age difference was not significant between groups (t(78) = 0.98, p = 0.33) and the groups did not significantly differ in terms of gender composition (χ2(1) = 2.68, p = 0.10). Children recruited at UCD and HUP were administered the Wechsler Intelligence Scale for Children, version 4 (WISC 4) (Wechsler, 2003) and children recruited at CHOP were administered the WISC 3 (Wechsler, 1991). As this was a sample of convenience, the Perceptual Organization (PO) from the WISC-3 and Perceptual Reasoning Index (PRI) from the WISC-4 as well as the Verbal Comprehension Index (VCI) from the WISC-3 and the VCI from the WISC-4 were treated as the same measures for the purpose of assessing participants’ cognitive function. IQ measures were not available for 6 children with chromosome 22q11.2DS and for 9 TD children.
Mean Full Scale, Verbal (VC or VCI), and Perceptual (PO or PRI) scores were compared between the 22q11.2DS and TD groups with univariate analyses of variance (ANOVA) with gender and age in months as covariates. Full scale [F(1,65) = 128.59, p < 0.0001; 22q11.2DS mean = 76.68, SD = 11.77; TD mean = 110.04, SD = 11.52], Verbal Comprehension [F(1,65) = 75.37, p < 0.0001; 22q11.2DS mean = 81.12, SD = 13.30; TD mean = 111.32, SD = 14.18], and Perceptual Organization/Reasoning [F(1,65) = 104.56, p < 0.0001; 22q11.2DS mean = 76.45, SD = 12.55; TD mean = 108.88, SD = 11.63] scores were lower in the 22q11.2DS group than the TD children. There were no significant effects of gender or age in months on any of the IQ measures [p values > 0.15].
2.2. Brain image acquisition
This was a retrospective study that aimed to take advantage of a relatively large number of brain image datasets collected at three separate institutions. Thirty-five scans (n = 18 22q11.2DS, n = 17 TD) were obtained at the Children’s Hospital at Philadelphia, 18 scans (n = 12 22q11.2DS, n = 6 TD) at the Hospital of the University of Pennsylvania, and 27 scans (n = 15 22q11.2DS, n = 12 TD) at the University of California, Davis Medical Center. We acknowledge that there are limitations inherent with combining multi-site data sets. Optimally, we would have intraclass correlation coefficients for a subgroup of participants or for a single phantom but given the retrospective nature of the study but this was not possible.
Three-dimensional high-resolution T1-weighted structural scans were acquired using magnetic resonance imaging (MRI) at three separate institutions. All three sites utilized a magnetization prepared rapid gradient echo (MP-RAGE) sequence for image acquisition. At the Children’s Hospital of Philadelphia, a 1.5-T Siemens MAGNETOM Vision scanner (Siemens Medical Solutions, Erlangen, Germany) was used with the following parameters: repetition time (TR) = 1.97 s, echo time (TE) = 4 s, flip angle = 12°, number of excitations = 1, matrix size = 256 × 256, slice thickness = 1.0 mm, 160 sagittal slices, in-plane resolution = 1 × 1 mm. At the Hospital of the University of Pennsylvania, a 3.0-T Siemens MAGNETOM Vision scanner (Siemens Medical Solutions, Erlangen, Germany) was used with the following parameters: TR = 1.62 s, TE = 3.87 s, flip angle = 15°, number of excitations = 1, matrix size = 192 × 256, slice thickness = 1.0 mm, 160 sagittal slices, in-plane resolution = 1 × 1 mm. At the University of California, Davis, a 3.0-T Siemens MAGNETOM Vision scanner (Siemens Medical Solutions, Erlangen, Germany) was used with the following parameters: TR = 1.82 s, TE = 2.93 s, flip angle = 12°, number of excitations = 1, matrix size = 256 × 256, slice thickness = 1 mm, 160 sagittal slices, in-plane resolution = 1 × 1 mm.
2.3 Image analysis
Images were transferred to a workstation for preprocessing and analysis. Image sets were aligned to the anterior commissure and posterior commissure (AC-PC) plane using Analyze 7.5 software (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN). All image tracing was performed by Y.Q. and V.N. with a high degree of inter-rater reliability on measures of CSP length (r2 = 0.99, p < 0.0001) and volume (r2 = 0.916, p < 0.0001).
Anteroposterior CSP length can be measured by summing the number of consecutive 1 mm slices through the coronal plane where a CSP is visible (Nopoulos et al., 2000). For example, a CSP that could be seen across 6 coronal slices would be approximately 6 mm long. It should be noted that accurate determination of length might be limited by acquisition of partial image volumes using this method. Participants’ anteroposterior CSP length was determined using this method and accordingly, participants where categorized into five groups following Nopoulos et al. (2000). Categories were defined in terms of the number of 1 mm slices (i.e. length) visible in the coronal plane. A CSP evident from 1 to 4 slices was labeled Normal; 5 to 6 slices as Borderline; 7 to 10 slices as Abnormal. If no CSP was evident, these cases where were labeled as None.
Next, to gain a more accurate measure of CSP volume, the visible CSP area was traced on each 1 mm slice through the coronal plane according to predefined boundaries using Multitracer (UCLA Laboratory of Neuroimaging, Los Angeles, CA). We operationally defined the boundaries of the CSP as follows: anteriorly by the genu of the corpus callosum, superiorly by the body of the corpus callosum, posteriorly by the anterior limb and pillars of the fornix, and inferiorly by the rostrum of the corpus callosum and the anterior commissure. When viewed in the coronal plane, the cavum is triangular with its base at the corpus callosum (Born et al., 2004). Voxels within the tracing boundaries for each slice were then summed to calculate volume in mm3.
3. Results
3.1 Prevalence of CSP
3.1.1 Prevalence by group and gender
The proportion children with CSP of any size was greater in children with 22q11.2DS (38 out of 45; 84.4%; χ2 (1) = 7.36, p = 0.007) versus TD controls (20 out of 35; 57.1%). The overall proportion of having any CSP did not statistically differ (χ2 (1) = 0.076, p = 0.78) between males (31 out of 42; 73.8%) and females (27 out of 38; 71.1%). Within each group, the proportion of males to females with any CSP did not differ from expectation in the 22q11.2DS (χ2 (1) = 0.85, p = 0.44) or TD (χ2 (1) = 0.57, p = 0.72) groups. There was also no effect of gender on severity of CSP within the 22q11.2DS (χ2 (5) = 2.13, p = 0.71) or TD (χ2 (3) = 2.04, p = 0.56) groups.
3.1.2 Prevalence by classification
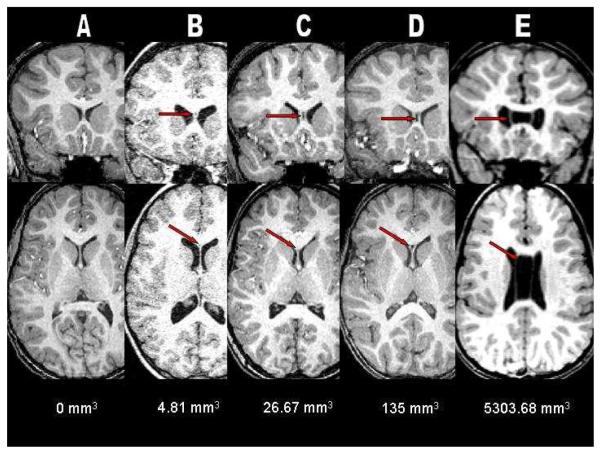
In the course of analyzing the images, we extended the classification scheme by Nopoulous et al. (2000) to include an Extreme category of CSP that is twice the anteroposterior length of the upper value of the Borderline classification (i.e., 12 mm or greater). These extremely large CSP (ranging from 12 to as much as 58 mm in length) occurred in 11 out of 45 of the 22q11.2DS group. Given that this was seen in 24.4% of our 22q11.2DS sample, we felt that this length range, albeit extreme, should be considered as a new category of CSP rather than as a set of outliers whose relevance is not considered further. In fact, the percentage of children with 22q11.2DS in the Extreme category was second only to those in the Normal category. Example images of each classification category are shown in Figure 1.
Figure 1.
A composite of coronal and axial T1-weighted anatomical magnetic resonance (MR) images illustrating an example from each of the five CSP classifications used in this study: A = None, B = Normal, C = Borderline, D = Abnormal, and E = Extreme. Note the complete nonfusion of the septal leaflets in E showing the presence and severe enlargement of cavum septum pellucidum/cavum verga in a patient with chromosome 22q11.2 deletion syndrome.
The proportion and frequencies of individuals that fell into the None, Normal, Borderline, Abnormal, and Extreme categories are shown in Table 1. There was a significant relationship between group and overall CSP classification (χ2 (4) = 13.00, p = 0.009). There was no detectable CSP in 15.6% (7/45) of the 22q11.2DS group compared to 42.9% (15/35) of the TD group, and it was statistically more likely that a TD participant would fall into the None CSP classification (χ2 (1) = 4.90, p = 0.027). We detected the Normal variant CSP in 44.4% (20/45) of the 22q11.2DS children and in 37.1% (13/35) of the TD group and the groups did not differ in the likelihood of this classification (χ2 (1) = 0.025, p = 0.87). Borderline CSP was present in 4.4% (2/45) of the 22q11.2DS and 11.4% (4/35) of the TD group, and this proportion was also not significantly different (χ2 (1) = 1.38, p = 0.24) between groups. Children with 22q11.2DS were no more likely to possess an Abnormal CSP (χ2 (1) = 0.71, p = 0.40) than TD children with 11.1% (5/45) of the 22q11.2DS and 8.6% (3/35) of the TD group meeting the Abnormal threshold. None of the TD children met the Extreme CSP criteria whereas 24.4% (11/45) of the children with 22q11.2DS had CSP 12 mm or longer indicating a statistically significant difference in the likelihood of CSP classification in the Extreme category (χ2 (1) = 7.42, p = 0.06).
Table 1.
The frequency of CSP in children with chromosome 22q11.2 deletion syndrome (22q11.2DS) and typically developing (TD) controls according to anteroposterior length classification scheme.
| Groups | None | Normal | Borderline | Abnormal | Extreme |
|---|---|---|---|---|---|
| (0 mm) | (1-4 mm) | (5-6 mm) | (7-10 mm ) | (12+ mm) | |
| N (% Group) | N (% Group) | N (% Group) | N (% Group) | N (% Group) | |
| 22q11.2DS (N = 45) | 7 (15.6) | 20 (44.4) | 2 (4.4) | 5 (11.1) | 11(24.4) |
| Male (N = 20) | 2 (4.4) | 10 (22.2) | 2 (4.4) | 1 (2.2) | 5 (11.1) |
| Female (N =25) | 5 (11.1) | 10 (22.2) | 0 (0.0) | 4 (8.9) | 6 (13.3) |
|
| |||||
| TD (N = 35) | 15 (42.9) | 13 (37.1) | 4 (11.4) | 3 (8.6) | 0 (0.0) |
| Male (N = 22) | 9 (25.7) | 10 (28.6) | 1 (2.9) | 2 (5.6) | 0 (0.0) |
| Female (N = 13) | 6 (17.1) | 3 (8.6) | 3 (8.6) | 1 (2.9) | 0 (0.0) |
3.2 CSP morphometry
3.2.1 CSP Lengths
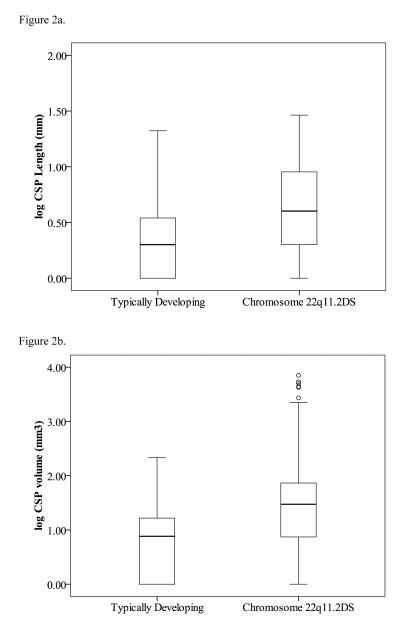
The anteroposterior CSP lengths (i.e. sum of 1 mm slices where CSP was evident) were compared between TD children (M = 2.31; SD = 2.60) and those with 22q11.2DS (M = 12.58; SD = 18.64) controlling for gender, age, and scanner site using analysis of variance (ANOVA) revealing a significant main effect of Group [F(1) = 10.11, p = 0.002]. There were no significant effects of age [F(1) = 0.58, p = 0.45], gender [F(1) = 0.011, p = 0.92], or scanner site [F(1) = 0.12, p = 0.73]. Box plots of mean log-transformed CSP length by group are illustrated in Figure 2a.
Figures 2a and 2b.
Boxplots of log-transformed CSP lengths (2a) and volumes (2b) for children with and without chromosome 22q11.2 deletion syndrome (22q11.2DS). Data points are labeled according to the CSP classification scheme. Note the number of CSP volumes that fell beyond the fourth quartile of the boxplot in the group of children with 22q11.2DS.
3.2.2 CSP volumes
Mean CSP volumes were calculated for children with 22q11.2DS (N = 45; M = 916.13 mm3; SD = 2413.68) and TD (N = 35; M = 12.83 mm3; SD = 19.21) children. Since CSP volumes ranged from 0 to 13054 mm3 and the distribution of volumes was extremely negatively skewed [skewness = 4.89, SE = 0.27], parametric analyses were not possible. Thus, we log transformed CSP values. Cava were larger in the 22q11.2DS group than in the TD group. Univariate ANOVA comparing logCSP volumes across Groups (22q11.2DS, TD) while controlling for gender, age in months, and scanner site revealed a significant main effect of Group [F(1, 75) = 14.93, p < 0.001]. There were no significant effects of age [F(1,75) = 1.30, p = 0.26], gender [F(1, 75) = 0.10, p = 0.75], or scanner site [F(1, 75) = 1.17, p = 0.28] in relation to CSP volume. Univariate ANOVA comparing overall log-transformed volumes across scanner sites did not indicate a statistically significant difference [F(2,79) = 1.16, p = 0.32]. Box plots of mean log-transformed CSP volume by group are illustrated in Figure 2b.
3.3 CSP and IQ
Potential relationships between IQ measures and log-transformed CSP volumes in each group was investigated using partial correlation controlling for gender and age in months. For the 22q11.2DS group, log-transformed CSP volume was not correlated with FSIQ [r2(32) = 0.07, p = 0.68], VC/VCI [r2(32) = 0.04, p = 0.82], or PO/PRI [r2(32) = 0.07, p = 0.69]. For the TD children, log-transformed CSP volume was not correlated with FSIQ [r2(44) = −0.07, p = 0.64], VC/VCI [r2(44) = −0.10, p = 0.54], or PO/PRI [r2(44) = 0.16, p = 0.92]. We also compared mean FSIQ, VIQ, and PIQ scores between the 11 children with 22q11.2DS who had extremely large CSP and the 34 other children with 22q11.2DS. No significant differences on any of the IQ measures was evident between the Extreme CSP subgroup and the other children with 22q11.2DS even when accounting for unequal group sizes using Welsh’s modification of Student’s t-test.
4. Discussion
Here, we report a detailed analysis of cavum septum pellucidum in children with chromosome 22q11.2 deletion syndrome in comparison to typically developing controls including, for the first time, volumetric CSP measurements. Consistent with the only previous report on CSP incidence in children with 22q11.2DS (Campbell et al., 2006), we found that atypical CSP length occurs with significantly greater frequency in children with 22q11.2DS than in TD controls. Based on the CSP classification scheme we used, children with 22q11.2DS and TD children did not differ in incidence of Normal or Borderline CSP variants. CSP in children with 22q11.2DS were more likely to be classified as Abnormal or Extreme than TD children.
Mean CSP volumes were significantly larger in children with 22q11.2DS. However, it is noted that 11 children with very large CSP volumes ranging from 66 to 13054 mm3 accounted for much of the groups’ differences. The very large CSP volumes were, however, only seen in the 22q11.2DS group. It is difficult to say whether these very large CSP volumes are representative of a very broad continuous distribution as only the most extreme volume (i.e. 13054 mm3) falls outside the upper quartile when log-transformed. However, given that 11 out of 45 or 24.4 percent of children with 22q11.2DS in our study fell under the Extreme CSP designation, we feel that these are clearly not outliers. Possibly, these 8 children represent a distinct variant within the 22q11.2DS neurocognitive endophenotype. Nevertheless, hemizygous deletion of chromosome 22q11.2 is neither necessary nor sufficient to explain the presence of even an unusually large CSP given that 7 TD children had Borderline or Abnormal CSP and 7 children with 22q11.2DS had no measurable CSP in the present sample.
How might CSP be maintained in children with 22q11.2DS?
Likely contributing factors include a generally decreased cerebral cortical mass which does not exert enough medial force combined with diminished downward pull exerted by smaller temporal lobe structures such as the hippocampus. Overall brain volume in children with 22q11.2DS is between 8 and 11% smaller than TD children, with reductions in both white and grey matter (Eliez et al., 2000; Kates et al., 2001; Simon et al., 2005b; Campbell et al., 2006) and specific volume reductions in the hippocampus (Eliez et al., 2001; DeBoer et al., 2007) and thalamus (Bish et al., 2004). Whole brain tissue volume reductions are accompanied by enlarged lateral ventricles (Simon et al., 2005b) and enlarged ventricles have been associated with displacement of the corpus callosum (Simon et al., 2005b). The hippocampus is attached to the fornix through the alveus, which is pulled backward and downward with hippocampal development. Lower mass from the developing hippocampi and proximal temporal lobe structures (e.g. amygdalae, parahippocampal gyri) may not be great enough to adequately pull the inferior leaflets of the septum pellucidum into apposition via a tractional effect on the fornix. The lower average masses of temporal lobe structures may thus help explain the greater incidence of CSP in children with 22q11.2DS versus TD children.
What are the functional implications of enlarged CSP?
It is well known that 22q11.2DS confers one of the highest genetic risks of developing schizophrenia (Murphy, 1999), a condition in which CSP has been extensively investigated (e.g. Kwon et al., 1998; Nopoulos et al., 2000; Filipović et al., 2004). Given the high incidence of CSP in patients with schizophrenia, surveying abnormalities in brain morphometry in childhood may help to characterize a “high-risk” endophenotype in children with 22q11.2DS. However, it has been shown that the presence of a large CSP does not distinguish patients with schizophrenia who have (van Amelsvoort et al., 2004) or who do not have (Flashman et al., 2007; Takahashi et al., 2007) 22q11.2DS from TD controls. Large CSP was, however, associated with volume reductions in left parahippocampal gyrus and bilateral amygdalae in participants with schizophrenia but not in healthy controls (Takahashi et al., 2007). In females with schizotypal personality disorder, both larger CSP and smaller hippocampi have been noted (Dickey et al., 2007) further suggesting a contributing role of abnormal hippocampal development in the development and maintenance of CSP. While there is a diathesis conferred via this chromosomal deletion, the likelihood of psychosis in young adulthood is clearly modulated by a multitude of variables. Standardized IQ measures can be a proxy measure of global intellectual function but we did not find any relationships between CSP incidence or size and intellectual ability. Thus, it appears that enlarged CSP, while reflecting anomalous midline brain development, does not necessarily reflect poorer brain function.
In summary, we used a slice-by-slice image tracing approach to accurately characterize CSP volumetric variability in a fairly large sample of children with 22q11.2DS. We replicated the single previous report of high rates of CSP in children affected by the deletion. We extended existing knowledge by showing that the most common category of abnormal CSP in children with 22q11.2DS is the new category that we labeled Extreme. CSP of this size have typically been set aside as outliers in other studies and, while that label may be appropriate for populations where relatively small enlargements occur, we present the view that it is not valid in the case of 22q11.2DS. The presence of an enlarged CSP was not predictive of intellectual ability in the current study and may be more indicative of anomalous brain development rather than predictive of functional impairment. Nevertheless, 22q11.2DS confers a 25-30 fold increased risk for developing schizophrenia versus the general population and the brains of children with 22q11.2DS and adults with schizophrenia share a number of morphological similarities, including enlarged CSP. Further studies into the maintenance of CSP and development of proximal brain structures are an important avenue for future investigation especially in older children who may be displaying symptoms prodromal to conversion to a psychotic disorder.
Acknowledgements
The authors would like to thank the children who took part in this study and their families. We would also like to thank the 22q and You Center at Children’s Hospital of Philadelphia for their support of the early phases of this study. Funding for this project was through grant NIHR01HD42974 awarded to Tony J. Simon. There are no conflicts of interest, financial or otherwise, for any of the authors.
Abbreviations
- 22q11.2DS
chromosome 22q11.2 deletion syndrome
- CSP
cavum septum pellucidum
- TD
typically developing
- IQ
intelligence quotient
- CV
cavum vergae
- SD
standard deviation
- WISC
Wechsler Intelligence Scale for Children
- FSIQ
full scale intelligence quotient
- PO
perceptual organization
- PRI
perceptual reasoning index
- VCI
verbal comprehension index
- CHOP
Children’s Hospital of Philadelphia
- HUP
Hospital of the University of Pennsylvania
- UCD
University of California, Davis
- χ2
chi-square
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antshel KM, Kates WR, Roizen N, Fremont W, Shprintzen RJ. 22q11.2 deletion syndrome: Genetics, neuroanatomy and cognitive/behavioral features. Neuropsychology, Development, and Cognition. Section C, Child Neuropsychology. 2005;11:5–19. doi: 10.1080/09297040590911185. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, Emannuel B, Cannon TD. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. Journal of Clinical and Experimental Neuropsychology. 2001;23:447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Bish JP, Ferrante S, McDonald-McGinn D, Zackai E, Simon TJ. Maladaptive conflict monitoring as evidence for executive dysfunction in children with chromosome 22q11.2 deletion syndrome. Developmental Science. 2005;8:36–43. doi: 10.1111/j.1467-7687.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- Bish JP, Nguyen V, Ding L, Ferrante S, Simon TJ. Thalamic reductions in children with chromosome 22q11.2 deletion syndrome. Neuroreport. 2004;15:1413–1415. doi: 10.1097/01.wnr.0000129855.50780.85. [DOI] [PubMed] [Google Scholar]
- Bodensteiner JB, Schaefer GB. Wide cavum septum pellucidum: a marker of disturbed brain development. Pediatric Neurology. 1990;6:391–394. doi: 10.1016/0887-8994(90)90007-n. [DOI] [PubMed] [Google Scholar]
- Bodensteiner JB, Schaefer GB. Dementia pugilistica and cavum septi pellucidi: born to box? Sports Medicine. 1997;24:361–365. doi: 10.2165/00007256-199724060-00002. [DOI] [PubMed] [Google Scholar]
- Born CM, Meisenzahl EM, Frodl T, Pfluger T, Reiser M, Möller HJ, Leinsinger GL. The septum pellucidum and its variants. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:295–302. doi: 10.1007/s00406-004-0496-z. [DOI] [PubMed] [Google Scholar]
- Brisch R, Bernstein H-G, Krell D, Stauch R, Trübner K, Dobrowolny H, Kropf S, Bielau H, Bogerts B. Volumetric analysis of septal region in schizophrenia and affective disorder. European Archives of Psychiatry & Clinical Neuroscience. 2007;257:140–148. doi: 10.1007/s00406-006-0697-8. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, Ng V, van Amelsvoort T, Chitnis X, Cutter W, Murphy DG, Murphy KC. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–1228. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- Crippa J.A.d.S., Zuardi AW, Busatto GF, Sanches RF, Santos AC, Araújo D, Amaro E, Hallak JEC, Ng V, McGuire PK. Cavum septum pellucidum and adhesio interthalamica in schizophrenia: an MRI study. European Psychiatry. 2006;21:291–299. doi: 10.1016/j.eurpsy.2005.09.010. [DOI] [PubMed] [Google Scholar]
- DeBoer T, Wu Z, Lee A, Simon T. Hippocampal volume reduction in children with chromosome 22q11.2 deletion syndrome is associated with cognitive impairment. Behavioral and Brain Functions. 2007;3:54. doi: 10.1186/1744-9081-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Xu ML, Seidman LJ, Voglmaier MM, Niznikiewicz MA, Connor E, Shenton ME. MRI abnormalities of the hippocampus and cavum septi pellucidi in females with schizotypal personality disorder. Schizophrenia Research. 2007;89:49–58. doi: 10.1016/j.schres.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGeorge A. A new concept of the cellular basis of immunity. Journal of Pediatrics. 1965;67:907. [Google Scholar]
- Driscoll DA, Budarf ML, Emanuel BS. A genetic etiology for DiGeorge syndrome: consistent deletions and microdeletions of 22q11. American Journal of Human Genetics. 1992;50:924–933. [PMC free article] [PubMed] [Google Scholar]
- Eliez S, Blasey CM, Schmitt EJ, White CD, Hu D, Reiss AL. Velocardiofacial syndrome: Are structural changes in the temporal and mesial temporal regions related to schizophrenia. American Journal of Psychiatry. 2001;158:447–453. doi: 10.1176/appi.ajp.158.3.447. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with Velocardiofacial Syndrome: A Volumetric Study. American Journal of Psychiatry. 2000;157:409–415. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Filipović B, Prostran M, Ilanković N, Filipović B. Predictive potential of cavum septi pellucidi (CSP) in schizophrenics, alcoholics and persons with past head trauma. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:228–230. doi: 10.1007/s00406-004-0483-4. [DOI] [PubMed] [Google Scholar]
- Flashman LA, Roth RM, Pixley HS, Cleavinger HB, McAllister TW, Vidaver R, Saykin AJ. Cavum septum pellucidum in schizophrenia: Clinical and neuropsychological correlates. Psychiatry Research: Neuroimaging. 2007;154:147–155. doi: 10.1016/j.pscychresns.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarza M, Merlo AB, Ingratta A, Albanese EF, Albanese AM. Cavum Septum Pellucidum and Its Increased Prevalence in Schizophrenia: A Neuroembryological Classification. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:41–46. doi: 10.1176/jnp.16.1.41. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Jabs EW, Rutberg J, Murphy AM, Grados M, Geraghty M, Kaufmann WE, Pearlson GD. Regional cortical white matter reductions in velocardiofacial syndrome: A volumetric MRI analysis. Biological Psychiatry. 2001;49:677–684. doi: 10.1016/s0006-3223(00)01002-7. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Shenton ME, Hirayasu Y, Salisbury DF, Fischer IA, Dickey CC, Yurgelun-Todd D, Tohen M, Kikinis R, Jolesz FA, McCarley RW. MRI Study of Cavum Septi Pellucidi in Schizophrenia, Affective Disorder, and Schizotypal Personality Disorder. Am J Psychiatry. 1998;155:509–515. doi: 10.1176/ajp.155.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Kirschner R, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, Moss EM, Solot CB, Wang PP, Jacobs I, Handler S, Knightly C, Heher K, Wilson M, Ming JE, Grace K, Driscoll DA, Pasquariello P, Randall P, LaRossa D, Emanuel BS, Zackai EH. The Philadelphia Story: The 22q11.2 deletion: Report on 250 patients. Genetic Counselling. 1999;10:11–24. [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, Emmanuel BS, Zackai EH, Wang PP. Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. Journal of Pediatrics. 1999;134:193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- Needelman H, Schroeder B, Sweney M, Schmidt J, Bodensteiner JB, Schaefer B. Ontogeny and Physiology of the Cavum Septum Pellucidum in Premature Infants. Journal of Child Neurology. 2006;21:298–301. doi: 10.1177/08830738060210041501. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Krie A, Andreasen NC. Enlarged Cavum Septi Pellucidi in Patients With Schizophrenia: Clinical and Cognitive Correlates. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:344–349. doi: 10.1176/jnp.12.3.344. [DOI] [PubMed] [Google Scholar]
- Oskarsdottir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Archives of Disease in Childhood. 2004;89:148–151. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M. The septum pellucidum: normal and abnormal. American Journal of Neuroradiology. 1989;10:989–1005. [PMC free article] [PubMed] [Google Scholar]
- Shashi V, Muddasani S, Santos CC, Berry MN, Kwapil TR, Lewandowski E, Keshavan MS. Abnormalities of the corpus callosum in nonpsychotic children with chromosome 22q11 deletion syndrome. Neuroimage. 2004;21:1399–1406. doi: 10.1016/j.neuroimage.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Shprintzen R,J. Velo-cardio-facial syndrome: 30 Years of study. Developmental Disabilities Research Reviews. 2008;14:3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate Journal. 1978;15:56–62. [PubMed] [Google Scholar]
- Simon TJ. A New Account of the Neurocognitive Foundations of Impairments in Space, Time and Number Processing in Children with Chromosome 22q11.2 Deletion Syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2008 doi: 10.1002/ddrr.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, McDonald-McGinn DM, Zackai E. Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex. 2005a;41:145–155. doi: 10.1016/s0010-9452(08)70889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. NeuroImage. 2005b;25:169–180. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Blundell M, Anyane-Yeboa K, Karayiorgou M. Networks of attention in children with the 22q11 deletion syndrome. Developmental Neuropsychology. 2004;26:611–626. doi: 10.1207/s15326942dn2602_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Vandeputte L, Cracco J, Maes B, Ghesquiere P, Devriendt K, Fryns JP. Neuropsychological, learning and psychosocial profile of primary school aged children with the velo-cardio-facial syndrome (22q11 deletion): evidence for a nonverbal learning disability? Child Neuropsychology. 1999;5:230–241. doi: 10.1076/0929-7049(199912)05:04;1-R;FT230. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Hagino H, Niu L, Zhou S-Y, Nakamura K, Tanino R, Kawasaki Y, Seto H, Kurachi M. Prevalence of large cavum septi pellucidi and its relation to the medial temporal lobe structures in schizophrenia spectrum. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:1235–1241. doi: 10.1016/j.pnpbp.2007.04.019. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort T, Daly E, Henry J, Robertson D, Ng V, Owen M, Murphy KC, Murphy DGM. Brain anatomy in adults with velocardiofacial syndrome with and without schizophrenia: Preliminary results of a structural magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:1085–1096. doi: 10.1001/archpsyc.61.11.1085. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort T, Daly E, Robertson D, Suckling J, Ng V, Critchley H, Owen MJ, Henry J, Murphy KC, Murphy DGM. Structural brain abnormalities associated with the deletion at chromosome 22q11. British Journal of Psychiatry. 2001;178:412–419. doi: 10.1192/bjp.178.5.412. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children III. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. WISC-IV technical and interpretive manual. The Psychological Corporation; San Antonio: 2003. [Google Scholar]
- Woodin MF, Wang PP, Aleman D, McDonald-McGinn DM, Zackai EH, Moss EM. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genetics in Medicine. 2001;3:34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]