Abstract
For best results with unrelated allogeneic transplantation of acute myeloid leukemia (AML) patients, high-resolution typing should be completed for human leukocyte antigens (HLA) A, B, C, and DR. In the absence of an HLA-identical sibling, an unrelated adult donor fully matched or with a single mismatch at these loci should be used. If such a donor is not available in a timely manner, cord blood mismatched at one or two loci may be used. Data also suggest that peripheral blood transplantation may be more permissive of an HLA mismatch than bone marrow transplants. Transplant decisions should also be based on a several factors, including availability of matched donors, but also patient age, performance status, and disease stage.
Keywords: human leukocyte antigen (HLA), match, allele, antigen, peripheral blood, bone marrow, cord blood, high-resolution typing, low-resolution typing, low-expression loci
INTRODUCTION
Unrelated donor transplants today use better matched donors than previously. Based on data from 1993-2007 collected by the Center for International Blood and Marrow Transplant Research (CIBMTR),1 almost 70% of unrelated donor transplants performed in 2007 used donors that were high-resolution matched at 8 of 8 human leukocyte antigens (HLAs), including A, B, C, and DR. Rates of better-matched transplants improved over the last 15 years as techniques developed to discriminate between allelic differences at class I and class II HLAs (A, B, and C vs DR, DP, and DQ, respectively).
The ability to identify and select better matched donors also accounts for a major part of the improvement in outcomes for unrelated donor transplantation over the past decade. According to data from CIBMTR and the National Marrow Donor Program (NMDP) for patients under the age of 50 years with chronic myeloid leukemia (CML), myelodysplastic syndromes, or acute leukemia receiving received hematopoietic cell transplantation (HCT) with myeloablative regimens, one-year survival after unrelated donor transplants improved progressively and is now similar to one-year survival after an HLA-identical sibling transplant.1 However, there is still more morbidity after unrelated donor transplantation.
WHAT DEGREE OF MATCHING IS NECESSARY?
The number of known alleles keeps growing, making it more difficult to find a fully matched donor and more complicated to determine how closely matched a donor must be to achieve optimal transplant outcomes. Three large, recent studies provide insight, though not a definitive answer. The first, a study by Lee and colleagues at the NMDP, included data from 3860 myeloablative transplants from 1988-2003.2 The NMDP had stored samples from donor recipients from the inception of the program. All of the samples were retrospectively typed using DNA methods. Low-resolution DNA typing was considered to be the antigen equivalent, while high-resolution typing was the allele equivalent. Samples were typed at HLA-A, B, C, DRB1, DQB1, DQA1, DPB1, and DPA1 alleles to define the degree of match at high resolution. Very few cases were fully matched or had 10/10 high-resolution matching at HLA-A, B, C, DRB1, and DQ. Median follow-up was 6 years.
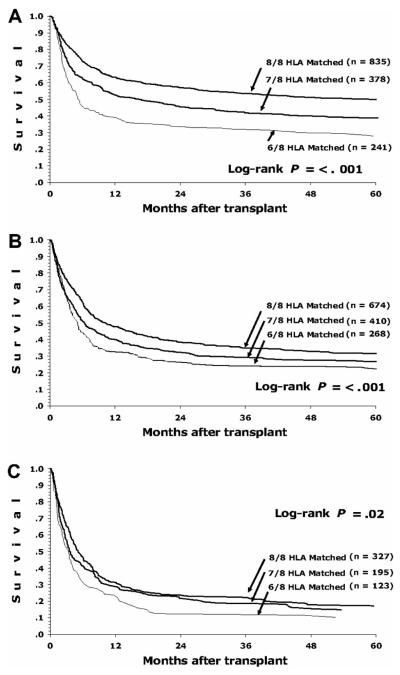
A single locus mismatch at any of those 10 loci except for DQ was associated with significantly worse overall survival, disease-free survival, transplant-related mortality, and acute graft-vs-host disease (GVHD).2 There was no statistical difference if the mismatch was detected at the low-resolution antigen or high-resolution antigen level, except at HLA-C. Allelic and antigenic differences at all loci had similar impacts on outcomes, except for HLA-C, which seemed to have a more negative impact with mismatch at the antigenic level. When examining effect of mismatching at specific loci on the specific outcomes of acute GVHD, transplant-related mortality, and overall mortality, mismatching at HLA-A and HLA-C significantly affected all outcomes, mismatching at HLA-DR affected transplant-related and overall mortality but did not significantly affect the risk of acute GVHD, while the effect of mismatching at HLA-B did not reach statistical significance for survival or transplant-related mortality, though numbers of HLA-B mismatched transplants were small. Cumulative effects of additional mismatches were also calculated.2 When compared to an 8/8 matched donor, those with 7/8 matches had a 25% increase in the relative risk of mortality, and those with 6/8 matches had a 65% increase in the risk of mortality. Donor age, cytomegalovirus status, gender, and parity were not significant risk factors for mortality. Among patients with early-stage disease (those with acute leukemia in first remission or CML in first chronic phase), each additional mismatch translates into a 10% absolute decrease in the likelihood of survival (Figure 1a). However, patients with early stage disease also have a lower risk of transplant-related mortality. Among those with intermediate-stage disease (those in second or subsequent remission or with accelerated phase CML), the absolute difference in survival is only 5% with each additional mismatch (Figure 1b). The absolute difference in survival is even less than 5% for those with advanced disease (those who are not in remission or who have CML in blast phase) (Figure 1c).
Figure 1.
Survival of patients after transplant by disease stage and degree of HLA matching.
PERMISSIVE MISMATCHES
There has been much discussion on permissive vs nonpermissive mismatches, ie, whether all HLA mismatches at the same antigen have the same effect. However, there are not sufficient data to form a clear opinion. In a study from Japan, specific mismatches of HLA-A2 seemed to have worse prognosis than other mismatches at HLA-A.3 Conversely, an analysis of 15 samples from the NMDP did not show a statistically significant change in relative risk with HLA-A2 mismatches.4 Some investigators have tried to predict biologically meaningful prognosis from insignificant mismatches from examination of the structure and function of the HLA molecule but no useful prognostic tool is yet available. Therefore, with the available data, there is not enough evidence to suggest whether certain mismatches are permissive.
LOW-EXPRESSION LOCI
The significance of low-expression class II loci HLA-DQ, DP, and DRB3/4/5 was recently studied in an NMDP analysis of 3853 patients who received bone marrow transplants.5 It was believed that the influence of the low-expression loci was weak, cumulative, and only demonstrable in combination with mismatches in other loci. In this population, mismatching at low-expression loci is more common in patients who are also mismatched at high-expression loci. Having 3 or more mismatches at low-expression loci was also more common in patients with HLA-DRB1 mismatch. There seems to be a further deleterious effect of multiple mismatching at low-expression loci for donor-recipient pairs who already have a mismatch at a high-expression locus.
PERIPHERAL BLOOD TRANSPLANTS
Of all the transplants studied in the previously cited studies, 94% were bone marrow grafts, though bone marrow is now the least frequently used graft source in allogeneic transplantation.5 Most patients now receive peripheral blood transplants. In 2008, the number of cord blood transplants exceeded the number of bone marrow transplants for the first time. Peripheral blood transplants are both qualitatively and quantitatively different than bone marrow grafts, as are cord blood grafts.
A study of 1933 peripheral blood transplants was conducted by the NMDP to determine the effect of HLA matching at HLA-A, B, C, DRB1, or DQB1 alleles.6 A third of the patients received reduced-intensity conditioning. In contrast to bone marrow grafts, there seemed to be a more adverse effect of antigen-level vs allele-level mismatching in this setting. Additional analysis of these data is ongoing.
RECOMMENDATIONS
Guidelines for selecting an unrelated door were recently published by NMDP.7 Selection of adult donors should be done using high-resolution typing at A, B, C, and DRB1 with the goal of identifying an 8/8 donor, if possible. When an 8/8 match is not available, 7/8 donors should be sought; evaluation of low-expression loci may be warranted. It must also be remembered that prognosis is determined by the sum of risks for adverse events, which includes many other criteria besides HLA matching. For example, the difference in survival between an 8/8 matched transplant and a 7/8 matched transplant is less than the difference between doing a transplant in early disease vs late disease.2
When faced with a patient who has an indication for hematopoietic stem cell transplantation, first search for a family donor. If there is no donor in the family, assess the availability of an unrelated adult donor or cord blood. If a suitably matched adult donor is not available in current registries, it is very unlikely that one will be recruited in a time frame that will be useful. Cord blood should also be considered as a graft source, especially in children and in adults without an 8/8 adult donor. Transplant decisions, such as whether, when, and how to do it, should take into account the predicted transplant outcome with available donors or cord blood units compared to the predicted outcome of nontransplant therapy. These predictions should be based on known prognostic factors that include but are not restricted to HLA matching (Table 1). Finally, the decision to transplant should not be delayed inordinately by waiting for a better matched donor.
Table 1.
Prognosis is determined by the sum of risks for adverse events
| Adverse events |
Recurrence | GVHD | Infection | Toxicity |
|---|---|---|---|---|
| Prognostic factors | Disease | Age | Donor type and match |
Age |
| Disease status | Donor type and match |
Graft type | Performance status |
|
| Disease duration | Donor-recipient sexmatch |
Disease | Conditioning regimen |
|
| Disease markers (eg, cytogenetics) |
Disease status and duration |
|||
| GVHD |
Abbreviations: GVHD, graft vs host disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karanes C, Nelson GO, Chitphakdithai P, et al. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:8–15. doi: 10.1016/j.bbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 3.Morishima Y, Kawase T, Malkki M, et al. Effect of HLA-A2 allele disparity on clinical outcome in hematopoietic cell transplantation from unrelated donors. Tissue Antigens. 2007;69(Suppl 1):31–35. doi: 10.1111/j.1399-0039.2006.759_3.x. [DOI] [PubMed] [Google Scholar]
- 4.Baxter-Lowe LA, Maiers M, Spellman SR, et al. HLA-A disparities illustrate challenges for ranking the impact of HLA mismatches on bone marrow transplant outcomes in the United States. Biol Blood Marrow Transplant. 2009;15:971–981. doi: 10.1016/j.bbmt.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Vina M, Klein J, Haagenson M, et al. The clinical significance of matching for alleles at the low expression of HLA loci DP, DQ and DRB3/4/5 in unrelated hematopoietic stem cell transplantation. Blood. 2008;112:210. abstr 561. [Google Scholar]
- 6.Woolfrey AE, Klein J, Haagenson MD, et al. Evaluation of human leukocyte antigen (HLA) matching requirements for unrelated peripheral blood stem cell (PBSC) transplantation. Blood. 2008;112:211. abstr 563. [Google Scholar]
- 7.Bray RA, Hurley CK, Kamani NR, et al. National marrow donor program HLA matching guidelines for unrelated adult donor hematopoietic cell transplants. Biol Blood Marrow Transplant. 2008;14:45–53. doi: 10.1016/j.bbmt.2008.06.014. [DOI] [PubMed] [Google Scholar]