Abstract
In the past, we viewed lack of response to asthma medications as a rare event. Based on recent studies, we now expect significant variation in treatment response for all asthma medications. However, little information is available about methods to predict favorable treatment response. Research conducted in the National Heart, Lung and Blood Institute (NHLBI) Asthma Clinical Research Network (ACRN) and the NHLBI Childhood Asthma Research and Education (CARE) Network verified this variability in response to several long-term control medications, specifically inhaled corticosteroids (ICS) and leukotriene receptor antagonists (LTRA), in adults and children with mild to moderate persistent asthma. The networks also identified potential methods to utilize patient characteristics, such as age and allergic status, and biomarkers, such as bronchodilator response, exhaled nitric oxide and urinary leukotrienes, to help predict response to ICS and LTRA and to determine which of the two treatments may be more effective in individual patients. This information now assists the clinician in personalizing asthma treatment at the time of initiating long-term control therapy.
Keywords: Asthma, treatment response, inhaled corticosteroids, leukotriene receptor antagonists, leukotriene modifiers, β-adrenergic agonists
Introduction
Great care has been applied to developing asthma guidelines that provide principles of asthma management to achieve optimal control (1, 2). The general tendency in preparing guidelines is to focus on the evidence-based conclusions of studies that often portray the average response derived from specific studies. However, clinicians recognize that there is great variability in patient response to treatment, that includes significant or obvious response, minimal response that raises question about patient adherence or inadequate effect, or even an adverse response to a medication that either prompts a reduction of the dose or discontinuation of the treatment.
Clinician guidance for treatment selection is usually derived from information reported in clinical trials and summarized in guidelines. This information is then applied in a medication trial and the clinical response is assessed from the patient report on symptom control. The clinician generally assumes that the patient took the medication including the appropriate technique and those questions are reviewed if there is an inadequate response.
There are several weaknesses in this current process of medication selection that can result in a delay in the patient receiving the best medication to obtain the optimal treatment response. Currently, most clinical trials place emphasis on reporting the average response to the medication in relation to either placebo or another treatment. Although information is provided on the degree of variability in response, most trials do not take the next step to understand this variability in response. If information regarding the variability of response to asthma treatment were available along with methods to predict treatment response, the clinician could select the medication most likely to provide a significant favorable response and thus reduce the time for achieving maximal symptom control.
This review will focus on the current understanding in variability of response to two long-term controller medications, inhaled corticosteroids (ICS) and oral leukotriene receptor antagonists (LTRA), that has been developed through a series of clinical trials. These specific trials were conducted in the NHLBI Asthma Clinical Research Network (ACRN) and the NHLBI Childhood Asthma Research and Education (CARE) Network, and the results indicate how this variability in response can be associated with patient characteristics and biomarkers that can be used to help predict treatment response in the clinical setting.
Measuring Response in a Clinical Trial: The Components of Treatment Response
In clinical studies, response to asthma therapy is traditionally assessed by measuring improvement in pulmonary function, especially in studies evaluating the efficacy of bronchodilator or quick-relief medications. However, as we turned our attention in the NIH asthma networks to measuring response to long-term controllers, especially ICS, it became apparent that improvement in pulmonary function response is highly variable and therefore, we need to look at the variability of other measures of clinical response including: reduction in symptoms and need for rescue therapy, increase in asthma control days, prevention of acute exacerbations and nocturnal exacerbations, reduction in airway hyper-responsiveness, and improvement in quality of life. All of these are components of asthma that are co-existent but not necessarily co-dependent features.
Therefore, careful studies must be done to identify a medication’s predominant effect on asthma management and also to evaluate other measures of response in order to obtain a more comprehensive picture of the medication’s potential benefits in asthma management and aide the clinician in medication selection.
Table 1 summarizes information that could be applied in a comprehensive clinical trial to measure the response to individual medications. Most of these measures are easily obtained in adult patients but some, for example spirometry and markers of inflammation are more difficult to obtain in children, especially young children.
Table 1.
Measures of a treatment response
| Clinical measures: |
Symptoms
|
Exacerbations
|
| Asthma control and quality of life questionnaires |
| Pulmonary function measures: |
| Percent change in FEV1 for short-term studies |
| Change in percent predicted FEV1 for long-term studies, especially in children |
| Post-bronchodilator FEV1 |
| Other objective measures of treatment response: |
Challenge techniques
|
Airway inflammation
|
Distal lung or small airways
|
Analyzing Variability in Treatment Response
One of the major questions during the 1990s centered on the comparative efficacy of ICS. Looking at traditional change in FEV1 values as a measure of clinical response to ICS therapy, it was very difficult to assess a dose-response relationship for any individual ICS, let alone compare two ICSs to each other. One of the initial studies conducted by the NHLBI ACRN was to carefully examine the dose-response relationship for available ICSs. Realizing the complexity of such an investigation, a trial called the “Measuring Inhaled Corticosteroid Efficacy (MICE)” study was designed to evaluate multiple treatment response parameters in order to decide which one would be most applicable to comparing response among available ICSs and then to examine the associated systemic effect with that response by measuring overnight plasma cortisol suppression (3).
Briefly, this study was a randomized, parallel, open-label, multicenter trial conducted in adults with mild to moderate asthma that examined the benefit-risk ratio of two ICSs (3). Benefit was assessed by measuring the improvement in FEV1 and methacholine FEV1 PC20 and risk was assessed by overnight plasma cortisol suppression. Thirty subjects were randomized to either beclomethasone dipropionate (BDP) 168, 672 and 1,344 mcg/day (n=15) or fluticasone propionate (FP) 88, 352, and 704 mcg/day, both administered by means of a metered dose inhaler (MDI) with chlorofluorocarbon propellant via spacer, in 3 consecutive 6-week intervals. This 18-week treatment course was followed by 3 weeks of FP dry powder inhaler (DPI) at 2,000 mcg/day.
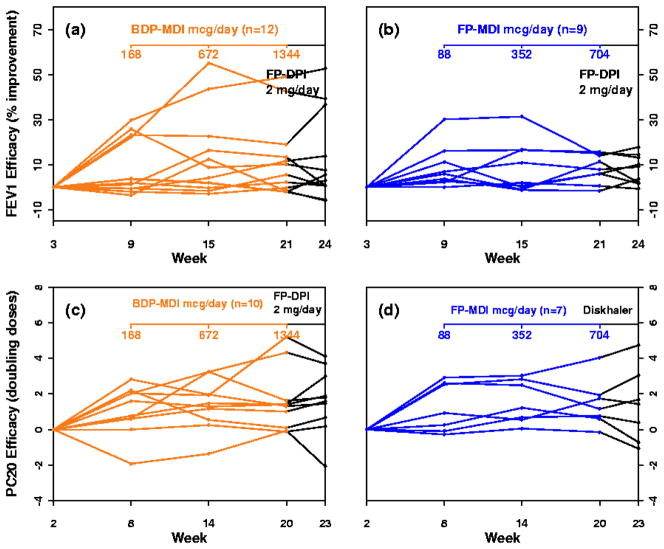
Several key observations were made from this relatively small but important study. First, maximal FEV1 response occurred with the low-dose for FP-MDI and the medium dose for BDP-MDI and was not further increased by treatment with high dose FP-DPI. The same pattern was seen with methacholine FEV1 PC20. Both BDP-MDI and FP-MDI caused dose-dependent cortisol suppression. Therefore, high-dose ICS therapy did not significantly increase the efficacy for these two measures but did increase the systemic effect measure, overnight cortisol suppression. Second, significant intersubject variability in response occurred with both FP-MDI and BDP-MDI (Fig. 1). Good (>15%) FEV1 response, in contrast to poor (<5%) FEV1 response, was found to be associated with high exhaled nitric oxide, high bronchodilator response, and a low FEV1/FVC ratio before treatment. In contrast, excellent (>3 doubling dilutions) improvement in methacholine FEV1 PC20, in contrast to poor (<1 doubling dilution) improvement, was found to be associated with high sputum eosinophil levels and older age of onset of asthma.
Figure 1.
Variability in FEV1 response (a and b) and methacholine PC20 response (c and d) for BDP-MDI (a and c) and FP-MDI (b and d) for the 3 study doses and the 2-mg/day dose of FP via Diskhaler. Only subjects with complete data sets are included. [Reprinted with permission from Szefler SJ et al. J Allergy and Clin Immunol 2002;109:410–8.]
This study alerted us to the fact that treatment response was indeed highly variable even in adults with mild to moderate persistent asthma and that there was some potential to relate treatment response to patient characteristics and biomarkers. The next set of questions were addressed in subsequent NHLBI asthma network studies including but not limited to the following: Could the patient characteristics and biomarkers identified in the small population included in the MICE study predict treatment response in a larger adult population of mild to moderate asthmatics? Does this variability in response to ICS noted in the MICE study also occur in children and are the potential predictors of response similar in adults and children? Does this variability in response also occur with other long-term controllers, such as LTRA? Can biomarkers and patient characteristics also relate to other measures of long-term controller treatment response?
Identifying Predictors of Response
Adults
Based on the provocative results of the MICE study, the ACRN developed a larger follow-up study, the “Predicting Response to Inhaled Corticosteroid Efficacy (PRICE)” trial, to evaluate potential biomarkers associated with short-term (6-week) response to ICS with subsequent evaluation of responders and non-responders to asthma control over a longer interval (16 additional weeks) (4).
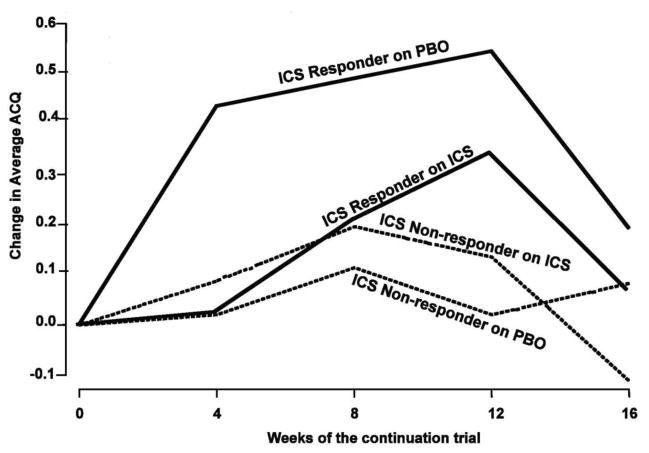
In the PRICE study, 83 subjects with asthma who were off steroid therapy were enrolled in this multicenter ACRN study. Biomarkers and asthma characteristics were evaluated as associated features of ICS response over a 6-week trial for changes in FEV 1 and methacholine FEV1 PC20. After this 6-week ICS treatment period, an additional 4-month trial evaluated asthma control. The key findings in this study included the following observations. First, although multiple baseline features had significant correlations with improvements for short-term ICS success, the only strong correlations (r ≥ ± 0.6) were albuterol reversibility (r ≥ ± 0.83; P<.001), FEV1/FVC (r ≥ ± −0.75; P<.001), and FEV1% predicted (r ≥ ± −0.71; P<.001). Second, for the non-responders (<5% FEV1 improvement), asthma control remained unchanged whether ICS were continued or were substituted with a placebo (Fig. 2). Third, the good short-term responders (>5% improvement in FEV1) maintained asthma control longer-term only if maintained on ICS. In this larger validation study, eNO was not identified as an associated biomarker of ICS pulmonary response in adults with mild to moderate persistent asthma as noted in the MICE study.
Figure 2.
Asthma control as measured by the ACQ over the 16-week ICS or placebo (PBO) continuation trial. The groups are categorized on the basis of the FEV1 results of the previous 6-week ICS trial: nonresponders ≤5% improvement on ICS; responders >5% improvement on ICS. The only significant within-group difference occurred between PBO and ICS responder groups (P = .007). [Reprinted with permission from Martin RJ et al. J Allergy and Clin Immunol 2007;119:73–80]. Note that a clinically significant change in the ACQ is considered to be 0.5 units.
Therefore, we concluded from the PRICE study that short-term response to ICS with regard to FEV1 improvement predicts long-term control. The clinical implications for these findings were that (1) the decision to use long-term ICS could be based on a short-term trial and (2) that different therapeutic strategies would need to be established for non-responders.
Children
As the NHLBI CARE Network was established, one of the first studies conducted sought to examine the variability in treatment response to ICS and LTRA in children in the “Characterizing Response to Leukotriene Receptor Antagonist and Inhaled Corticosteroids (CLIC)” study (5). A main driving force to conduct the CLIC study was the experience derived from the NHLBI MICE study (3) in identifying variable response to ICS in adult asthmatics as well as other reports showing variable response to LTRA in adult asthmatics (6). It was decided to conduct a cross-over study with these two treatments to determine whether response to ICS and LTRA were concordant for individuals or whether asthmatic patients who do not respond to one medication have a response to the other medication.
For the CLIC study, children 6 to 17 years of age with mild-to-moderate persistent asthma. These children were required to have asthma symptoms or rescue bronchodilator use on average of 3 or more days per week during the previous 4 weeks and improvement in FEV1 of 12% or greater after maximal bronchodilation or methacholine PC20 of 12.5 mg/ml. The children were randomized to one of 2 crossover sequences, including 8 weeks of an ICS, fluticasone propionate [FP] (100 mcg twice daily), and 8 weeks of an LTRA, montelukast (5 – 10 mg nightly depending on age), in a multicenter, double-masked, 18-week trial. In the primary analysis, response was assessed on the basis of improvement in FEV1 (7.5% or greater) and assessed for relationships to baseline asthma phenotype-associated biomarkers.
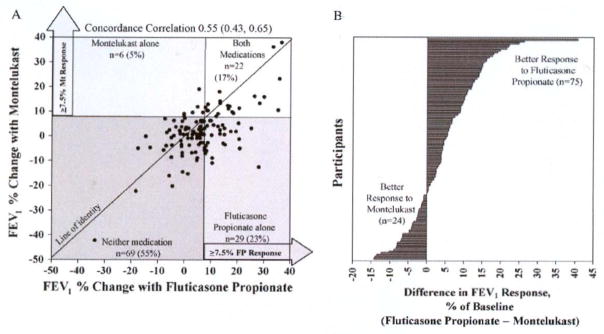
Once again, several key observations were reported in this study. First, 17% of the 126 participants responded to both medications, 23% responded to fluticasone alone, 5 % responded to montelukast alone, and 55% responded to neither medication (Fig. 3A). Second, compared with those who responded to neither medication, favorable response to FP alone was associated with higher levels of exhaled nitric oxide (eNO), total eosinophil counts, levels of serum IgE, and levels of serum eosinophilic cationic protein (ECP) and lower levels of methacholine FEV1 PC20 and pulmonary function; favorable response to montelukast alone was associated with younger age, higher urinary leukotrienes, and shorter duration of disease. Third, greater differential response to FP over montelukast (Fig. 3B) was associated with higher bronchodilator use, bronchodilator response, eNO levels, and ECP levels, and lower methacholine FEV1 PC20.
Figure 3.
A, Variability of response and differential response to fluticasone and montelukast, as measured by change in FEV1. Four regions show categories of response, defining a favorable response as 7.5% or greater. FP, Fluticasone propionate; Mt, montelukast. The line of identity is designated, with patients favoring montelukast falling above the line, and those favoring fluticasone falling below the line. The concordance correlation with 95% CIs is displayed. B, Difference in FEV1 response between fluticasone propionate and montelukast for individual participants. Each line designates a single participant. [Reprinted with permission from Szefler SJ et al. J Allergy and Clin Immunol 2005;115:233–42].
Therefore, we concluded form the CLIC study that response to FP and montelukast vary considerably in children with mild to moderate asthma. Also, children with low pulmonary function or high levels of markers associated with allergic inflammation should receive ICS therapy.
A subsequent expanded analysis of the CLIC study focused on the assessment of intraindividual and interindividual response profiles and predictors of response to evaluate clinical, pulmonary and inflammatory responses to ICS and LTRA. Zeiger et al (7) from the CARE Network derived several new clinical findings from the CLIC study. First, improvements in most clinical asthma control measures occurred with both controllers. Second, clinical outcomes, pulmonary responses, and inflammatory biomarkers, specifically eNO, improved significantly more with FP than with montelukast treatment. Third, eNO was both a predictor of asthma control days (ACD) and a response indicator in discriminating the difference in ACD response between FP and montelukast. In other words, the higher the participant’s eNO, the greater the response to FP as compared to montelukast in that individual participant.
The clinical implication of this subsequent analysis was that more favorable clinical, pulmonary, and inflammatory response to an ICS than to an LTRA provide pediatric-based group evidence to support ICSs as the preferred first-line therapy for mild-to-moderate persistent asthma in children. Also, eNO, as an associated feature of favorable response to ICS, might help to identify individual children not receiving controller therapy who achieve a greater improvement in ACDs with an ICS compared with an LTRA.
The findings of the CLIC study prompted an evaluation of a subsequent study conducted in the CARE Network, entitled the “Pediatric Asthma Controller Trial (PACT)” to identify phenotypic characteristics having potential value for identifying the difference in treatment responses between twice daily FP and once-daily montelukast (8, 9). The PACT trial compared the effectiveness of 3 regimens in achieving asthma control in a total of 285 children, ages 6 to 14 years, with mild-to-moderate persistent asthma on the basis of symptoms, and with an FEV1 ≥ 80% predicted and methacholine FEV1 PC20 ≤ 12.5 mg/ml (8). The children were randomized to 1 of 3 double-blind 48-week treatments: fluticasone 100 mcg twice daily (fluticasone monotherapy; Flovent Diskus; Glaxo Smith Kline, Research Triangle Park, NC), fluticasone 100 mcg/salmeterol 50 mcg (Advair Diskus; Glaxo Smith Kline) in the morning and salmeterol 50 mcg (Serevent Diskus; Glaxo Smith Kline) in the evening (PACT combination), and montelukast 5 mg (Singulair; Merck, Whitehouse Station, NJ) in the evening. Outcomes included asthma control days (primary outcome), exacerbations, quality of life measurements, and pulmonary function.
The general conclusions derived from the PACT study included the following observations (8). Both fluticasone monotherapy and the PACT combination achieved greater improvements in asthma control days than montelukast. However, fluticasone monotherapy was superior to the PACT combination in achieving greater improvement in other dimensions of asthma control. A subsequent analysis of the PACT study conducted by Knuffman et al (9) in the CARE Network sought to identify phenotypic characteristics associated with response to FP monotherapy and montelukast.
Data from the PACT trial were assessed with multivariate analysis. Outcomes included the change in ACDs, FEV1, peak expiratory flow, and time to first asthma exacerbation measured over the one-year treatment period. Key findings in this secondary analysis included the following observations. First, a history of parental asthma best predicted the expected treatment benefit with FP compared with montelukast in terms of gain in ACDs and time to first exacerbation. Second, increased baseline eNO levels were associated with the differential treatment response for FP regarding the gain in ACDs. Third, prior ICS use and low methacholine FEV1 PC20 values were associated with the benefit of FP over montelukast regarding the time to first exacerbation. Fourth, no phenotypic characteristic was associated with treatment benefits for montelukast over fluticasone for either outcome.
Therefore, the clinical implications of the PACT secondary analysis are that in children with mild-to-moderate persistent asthma, a parenteral history of asthma, airway hyperresponsiveness, or increased measures of inflammation might have a superior response to an ICS over an LTRA. These findings helped to corroborate and extend the initial observations obtained in the CLIC study and the two studies combined suggested that eNO could be a better predictor of ICS treatment response in children as compared to the experience in our adult study (4).
Young children
It is also desirable to determine whether patient characteristics could be associated with response to long-term controller medications in young children. To evaluate this question, a secondary analysis was performed on the “Prevention of Asthma in Kids (PEAK)” trial conducted in the CARE Network (10). The PEAK study was a randomized trial conducted in 285 participants two to three years of age with a positive asthma predictive index who were assigned to treatment with either FP at a dose of 88 mcg twice daily or masked placebo for two years, followed by a one-year period without study medication. The primary outcome for this study was the proportion of episode-free days during the one-year observation period. The primary conclusion of this study was that in preschool children at high risk for asthma, two years of ICS therapy did not change the development of asthma symptoms or lung function during the third, treatment-free year. However, during the treatment period, as compared to placebo, use of ICS was associated with a greater proportion of episode-free days and a lower rate of exacerbations and of supplementary use of controller medication.
Following the primary outcome report, a secondary analysis was conducted by Bacharier et al (11) in the CARE Network to determine if demographic and atopic features were associated with a favorable response to ICS in the PEAK trial. This analysis revealed two important findings. First, significantly greater improvement with FP than placebo in terms of episode-free days occurred among boys, white subjects, participants with an Emergency Department (ED) visit or hospitalization within the past year, and those who experienced more symptomatic days at baseline. Second, children with aeroallergen sensitization experienced greater benefits in terms of oral corticosteroid use, urgent care and ED visits, and use of supplemental controller medications. The clinical implication of this analysis was that preschool children at high risk for asthma experience favorable responses to ICS therapy, particularly when indicators of greater disease severity and aeroallergen sensitization are present.
Smokers
Another important predictor of long-term controller response in adults was identified in the ACRN “Smoking Modulates Outcomes of Glucocorticoid Therapy (SMOG)” study (12). The purpose of this study was to determine if the response to an ICS or a LTRA is attenuated in individuals with asthma who smoke. This was a multicenter, placebo-controlled, double-blind, crossover trial, with 44 nonsmokers and 39 light smokers with mild asthma who were assigned randomly to treatment twice daily with inhaled BDP and once daily with oral montelukast. The primary outcome was change in pre-bronchodilator FEV1 in smokers versus nonsmokers. This study provided the following insights into treatment response. First, in subjects with mild asthma who smoke, the response to ICS is attenuated, suggesting that adjustments to standard therapy may be required to attain asthma control. Second, greater improvement in some outcomes in smokers treated with montelukast, specifically an increase in A.M. peak flow 12.6 L/min (p = 0.002), but not in nonsmokers, suggested that LTRA may be useful in this group of patients. The SMOG study confirmed the presence of corticosteroid insensitivity in patients with asthma who smoke, and suggested that leukotriene modifiers may be beneficial for these patients. However, large prospective studies are required to determine whether leukotriene modifiers can be recommended for managing asthma in patients who smoke.
Clinical Implications of Variability in Treatment Response
The results of the studies conducted in the ACRN and CARE Networks provide new insights into individualizing asthma therapy at the Step 2 level of the asthma guidelines. The Step 2 treatment step recognizes ICS as the preferred medication with other long-term controllers, including leukotriene modifiers, identified as alternative treatments (1, 2). Despite evidence from multiple studies showing that ICS are indeed the preferred long-term controller for adults and children with asthma, patients, parents and clinicians are still concerned regarding the long-term safety of ICS and require additional information to support the use of ICS as the preferred treatment for the specific individual being treated. They may also be concerned about reported adverse effects associated with the LTRA. This is indeed the heart of “individualized” or “personalized” medicine. Therefore, is there specific information that the clinician can use for either reinforcing the need for the preferred medication, in this case ICS, or that an alternative medication, for example LTRA, may provide an equivalent or perhaps even better response to the preferred treatment?
The observations from the NIH asthma network studies help to address this question for Step 2 therapy and therefore set the stage for incorporating personalized medicine into the asthma guidelines. Studies are currently being conducted in ACRN and the CARE Network to understand individual treatment response at the Step 3 level and could be the subject of a future review as this information becomes available.
The following conclusions can be derived from the studies summarized in this review (Text Box). First, the clinician should expect variability in treatment response to clinical, physiologic and inflammatory indicators of response for ICS and LTRA in all patients including children and adults. Second, low pulmonary function, bronchodilator response and airway hyperresponsiveness are associated with a favorable response to ICS therapy in all patients and indicators of allergic inflammation, specifically eNO, are associated with a favorable response to ICS in children but not adults. Third, younger age, higher urinary leukotrienes, and shorter duration of disease are linked to a favorable response to LTRA in children. Fourth, particularly in children, a greater pulmonary response to ICS over LTRA is observed with higher eNO as well as higher bronchodilator use, and lower methacholine PC20 and pulmonary function. Fifth, adult patients with asthma who smoke may have a favorable response to LTRA over ICS.
Text box
What do we know?
There are multiple measures of response that can be considered in determining whether a medication can be effective in an individual patient
Variability in response to all forms of treatment should be anticipated
Patient characteristics and biomarkers can be used to predict treatment response and to select medications
What is still unknown?
Biomarkers that measure disease activity associated with ongoing loss of pulmonary function
Genetic features that could predict who will get severe asthma and how to target therapy to manage and even prevent severe asthma
Whether genetics and/or biomarkers can be used to predict treatment response for reducing asthma exacerbations
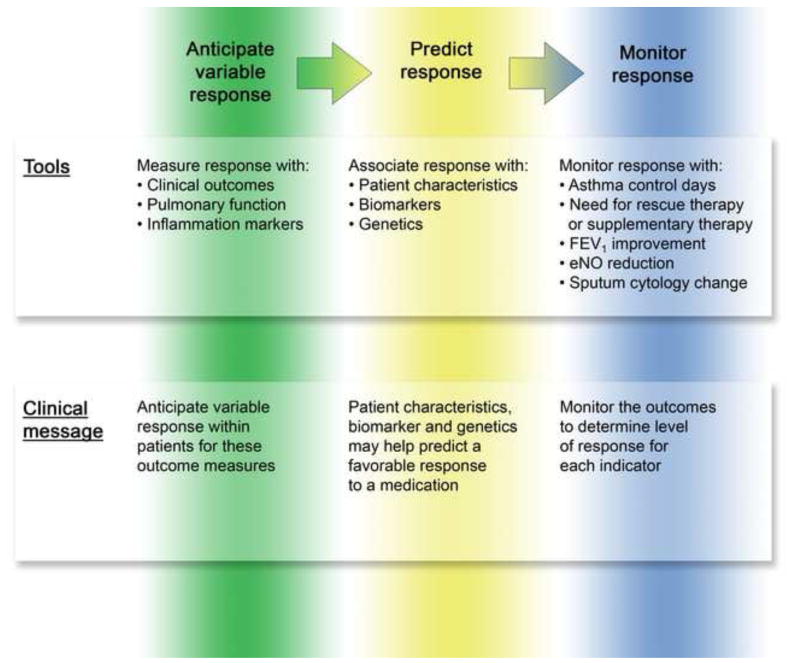
Based on the observations summarized in this review, it is now possible to utilize a combination of patient characteristics and biomarkers to help direct long-term controller therapy in children and adults. This is primarily based on outcome measures of clinical symptoms, pulmonary function and inflammation markers. Figure 4 summarizes the key steps necessary to link variability in treatment response to indicators associated with favorable response derived from clinical trials and then to utilize this information to predict and ultimately monitor outcomes in the clinical setting. All preliminary observations based on retrospective data analysis should be tested with follow-up large, prospective studies. They should also be evaluated for their cost effectiveness in clinical management.
Figure 4.
Application of clinical trial information to clinical care. Based on the studies presented, the clinician should anticipate that variability to response will occur with each treatment, that this variability in response can be associated with patient characteristics, biomarkers and genetics, and that response to treatment should be monitored for the various outcomes measures, especially symptom control and pulmonary function to assure rapid and sustained achievement of asthma control.
In regards to biomarkers, the most easily measured and readily available biomarker for clinical application is exhaled nitric oxide (eNO). Many lessons can be derived from experience with eNO as a biomarker. For example, measurements of eNO prior to beginning treatment may be useful in predicting response to ICS therapy in children but not adults. In addition, there is not a clear set of rules for applying a biomarker to clinical practice in the setting of predicting treatment response. Information to date is gathered from a body of evidence and not a rigorous set of protocols defined by a regulatory agency, such as the Food and Drug Administration.
In the future, we could benefit from such a standardized set of rules to evaluate biomarkers as a tool for clinical practice (Text Box). In addition, we could benefit from the discovery of patient characteristics, biomarkers, or genetics that are linked to a higher risk of disease progression or severe asthma. Biomarkers to measure key pathways associated with asthma progression would be useful to monitor disease activity and also serve as an indicator of treatment resistance and perhaps as a therapeutic target for medication selection.
Another weakness in our current body of knowledge is the ability to predict who is susceptible to an asthma exacerbation and measures of an impending asthma exacerbation that could be used to signal early intervention to prevent an asthma exacerbation. For example in an NIAID Inner City Asthma Consortium study entitled the “Asthma Control Evaluation (ACE)” Study, the investigators demonstrated that regular measures of eNO added to an asthma guideline-based approach did not add significant benefit over the guidelines-based approach alone in proving asthma control (13). Therefore, a single biomarker may address some questions related to asthma management, such as predicting treatment response, but additional tools will be necessary to address other important questions, such as the ability to select medications most likely to prevent an asthma exacerbation or prevent asthma progression.
Despite the ongoing needs for future management, much has been gained in the past twenty years to assist the clinician in “individualizing” or “personalizing” asthma therapy. We should now look to further refine that knowledge around the application of these techniques, including cost effectiveness studies, to continue to improve overall asthma care, perhaps even developing methods to anticipate and prevent asthma exacerbations and to induce full remission of persistent and even emerging asthma.
Acknowledgments
Supported in part by Public Health Services Research Grants HR-16048, HL64288, HL 51834, AI-25496, HL081335, HL075416, HL087811, the Colorado CTSA grant 1 UL1 RR025780 from the National Institutes of Health (NIH) and National Center for Research Resources (NCRR) and the Colorado Cancer, Cardiovascular and Pulmonary Disease Program
The authors would like to thank their colleagues in the NHLBI ACRN and CARE Networks including the research staff and investigators for their ongoing collaboration in the design, implementation and publication of the network studies. In addition, we would like to thank the NHLBI for their foresight in setting up the asthma networks to address key gaps in information related to asthma management and allow the investigators to design and interpret the data generated from these studies. We would also like to thank the pharmaceutical firms that provided medication and placebo to conduct these high quality studies. Most of all we would like to thank the study participants including children and parents for their dedication to improving asthma care.
Abbreviations
- ACD
asthma control days
- ACRN
Asthma Clinical Research Network
- BDP
beclomethasone dipropionate
- CARE Network
Childhood Asthma Research and Education Network
- ECP
eosinophilic cationic protein
- ED
Emergency Department
- eNO
exhaled nitric oxide
- FP
fluticasone propionate
- ICS
inhaled corticosteroids
- LABA
long-acting β-adrenergic agonists
- LTRA
leukotriene receptor antagonists
- MICE
Measuring Inhaled Corticosteroid Efficacy Trial
- NHLBI
National Heart, Lung and Blood Institute
- PACT
Pediatric Asthma Controller Trial
- PEAK
Prevention of Asthma in Kids Trial
- PRICE
Predicting Response to Inhaled Corticosteroid Efficacy Trial
- SMOG
Smoking Modulates Outcomes of Glucocorticoid Therapy Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stanley J. Szefler, Helen Wohlberg & Herman Lambert Chair in Pharmacokinetics, Divisions of Pediatric Clinical Pharmacology and Allergy and Immunology, Department of Pediatrics, National Jewish Health, Denver, Colorado 80206
Richard J. Martin, Chairman, Department of Medicine, National Jewish Health, Denver, Colorado 80206.
References
- 1.National Institutes of Health. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. [Accessed August 27, 2009];Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 August; NIH Publication No. 07-4051, http://www.nhlbi.nih.gov/guidelines/asthma/index.htm.
- 2.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma – Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 4.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunology. 2007;119:73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Clin Allergy Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Malmstrom K, Rodriguez-Gomez G, Guerra J, Vilaran C, Pineiro A, Wei LX, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study group. Ann Intern Med. 1999;130:487–95. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 7.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild to moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Sorkness CA, Lemanske RF, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. J Allergy Clin Immunology. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Knuffman JE, Sorkness CA, Lemanske RF, Mauger DT, Boehmer SJ, Martinez FD, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunology. 2009;123:411–6. doi: 10.1016/j.jaci.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Two year inhaled corticosteroid treatment on subsequent asthma in high-risk toddlers. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 11.Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol. 2009;123:1077–82. doi: 10.1016/j.jaci.2008.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175:783–790. doi: 10.1164/rccm.200511-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Adding exhaled nitric oxide to guideline-based asthma treatment in inner-city adolescents and young adults: a randomized controlled trial. Lancet. 2008;372:1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]