Abstract
Background
Radioiodine is efficiently concentrated by tissues expressing the human sodium iodide symporter (hNIS). The aim of this study was to analyze the effects of 131I on acute cardiac allograft rejection after hNIS ex-vivo gene transfer in a rat model of cardiac allotransplantation.
Methods
Hearts from Brown Norway rats were perfused ex vivo either with UW solution (n=9) or with UW solution containing 1 × 109 pfu/ml adenovirus 5+NIS (Ad-NIS) (n=18). Donor hearts were transplanted heterotopically into the abdomen of Lewis rats and recipients were treated on post operative day 3 with either 15,000 μCi 131I or saline. The hearts were explanted when no longer beating and were evaluated histologically for evidence of rejection and other changes.
Results
Grafts perfused with the Ad-NIS vector survived significantly longer in recipients injected with 131I (11.3±1.9 days) compared to control animals not treated with 131I (5.7±0.65 days; p<0.001). 131I treatment did not prolong graft survival in recipients of hearts that were not perfused with Ad-NIS (5.5±1.0 vs 5.3±0.8 days). In Ad-NIS 131I treated transplants, the level of myocardial damage on day 6 after surgery, when control hearts rejected, was significantly lower (60.8±28.0 vs 99.7±0.8; p<0.05).
Conclusions
This study indicates that 131I, after NIS gene transfer, can effectively prolong cardiac allograft survival. To our knowledge, this is the first report of the use of NIS-targeted 131I therapy in cardiac transplantation. Further studies are required to determine the mechanism of this effect and its potential for clinical application.
Keywords: Gene therapy, heart transplantation, sodium iodide symporter (NIS), single photon emission computed tomography (SPECT), molecular imaging, Iodine 131
Introduction
In the field of organ transplantation, maintenance of a functional allograft requires life-long immunosuppression to prevent rejection by the immune system. Currently used immunosuppressive drugs are associated with significant side effects including renal toxicity, dyslipidemia, diabetes and increased risk of infections and malignancies.
Gene therapy opens up the possibility of augmenting the effectiveness of immunosuppression or ameliorating the toxic effects of current immunosuppressive agents in the setting of solid organ transplantation, because it can be carried out ex-vivo in the donor organ under well-controlled conditions immediately before transplantation, and because potentially therapeutic agents can be introduced directly into the graft, where it may have its greatest effect and least systemic toxicity.
In 1996 the sodium iodide symporter gene(NIS) 1, 2, was cloned. Since that time many investigators have explored the ability of NIS gene transfer to facilitate targeted radioiodide therapy of non thyroidal cancers. As a result, NIS gene transfer has been demonstrated to increase radioactive iodine uptake several hundred fold in a variety of tumor systems 3–6. In addition to acting therapeutically and arresting tumor cell growth or killing them outright, the use of 131I allows in vivo tumor imaging 3, 7, 5, 8, 9. The ability to deliver a radio nuclide directly to cancer cells which could then kill those cells, made us speculate about the possibility to use this virus encoded gene as a reporter and therapeutic tool to target effector cells in the setting of allograft rejection. The aim of the study, therefore, was to analyze the effects of iodine 131 after hNIS ex-vivo gene transfer in a rat model of acute allo-rejection.
Materials and Methods
Inbred Lewis rats (300–350g, Harlan®, IN) and Brown-Norway rats (250–300g, Harlan®, IN) were used as recipients and donors, respectively, for allogenic abdominal heterotopic heart transplantation. Procedures and handling of animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic and Foundation in compliance with “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (National Institutes of Health publication No. 86–23, revised 1996).
Recombinant Adenovirus Production
A replication-deficient human recombinant adenovirus serotype 5 (Ad) construct containing human NIS under the control of the CMV promoter (Ad5/CMV/hNIS) was produced in collaboration with the Mayo Clinic Vector Production Facility using previously described methods10. Following plaque purification, the recombinant adenovirus Ad5/CMV/NIS was expanded in 293 cells and purified by banding on CsCl density gradients, followed by dialysis. Purified virus was diluted in cold University of Wisconsin (UW) solution to a concentration of 109 pfu/ml just prior to ex vivo perfusion of donor hearts.
Donor operation
Donor hearts were harvested using standard technique as previously described 11. After harvesting, the heart was stored in cold UW solution at 4°C. Prior to transplant all hearts were perfused with UW solution or UW solution containing Ad-NIS virus using a Langendorff perfusion apparatus as perviously described 11.
Experimental groups and gene transfer
Experiment 1
Hearts were perfused ex vivo for 30 minutes at 4°C with 5 ml of either UW solution (n=6) or with UW solution containing Ad-hNIS at 1× 109 pfu/ml (n=6) 11. To confirm expression of Ad-NIS all animals underwent SPECT/CT imaging of the donor hearts on post-operative day 4 after tail vein injection of 1000 μCi 123I. After imaging all animals were sacrificed.
Experiment 2
Twenty-seven transplants were performed. For group A (n=3) and B (n=6) the donor hearts were perfused for 30 minutes at 4°C with 5 ml of UW solution. Donor hearts for groups C, D and E (n=6 animals in each group), were perfused with 5 ml of Ad-NIS diluted in UW solution at a concentration of 1× 109 C while the recipient was prepared. Groups B, D and E were infused by tail vein injection with 15000 μCi of 131I three days after transplant. The remaining animals, group A and C, were injected with 0.1 ml of saline.
The status of the transplanted hearts was monitored by palpation and scored on a scale from 0 (non-beating) to 6 (vigorous, normal contractility). Transplanted hearts were harvested when the palpation scores fell to zero. Hearts from animals belonging to group D were harvested electively six days after surgery for histologic comparisons to the other groups.
Recipient operation
Heterotopic transplants were performed using standard techniques 11. All rats received analgesia postoperatively (Buprenorphine administered subcutaneously) and recovered with oxygen in a warm environment.
Imaging with micro-SPECT
Micro-SPECT/CT imaging was performed on POD 4 under sedation (intraperitoneal pentobarbital 50mg/kg). 20 minutes after tail vein injection of 37 MBq of 123I. The dose of infused 123I was corrected for residual material that remained within the syringe. Micro-SPECT images were obtained under a high-resolution gamma camera (X-SPECT, Gamma Medica-Ideas Inc., CA) with a low energy high resolution parallel hole collimator with 64 projections at a rate of 10s per projection and an acquisition time of 13m 46s. CT images were obtained under a circular orbit at a thickness of 50μM per slice under the same scanner without moving the animal in between. This ensured accurate co-registration of the axial CT images with the micro-SPECT maps.
In Vivo Image Analysis
SPECT and CT images were stored and pixel-intensity of the images was quantified as described in earlier studies 12. Briefly, the regions of interest (ROI) were defined manually around the heart shadows on the reconstructed axial tomographic images and quantification of 123I uptake was performed on the co-registered micro-SPECT images. Cumulative pixel counts within the defined region of interest (ROI) were converted to activity using a conversion factor of 1.63 × 10−7 MBq/measured count derived from our previous studies 13, 14. Values were expressed as a percentage of the injected dose per gram of heart tissue (%ID/g).
Measurements were obtained by an independent observer blinded to the origin of the images. Following the last imaging procedure, the rats were euthanized by exanguination and the transplanted hearts explanted and weighed.
Histologic Analysis
Under direct visualization full thickness mid-ventricular sections of the explanted hearts were collected and processed for routine light microscopy. Sections of all grafts were reviewed in a blinded fashion by an experienced cardiac pathologist. Acute rejection was scored according to the International Society for Heart and Lung Transplantation (ISHLT) grading system 15. Due to the severity of the changes, in addition to using the scoring system, the amount of damaged myocardium involved was quantified as a percent of total area examined by a visual estimate.
Detection of CD4 and CD8 was determined by immunohistochemistry (IHC). The paraffin-embedded (FFPE) samples were deparaffinized with 3 changes of xylene and rehydrated in a series of alcohols (100%, 95%, then 70% EtOH) and rinsed well in running distilled water. Slides were then placed in a preheated 0.1mM EDTA, pH 8.0 retrieval buffer for 30 minutes then cooled in the buffer for 5 minutes followed by a 5 minute rinse in running distilled water. After heat inactivated epitope retrieval (HIER), slides were placed on a DAKO (Carpenteria, CA) Autostainer for the following procedure (at room temperature). Sections were incubated with 3% H2O2 in ethanol for 5 min. to inactivate the endogenous peroxides, then incubated in 1:20 CD4 or CD8 (NCL-CD4-368, Novocastra Laboratories, Newcastle, United Kingdom and M7103, DAKO, respectively) for 30 minutes, followed by a rinse with TBST wash buffer. Secondary incubation was with DUAL+/HRP labeled polymer (K4061, DAKO) for 15 minutes. The slides were rinsed with TBST wash buffer. Sections were then incubated in 3,3′-diaminobenzidine(DAB+)(K3467, DAKO) for 5 minutes, counterstained with modified Schmidts’s Hematoxylin for 5 minutes and then rinsed for 3 minutes in tap water to blue sections, dehydrated through graded alcohols and cleared in 3 changes of xylene prior to mounting.
Statistics
T-test analysis was used to compare image intensity between Ad-NIS perfused hearts and controls in experiment 1 and to compare daily palpation scores, from POD 0 to POD 6, between Ad-NIS transduced hearts implanted in recipients 131I injected (Group D and E) and controls (Group A, B and C) in experiment 2. Graft survival is shown as mean survival time (±SD) and by Kaplan-Meier cumulative survival curves. Statistical significance of differences in graft survival between groups A, B, C and E (experiment 2) was analyzed using product limit (Kaplan-Meier) survival estimates by Log-Rank statistics. A p value of less than 0.05 was considered significant.
Results
There were no operative deaths in the 33 recipients in the study and no deaths occurred during the follow up period.
Experiment 1: Gene transfer confirmation
All 12 animals underwent SPCT/CT imaging on post-operative day 4 to confirm gene transfer. Significantly higher image intensity was noted in the hearts perfused with UW and Ad-NIS (1.0±0.2) compared with controls (0.45±0.12) (%ID/g; mean±SD; p<0.05) (Figure 1). These data confirm the gene was transferred after ex-vivo perfusion with a solution containing Ad-NIS.
Figure 1.
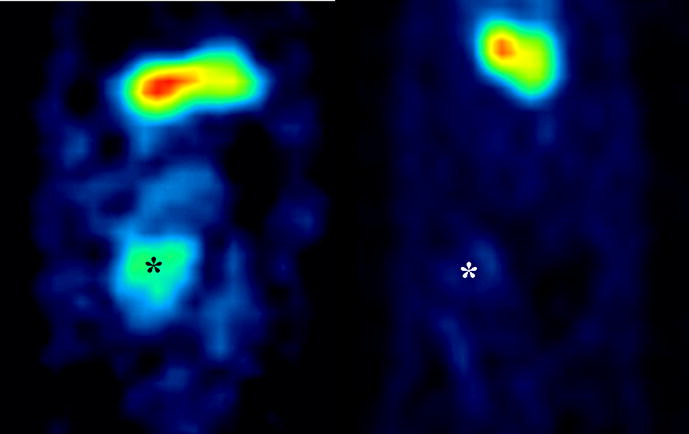
Micro-SPECT antero-posterior view of the abdomen showing the transplanted heart on POD 4. Image intensity of UW+Ad-NIS perfused hearts (left) was significantly higher than controls (right), 1.0±0.2 vs 0.45±0.12 respectively (%ID/g; mean±SD; p<0.05). The “hot spot” corresponds to the position of the transplanted heart (*). The “hot spot” above the transplanted heart corresponds to the 123I concentration in the stomach due to the inherent presence of NIS in the gastric mucosa. The ROI were defined manually around the heart shadows on the reconstructed axial tomographic images in all sections in which the heart was visualized, and quantification performed on the co-registered micro-SPECT images. This ensured accurate co-registration of the axial CT images with the micro-SPECT maps.
Experiment 2: Graft Survival and Histology
Graft survival was significantly higher in recipients of Ad-NIS transduced donor hearts that were injected with 131I (Group E; mean survival 11.3±1.9 days) compared to control transplants without Ad-NIS transduction or 131I injection (Group A), recipients treated with 131I but transplanted with hearts lacking Ad-NIS perfusion (Group B), or recipients transplanted with Ad-NIS transduced hearts but not injected with 131I (Group C) (5.7±0.6, 5.5±1.0 and 5.3±0.8) respectively for groups A, B and C p< 0.001 (Figure 2A).
Figure 2.
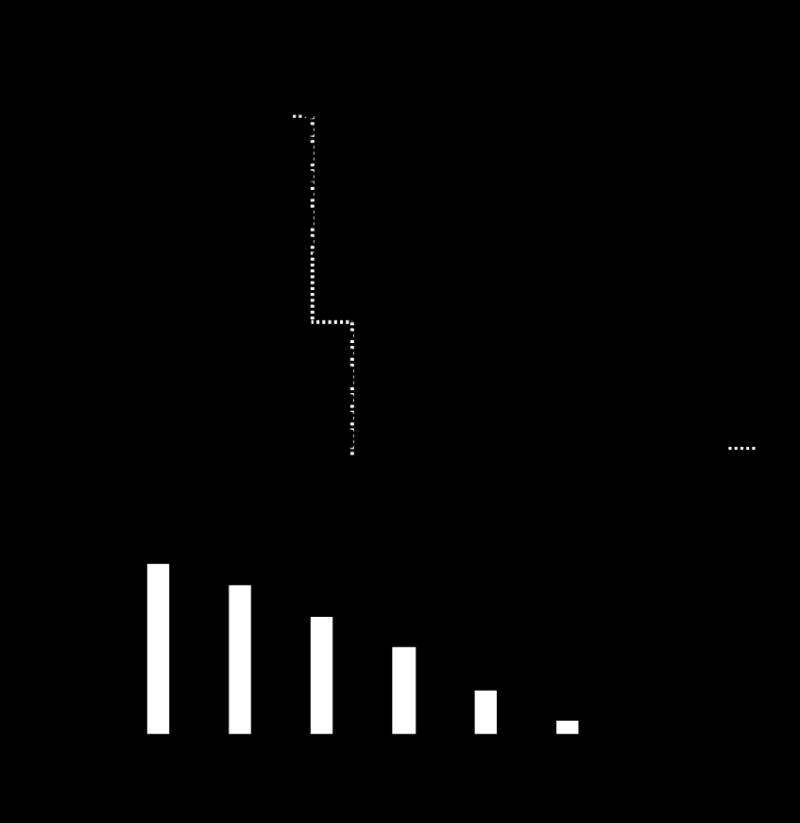
A. Prolonged survival of cardiac allografts after ex-vivo perfusion with solution of UW+Ad-NIS and 131I injection on POD 3 (Group E) (p<0.0001 vs all the other grafts grouped together, Kaplan-Meier log-rank statistical analysis). B Transplanted hearts were monitored daily by palpation and scored on a scale from 0 (non-beating) to 6 (vigorous, normal contractility). The mean value with the standard deviation is given for controls (white bars: group A, B, and C) and Ad-NIS transduced donor hearts in recipients that were injected with 131I on POD 3 (black bars: group D and E). Palpation score was significantly higher in group D and E respect to the controls (*p<0.0001). POD: post-operative day.
The range of mean survival of the controls (group A, B and C) was 5.3 to 5.7 days. On the base of this finding from POD 0 to POD 6 mean daily palpation score of group A, B and C was compared with group D and E. Respect to controls, palpation score was significantly higher from POD 4 in those Ad-NIS hearts transplanted into recipients treated with 131I on POD 3 respect to controls (p<0.0001) (Figure 2B).
Rejected grafts from groups A, B, C and E, which were explanted when no longer beating, showed widespread coagulative myocyte necrosis, with hemorrhage and edema. (Figure 3, left panel). Areas of organization were also present in some hearts. There was minimal cellularity and most cells present were macrophages. Significant numbers of T cells were not observed (data not shown). In contrast hearts from group D (Ad-NIS perfused hearts transplanted into recipients 131I injected on POD 3), which were still beating at the time of elective explant (POD 6), had a palpation score of 3.7±1.2 (mean±SD). Histologically these hearts showed focal areas of coagulative necrosis, but no cellular infiltrates. The average percentage of myocardial damage of group D grafts was 60.8%, significantly lower than the degree of rejection observed in all the other transplanted hearts (p<0.05) (Figure 3, right panel).
Figure 3.
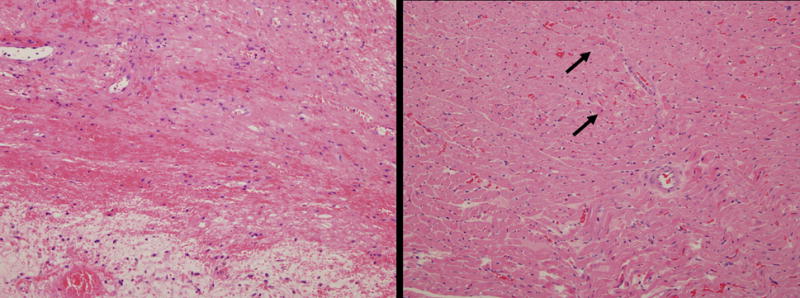
Representative pathology of grafts. The left panel shows representative histology from groups A, B, C and E. The myocardium has marked coagulative necrosis with hemorrhage, edema and organization. There is minimal inflammation and most cells present are macrophages. These features are consistent with end stage rejection. The right panel shows hearts from Group D (those perfused with a solution of UW and Ad-NIS + 131I injection on POD 3). There are only focal areas of coagulative necrosis (arrows), but no cellular infiltrates. (Hematoxylin and eosin. Magnification × 200, both panels).
Discussion
This study demonstrates that adeno-mediated myocardial expression of the hNIS gene coupled to 131I injection can prolonged cardiac graft survival in a rat allo-transplantation model. This finding is confirmed by a significantly higher palpation score of Ad-NIS perfused hearts after 131I injection respect to controls (either no Ad-NIS +/− 131I or Ad-NIS transfected hearts + no 131I) that showed a progressive decline to 0 of the contractility within 6 days after transplant. The palpation score, as a direct measure of cardiac vitality and effective contractility, proved to be a reliable and convenient test for the definition of the endpoint for graft survival with no variability or bias in the evaluations of independent observers.
The histology of grafts perfused with solution of UW and Ad-NIS, in animals that were 131I injected on POD 3, showed a lower percentage of myocardial damage compared to the controls. The myocardial damage observed in these hearts consisted predominantly of coagulative necrosis with hemorrhage and edema. There was minimal cellularity and so rejection could not be graded according to the ISHLT criteria. This is likely due to the fact that these animals were not immunosupressed and the features we observed were those of an endstage rejection process. To our knowledge this is the first time that NIS-targeted 131I therapy has been utilized in the setting of cardiac transplantation.
The effectiveness of NIS as non-invasive reporter gene has been validated in several studies in tumor models of gene transfer16, 17. In a previous study from our group we demonstrated that hNIS is an excellent reporter gene for the transplanted heart. The expression level of hNIS can be accurately and non-invasively monitored by serial radioisotopic SPECT imaging. In addition hNIS gene transfer permits sequential real-time detection and quantification of reporter gene expression in the transplanted heart (manuscript submitted).
With the experience obtained with non-invasive radioiodine imaging for quantitation of NIS reporter gene expression and the proven utility of NIS-targeted radionuclide in cancer therapy we think that this virus-encoded gene could be effectively used as both a reporter and a therapeutic gene in cardiac transplantation.
The administration of iodine 131 in the millicurie quantities (15 mCi), as were used in this study, raises a question regarding radiation dose and its effects on the cardiac tissue. Our study showed that cardiac tissues accumulated about 1% of the injected dose per gram of tissue. There are several factors that contribute to the cardiac radiation dose, including the frequency of Ad-NIS transduction in the tissue, the rate of 131I uptake and release from the heart, and the decay rate of 131I to name a few. The biological half-life of 131I shows substantial variation between tissues with an average of approximately 10h 6, 16, 18–24. When the decay rate of 131I (196 hours) is included this average biological half life decreases to 9.6 hours. As recommended by the Medical Internal Radiation Dosimetry Committee using the equation D = 1.44AS where D is dose in rad, A is accumulated activity in microcuries, and S is the amount of radiation deposited in a target tissue 21, we estimate the radiation dose for the heart to be 56.9 rad (0.57 Gy) after 20 minutes from injection, which is well below the dose documented to be a threshold for radiation side effects on the heart 25. Thus, a higher cardiac dose of iodine 131 could be used without direct radiation induced damage to the heart. However in the rat a whole body dose of 15 mCi is the maximum that can be administered before having complications due to 131I induced gastritis 26. Within the time frame of our study none of the rats exhibited weight loss and the histology of other organs (liver kidney lung and spleen) was normal. This suggests for this model that a higher cardiac dose of 131I could only be achieved through more efficient Ad virus transduction of the heart during perfusion.
Study limitations
The variability of NIS expression could represent a disadvantage of the Ad vector system that is well recognized. Ad dependence on Coxsackie virus and adenovirus receptor (CAR) to transfect cells contributes to the unpredictability and inefficiency of this system. In addition adenoviral vectors have been shown to suffer a number of concerns including induction of an inflammatory response and a limited duration of transgene expression. Most studies using adenoviral mediated gene transfer have reported a peak of gene expression around day 7 followed by total loss of gene expression between 3 to 4 weeks after gene delivery27–29. However, the need for immunosuppression in transplantation may modify this immune response and facilitate prolonged gene expression. Alternatively it would be possible to use recombinant adeno-associated virus (rAAV) vectors. These vectors have been shown to result in prolonged transgene expression without inflammation 30–35. A long acting vector could allow to treat in the same way also further episodes of rejection.
This is not a dose/response study. It would be necessary, especially in a bigger animal model, to define the safest and most effective dose of iodine 131 to inject.
The mechanism by which iodine 131 after hNIS ex-vivo gene transfer prolonged allograft survival is not clear and still need to be analyzed in this model. We believe that there are three possible pathways, first a direct immunosuppressive effect induced by the concentration of the radionuclide in the transplanted heart favoured by the presence of genetically transferred hNIS, second an indirect effect through radiation inducible promoters that modulate the immune response activating cytoprotective and immunomodulatory molecules, third a combination of the two mechanisms together.
In conclusion this study indicates that 131I, after NIS gene transfer, can effectively prolong graft survival. This finding provides the rationale to explore the use of gene transfer in combination with other therapeutic modalities as an approach for treating acute rejection. Further study is required to determine the mechanism of this effect and its potential for clinical application.
Acknowledgments
This research supported by: NIH Grant HL66598, William J von Liebig Foundation
We wish to acknowledge the contributions of Dr. Kent Bailey and Brian Lahr for statistical analysis, Teresa Decklever for imaging assistance, Dr Mark Federspiel for viral vectors procurement, Kelly Classic for suggestions on radiation safety concerns and Karen Schumacher for writing assistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996 Feb 1;379(6564):458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 2.Smanik PA, Liu Q, Furminger TL, et al. Cloning of the human sodium lodide symporter. Biochem Biophys Res Commun. 1996 Sep 13;226(2):339–345. doi: 10.1006/bbrc.1996.1358. [DOI] [PubMed] [Google Scholar]
- 3.Cho JY, Shen DH, Yang W, et al. In vivo imaging and radioiodine therapy following sodium iodide symporter gene transfer in animal model of intracerebral gliomas. Gene Ther. 2002 Sep;9(17):1139–1145. doi: 10.1038/sj.gt.3301787. [DOI] [PubMed] [Google Scholar]
- 4.La Perle KM, Shen D, Buckwalter TL, et al. In vivo expression and function of the sodium iodide symporter following gene transfer in the MATLyLu rat model of metastatic prostate cancer. Prostate. 2002 Feb 15;50(3):170–178. doi: 10.1002/pros.10046. [DOI] [PubMed] [Google Scholar]
- 5.Mandell RB, Mandell LZ, Link CJ., Jr Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res. 1999 Feb 1;59(3):661–668. [PubMed] [Google Scholar]
- 6.Shimura H, Haraguchi K, Miyazaki A, et al. Iodide uptake and experimental 131I therapy in transplanted undifferentiated thyroid cancer cells expressing the Na+/I− symporter gene. Endocrinology. 1997 Oct;138(10):4493–4496. doi: 10.1210/endo.138.10.5571. [DOI] [PubMed] [Google Scholar]
- 7.Haberkorn U, Henze M, Altmann A, et al. Transfer of the human NaI symporter gene enhances iodide uptake in hepatoma cells. J Nucl Med. 2001 Feb;42(2):317–325. [PubMed] [Google Scholar]
- 8.Carlin S, Cunningham SH, Boyd M, et al. Experimental targeted radioiodide therapy following transfection of the sodium iodide symporter gene: effect on clonogenicity in both two-and three-dimensional models. Cancer Gene Ther. 2000 Dec;7(12):1529–1536. doi: 10.1038/sj.cgt.7700264. [DOI] [PubMed] [Google Scholar]
- 9.Spitzweg C, Zhang S, Bergert ER, et al. Prostate-specific antigen (PSA) promoter-driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res. 1999 May 1;59(9):2136–2141. [PubMed] [Google Scholar]
- 10.Spitzweg C, Dietz AB, O’Connor MK, et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther. 2001 Oct;8(20):1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrini C, Jeppsson A, Taner CB, et al. Highly efficient ex vivo gene transfer to the transplanted heart by means of hypothermic perfusion with a low dose of adenoviral vector. J Thorac Cardiovasc Surg. 2000 Mar;119(3):493–500. doi: 10.1016/s0022-5223(00)70128-0. [DOI] [PubMed] [Google Scholar]
- 12.Carlson SK, Classic KL, Hadac EM, et al. In vivo quantitation of intratumoral radioisotope uptake using micro-single photon emission computed tomography/computed tomography. Mol Imaging Biol. 2006 Nov–Dec;8(6):324–332. doi: 10.1007/s11307-006-0058-z. [DOI] [PubMed] [Google Scholar]
- 13.Rao VP, Miyagi N, Ricci D, et al. Sodium iodide symporter (hNIS) permits molecular imaging of gene transduction in cardiac transplantation. Transplantation. 2007 Dec 27;84(12):1662–1666. doi: 10.1097/01.tp.0000295932.26883.ba. [DOI] [PubMed] [Google Scholar]
- 14.Ricci D, Mennander AA, Pham LD, et al. Non-invasive radioiodine imaging for accurate quantitation of NIS reporter gene expression in transplanted hearts. Eur J Cardiothorac Surg. 2008 Jan;33(1):32–39. doi: 10.1016/j.ejcts.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005 Nov;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer RM, Bergert ER, O’Connor MK, et al. Sodium iodide symporter-mediated radioiodide imaging and therapy of ovarian tumor xenografts in mice. Gene Ther. 2006 Jan;13(1):60–66. doi: 10.1038/sj.gt.3302599. [DOI] [PubMed] [Google Scholar]
- 17.Mitrofanova E, Unfer R, Vahanian N, et al. Rat sodium iodide symporter for radioiodide therapy of cancer. Clin Cancer Res. 2004 Oct 15;10(20):6969–6976. doi: 10.1158/1078-0432.CCR-04-0687. [DOI] [PubMed] [Google Scholar]
- 18.Dadachova E, Nguyen A, Lin EY, et al. Treatment with rhenium-188-perrhenate and iodine-131 of NIS-expressing mammary cancer in a mouse model remarkably inhibited tumor growth. Nucl Med Biol. 2005 Oct;32(7):695–700. doi: 10.1016/j.nucmedbio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Dingli D, Peng KW, Harvey ME, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004 Mar 1;103(5):1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer RM, Bergert ER, O’Connor MK, et al. Adenovirus-mediated and targeted expression of the sodium-iodide symporter permits in vivo radioiodide imaging and therapy of pancreatic tumors. Hum Gene Ther. 2006 Jun;17(6):661–668. doi: 10.1089/hum.2006.17.661. [DOI] [PubMed] [Google Scholar]
- 21.Howell RW, Wessels BW, Loevinger R, et al. The MIRD perspective 1999. Medical Internal Radiation Dose Committee. J Nucl Med. 1999 Jan;40(1):3S–10S. [PubMed] [Google Scholar]
- 22.Petrich T, Helmeke HJ, Meyer GJ, et al. Establishment of radioactive astatine and iodine uptake in cancer cell lines expressing the human sodium/iodide symporter. Eur J Nucl Med Mol Imaging. 2002 Jul;29(7):842–854. doi: 10.1007/s00259-002-0784-7. [DOI] [PubMed] [Google Scholar]
- 23.Smit JW, Schroder-van der Elst JP, Karperien M, et al. Iodide kinetics and experimental (131)I therapy in a xenotransplanted human sodium-iodide symporter-transfected human follicular thyroid carcinoma cell line. J Clin Endocrinol Metab. 2002 Mar;87(3):1247–1253. doi: 10.1210/jcem.87.3.8307. [DOI] [PubMed] [Google Scholar]
- 24.Spitzweg C, O’Connor MK, Bergert ER, et al. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000 Nov 15;60(22):6526–6530. [PubMed] [Google Scholar]
- 25.Byrne GW, Davies WR, Oi K, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006 Dec 27;82(12):1787–1791. doi: 10.1097/01.tp.0000251387.40499.0f. [DOI] [PubMed] [Google Scholar]
- 26.Shen DH, Marsee DK, Schaap J, et al. Effects of dose, intervention time, and radionuclide on sodium iodide symporter (NIS)-targeted radionuclide therapy. Gene Ther. 2004 Jan;11(2):161–169. doi: 10.1038/sj.gt.3302147. [DOI] [PubMed] [Google Scholar]
- 27.Gilgenkrantz H, Duboc D, Juillard V, et al. Transient expression of genes transferred in vivo into heart using first-generation adenoviral vectors: role of the immune response. Hum Gene Ther. 1995 Oct;6(10):1265–1274. doi: 10.1089/hum.1995.6.10-1265. [DOI] [PubMed] [Google Scholar]
- 28.Li JJ, Ueno H, Pan Y, et al. Percutaneous transluminal gene transfer into canine myocardium in vivo by replication-defective adenovirus. Cardiovasc Res. 1995 Jul;30(1):97–105. [PubMed] [Google Scholar]
- 29.Muhlhauser J, Jones M, Yamada I, et al. Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Ther. 1996 Feb;3(2):145–153. [PubMed] [Google Scholar]
- 30.Du L, Kido M, Lee DV, et al. Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors. Mol Ther. 2004 Sep;10(3):604–608. doi: 10.1016/j.ymthe.2004.06.110. [DOI] [PubMed] [Google Scholar]
- 31.Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006 Jul;14(1):45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakai H, Fuess S, Storm TA, et al. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005 Jan;79(1):214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schirmer JM, Miyagi N, Rao VP, et al. Recombinant adeno-associated virus vector for gene transfer to the transplanted rat heart. Transpl Int. 2007 Jun;20(6):550–557. doi: 10.1111/j.1432-2277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- 34.Su H, Huang Y, Takagawa J, et al. AAV serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect. Gene Ther. 2006 Nov;13(21):1495–1502. doi: 10.1038/sj.gt.3302787. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005 Mar;23(3):321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]


