Abstract
Regulatory T cells (Tregs) play an important role in the maintenance of peripheral tolerance. Several molecules including TGF-β have been linked to the function and differentiation of Tregs. In this study, we describe a unique population of T cells expressing a membrane bound form of TGF-β, the latency-associated peptide (LAP), and having regulatory properties in human peripheral blood. These CD4+LAP+ T cells lack Foxp3 but express TGF-βR type II and the activation marker CD69. CD4+LAP+ T cells are hypoproliferative compared with CD4+LAP− T cells, secrete IL-8, IL-9, IL-10, IFN-γ, and TGF-β upon activation, and exhibit TGF-β– and IL-10–dependent suppressive activity in vitro. The in vitro activation of CD4+LAP− T cells results in the generation of LAP+ Tregs, which is further amplified by IL-8. In conclusion, we have characterized a novel population of human LAP+ Tregs that is different from classic CD4+Foxp3+CD25high natural Tregs.
Central and peripheral mechanisms of tolerance prevent the development of autoimmune disorders in the mature immune system. Central mechanisms of tolerance are based on the deletion or inactivation of self-reactive clones (1). Peripheral mechanisms of tolerance are based on the activity of a specialized subset of lymphocytes endowed with suppressive activity (2, 3). The importance of regulatory T cells (Tregs) for the maintenance of immune homeostasis is highlighted by the autoimmune pathology that develops as a result of deficits in Treg activity (4).
The immunoregulatory activity of Tregs has been linked to several molecules, such as Foxp3, CTLA-4, TGF-β, and IL-10 (5–7). TGF-β has been shown to play an important role in the differentiation, maintenance, and function of natural Tregs (8–12). We have identified a murine Treg population that expresses latency-associated peptide (LAP) on the cell surface (13). LAP is a propeptide that is noncovalently associated to the amino terminal domain of TGF-β, forming a latent TGF-β complex. Mouse CD4+LAP+ Tregs suppress effector T cell function in a TGF-β–dependent manner both in vitro and in vivo and have been shown to suppress murine autoimmunity in experimental models of multiple sclerosis, systemic lupus erythematosus, and diabetes (14–17). CD4+LAP+ Tregs are present in healthy mice and can be expanded by stimulation through the mucosal route, suggesting that these cells represent an induced Treg population (14, 15). Recently, Andersson et al. and Tran et al. (18, 19) showed that LAP is expressed by activated mouse and human Foxp3+ Tregs. We have recently reported the expression of LAP on the surface of immature tolerogenic dendritic cells (20) in human peripheral blood. However, to date, LAP+ T cells have not been detected in human peripheral blood.
In this study, we report the characterization of Foxp3-negative CD4+LAP+ Tregs present in human peripheral blood. In vitro, the suppressive activity of CD4+LAP+ Tregs is dependent on both TGF-β and IL-10. CD4+LAP+ Tregs are induced in vitro by the activation of naive T cells, and their generation is amplified by IL-8. Thus, CD4+LAP+ T cells are a novel population of activation-induced Tregs that are different from classic CD4+Foxp3+CD25high natural Tregs.
Materials and Methods
Subjects
We collected blood from healthy controls (age 23–38 y) upon informed consent. This work was approved by the institutional review board at Brigham and Women's Hospital, Boston, MA.
Abs and reagents
Abs to CD3, CD28, IL-10, CD4, CD69, HLA-DR, and TGF-βRII and dead cell indicators (annexin-PE/FITC and aminoactinomycin D [AAD]) were obtained from BD Biosciences (San Jose, CA). Abs to Foxp3, IL-8, and IL-17 were obtained from eBioscience (San Diego, CA). Ab to LAP (clone 27232) and rLAP protein were obtained from R&D Systems (Minneapolis, MN). All RT-PCR primers and reagents were obtained from Applied Biosystems (Foster City, CA). Human rIL-2 was obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, Bethesda, MD.
Isolation of LAP+ T cells
PBMCs were obtained by Ficoll density gradient. Total CD4+ T cells were purified using a Miltenyi Biotec (Auburn, CA) negative selection kit. CD4+LAP+, CD4+LAP−, and CD4+LAP−CD25high T cells were obtained by FACS sorting using FACSAria (BD Biosciences) excluding dead and dying T cell subpopulations to typical 96–98% purity in postsort analysis.
Suppression assays
Responder T cells (CD4+LAP−CD25int/lowT cells) were activated with anti-CD3− (1 μg/107 beads) and anti-CD28− (1 μg/107 beads) coated beads for 5 d in the presence of Tregs (CD4+LAP+ or CD4+LAP−CD25high T cells) at a 2:1 (responder:regulatory) ratio. CD4+LAP− T cells were used as a control. Cells were pulsed with [3H]thymidine (1 μCi/well) for 16–24 h at the end of the incubation period.
Cytokine signaling pathways
Cytokine signaling pathways were analyzed using reverse-phase protein arrays as previously described (21). Abs directed against proteins and phosphoproteins involved in several signaling pathways are listed in Supplemental Table I.
Induction of LAP+ T cells
CD4+LAP−CD25int/low T cells were FACS sorted and activated with plate-bound Abs to CD3 (1 μg/ml), soluble anti-CD28 (1 μg/ml), and recombinant human IL-2 (50 U/ml) in the presence of either no cytokine, IL-8 (100 ng/ml), or IL-17 (10 ng/ml). After 6 d of differentiation, the cells were stained with Abs to LAP and were either analyzed by FACS or FACS sorted for suppression assays.
Results and Discussion
Identification of CD4+LAP+ T cells in human peripheral blood
To investigate the existence of CD4+LAP+ T cells in human peripheral blood, we isolated T cells and stained them with a murine anti-human LAP mAb. We found that a small percentage (1.32%) of CD4+ cells express LAP (Fig. 1A). The specificity of LAP staining was confirmed by competing with an excess of rLAP (Fig. 1A). Among different individuals, we found that the range of CD4+LAP+ T cells was 0.1–1.5% compared with 0.75–3.0% for CD4+CD25high T cells (Fig.1B). We also noted that CD4+CD25high T cells have the highest percentage of cells that express LAP (7.62%) compared with CD4+CD25int (2.09%) and CD4+CD25low (1.91%) T cells (Fig. 1C). We observed increased expression of LAP on CD4+CD25high T cells in all the individuals tested (range, 4–10%) as compared with CD4+CD25int/low T cells (range, 0.5–3%).
FIGURE 1.
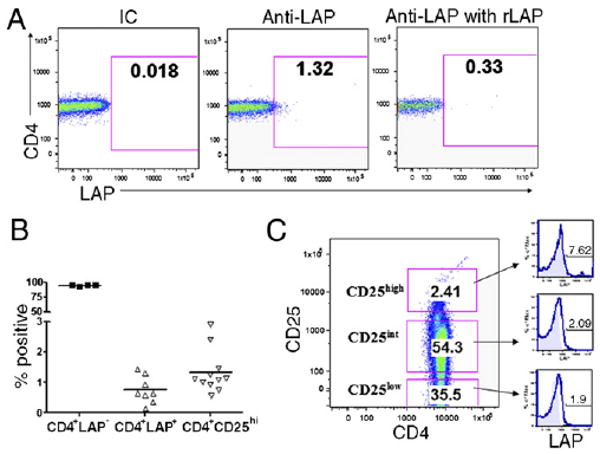
Identification of LAP+CD4+ T cells in human peripheral blood. Human PBMCs were isolated and analyzed by FACS. A, Left panel shows annexin−AAD−CD3+CD4+ T cells stained with anti-LAP Ab. Right panels show T cells stained with anti-LAP Ab in presence of rLAP. B, Percentage of CD4+LAP+, CD4+LAP−, and CD4+CD25high T cells among different individuals. C, Left panel shows gating criteria based on CD25 expression into CD25high, CD25int, and CD25low subpopulations. Right panels indicate overlay histogram plots representing the LAP+ T cell subpopulation in respective CD25high, CD25int, and CD25low T cell subpopulations. The isotype control is the filled profile, and anti-LAP staining is the empty profile. The percentages of LAP+ T cells are indicated in each plot. Results are representative of five independent experiments.
Selective expression of activation and regulatory markers on CD4+LAP+ T cells
Based on the reported expression of LAP by in vitro activated Foxp3+ Tregs (18, 19), we examined the coexpression of LAP and Foxp3 in ex vivo CD4+LAP+ T cells by FACS. We did not detect Foxp3 expression in any of the CD25 subsets of CD4+LAP+ T cells (Fig. 2A). To further confirm the lack of Foxp3 expression by CD4+LAP+ T cells, we quantified Foxp3 mRNA by real-time PCR. In agreement with our FACS results, we found no expression of Foxp3 mRNA by CD4+LAP+CD25int and CD4+LAP+CD25low, but markedly reduced Foxp3 expression in CD4+LAP+CD25high T cells as compared with CD4+LAP−CD25high T cells (Fig. 2B). Also, the CD4+LAP+ T cells did not transiently express Foxp3 at 48 h following activation (data not shown). In mice, Foxp3 directly correlates with the suppressive properties of Tregs. In humans, however, the Foxp3 expression is not restricted to Tregs and can be detected in activated effector T cells (22). Moreover, multiple subsets of Foxp3− Tregs, such as Tr1 cells, have been described (23). Our results show that CD4+LAP+ Tregs are Foxp3-negative, suggesting that they are different from naturally occurring CD4+Foxp3+CD25high Tregs.
FIGURE 2.
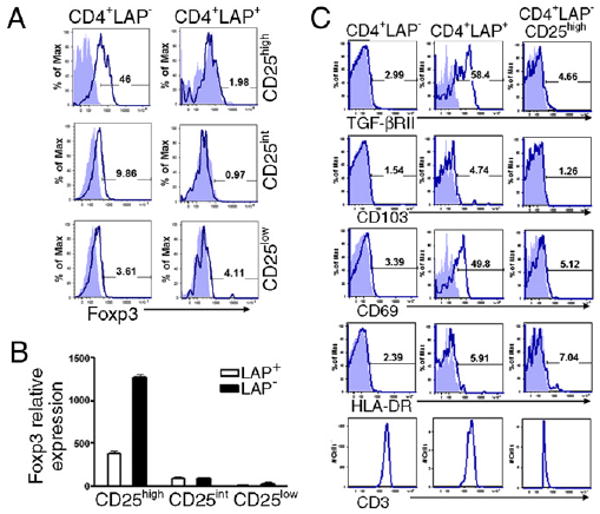
Selective expression of activation and regulatory markers on CD4+LAP+ T cells. A, Foxp3 staining of CD25high, CD25int, CD25low LAP+, and LAP- T cells. Cells were stained with AAD, annexin, CD3, CD4, CD25, and LAP followed by intracellular staining for Foxp3. B, RT-PCR analysis to determine Foxp3 expression in respective FACS-sorted populations. C, Human PBMCs were stained with AAD, annexin, CD3, CD4, CD25, LAP, and specific markers including TGF-βRII, CD69, CD103, HLA-DR, and CD3. Filled blue profiles represent isotype control; empty profiles represent specific staining for different Abs on selected populations. The percentage of positive cells are indicated in each plot. Results are representative of five independent experiments.
To further characterize CD4+LAP+ T cells, we analyzed CD4+LAP−, CD4+LAP+, and CD4+LAP−CD25high T cells by FACS with a panel of Abs to molecules associated with Tregs. We found high levels of TGF-βRII expression (56 ± 7.4%; p < 0.05) only on CD4+LAP+ T cells as compared with CD4+LAP− or CD4+LAP−CD25high T cells (Fig. 2C). TGF-βRII expression is reported to be important for the peripheral maintenance of Treg numbers (24, 25). In comparison with both CD4+LAP− and CD4+LAP−CD25high T cells, CD4+LAP+ T cells expressed the early activation marker CD69 (38 ± 16.3%; p < 0.05; Fig. 2C), suggesting that a subset of LAP+ cells consists of activated or induced Tregs. CD69 is also expressed by murine CD4+LAP+ Tregs (15). Although CD69 is considered as an early activation marker (26, 27), CD69 signaling can also induce the synthesis of TGF-β (28). Indeed, CD69+Foxp3−CD4+ Tregs have been recently described and shown to inhibit T cell proliferation in vitro via membrane-bound TGF-β (29). CD4+LAP+ and CD4+LAP−CD25high T cells expressed slightly increased levels of CD103 (1.7 ± 0.58% and 1.8 ± 0.84%, respectively) than CD4+LAP− T cells (0.74 ± 0.51%; Fig. 2C). CD4+LAP+ T cells showed decreased or similar percentages of HLA-DR+ T cells (6.4 ± 6.3%) compared with CD4+LAP−CD25high T cells (11.5 ± 7.5%; Fig. 2C). In addition, LAP+ T cells have increased expression of gluco-corticoid-induced tumor necrosis factor receptor, CTLA-4, and programmed death-1 compared with LAP− T cells or CD4+LAP−CD25high T cells (Supplemental Fig. 1). We observed similar expression levels of CD3 among all T cell subpopulations (Fig. 2C). Taken together, our data suggest that CD4+LAP+ T cells are induced Tregs for which numbers and activity are associated with Treg-related markers including TGF-βRII, CD69, and CTLA-4.
Proliferation, cytokine profile, and suppressive properties of CD4+LAP+ T cells
Next, we investigated the proliferation potential of CD4+LAP+ T cells. CD4+LAP+ T cells showed a significant (p < 0.05) decrease in their proliferative response to activation with Abs to CD3 and CD28 compared with CD4+LAP− T cells in the presence of IL-2. CD4+LAP+ T cells seemed to proliferate more than CD4+LAP−CD25high T cells, but this difference did not achieve statistical significance (Fig. 3A).
FIGURE 3.
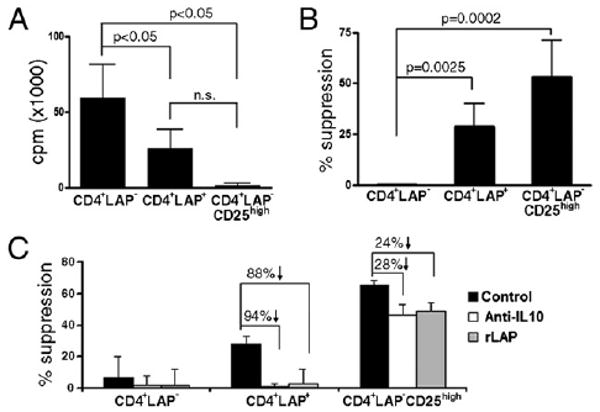
Proliferation and in vitro regulatory activity of CD4+LAP+ T cells. A, CD4+LAP−, CD4+LAP+, and CD4+LAP−CD25high T cells were sorted and stimulated with anti-CD3 and anti-CD28 for 5 d. Proliferation of respective T cell subpopulations is represented as a mean (±SD) of three independent experiments. B, Responder T cells were cocultured with Treg subpopulations in presence of anti-CD3− and anti-CD28−coated beads. Percent suppression for each population is represented as mean (±SD) of three independent experiments. C, Suppressive activity of CD4+LAP+ T cells and CD4+LAP−CD25high T cells reversed by blocking TGF-β (rLAP, 25 μg/ml) and anti–IL-10 (20 μg/ml). Results are representative of three independent experiments.
To determine the cytokine profile of human CD4+LAP+ T cells, we FACS sorted ex vivo-isolated T cells into CD4+LAP−, CD4+LAP+, and CD4+LAP−CD25high T cells and stimulated them with Abs to CD3 and CD28 in presence of IL-2 for 5 d. CD4+LAP+ T cells secreted an array of cytokines including IL-8, IL-9, IL-10, IFN-γ, and TGF-β (Table I). We did not detect the secretion of IL-8, IL-9, IL-10, and IFN-γ by CD4+LAP−CD25high T cells. CD4+LAP−T cells secreted lower levels of IL-8 and IL-9 as compared with CD4+LAP+ T cells. In mice, IL-9 is mainly produced by Th2 cells (30) and has been associated with the promotion of inflammation (31). In addition, IL-9 can also be produced by Tregs (32) and can increase their suppressive function (33). In human T cells, the function of IL-9 is still unknown.
Table I. Cytokine production by purified activated populations.
| Cytokine | Subject No. | CD4+LAP− | CD4+LAP+ | CD4+LAP−CD25high |
|---|---|---|---|---|
| IL-8 | 1 | 175 | 915 | 8 |
| 2 | 5 | 275 | 21 | |
| 3 | 16 | 2120 | 2 | |
| IL-9 | 1 | 1931 | 7034 | 191 |
| 2 | 98 | 1382 | 84 | |
| 3 | 64 | 890 | 18 | |
| IL-10 | 1 | 160 | 241 | 42 |
| 2 | 1 | 120 | 26 | |
| 3 | 104 | 732 | 40 | |
| IFN-γ | 1 | 530 | 525 | 3 |
| 2 | 300 | 335 | 9 | |
| 3 | ND | ND | ND | |
| TGF-β1 | 1 | 1075 | 2475 | 1962 |
| 2 | 1975 | 2208 | 2278 | |
| 3 | ND | ND | ND |
Cytokine values are in pg/ml.
To investigate the in vitro suppressive function of human CD4+LAP+ T cells, we carried out coculture experiments with CD4+LAP−CD25int/low responder T cells. We observed 28 ± 11% (range, 14–42%) suppression by CD4+LAP+ T cells as compared with 53 ± 18% (range, 30–75%) by CD4+LAP−CD25high T cells (Fig. 3B). We observed similar effects when measured by CFSE dilution (Supplemental Fig. 2A). Suppression mediated by CD4+LAP+ T cells or expression of LAP did not change by activation of these cells in presence of anti-CD3, anti-CD28, and IL-2 (Supplemental Fig. 2B, 2C). Furthermore, we observed no significant differences in secretion of IFN-γ and only a slight decrease in secretion of IL-2 (p < 0.05) during coculture of responders T cells with CD4+LAP+ T cells when compared with responders T cells alone (Supplemental Fig. 2D). Because murine CD4+LAP+ T cells mediate suppression via both IL-10 and TGF-β (13–15), we investigated whether these cytokines participate in the suppressive activity of human CD4+LAP+ T cells. To address this question, we employed anti–IL-10 Ab and rLAP, a potent neutralizer of TGF-β activity that binds to active TGF-β and inhibits the binding of the LAP/TGF-β complex to TGF-β binding factors (18, 20). We found that both TGF-β and IL-10 neutralization interfered with the suppressive activity of CD4+LAP+ T cells (>85% reversal of suppression; Fig. 3C). In contrast, neutralization of TGF-β or IL-10 resulted in a partial blockade of the suppressive activity of CD4+LAP−CD25high T cells (25% reversal of suppression; Fig. 3C), suggesting the involvement of an additional contact-dependent suppressive mechanism. Taken together, these data demonstrate that CD4+LAP+ T cells and CD4+LAP−CD25high T cells use different mechanisms to control their target cells. Suppression mediated by CD4+LAP+ T cells was reversed by separating responder T cells from the Treg population by a transwell, demonstrating that both soluble and contact-dependent mechanisms are involved in CD4+LAP+ T cell-mediated suppression (Supplementary Fig. 2E).
In vitro induction of CD4+LAP+ T cells
To investigate the conditions that promote the generation of CD4+LAP+ T cells, we studied the signaling pathways active in CD4+LAP+ T cells using reverse protein arrays (21). We found increased levels of IL-8–dependent signaling in CD4+LAP+ T cells (Fig. 4A). To test whether IL-8 plays a role in the generation of CD4+LAP+ T cells, we activated CD4+LAP− T cells in presence of IL-8, IL-17, or no cytokines as control. The activation of CD4+LAP− T cells with Abs to CD3 and CD28 in the presence of IL-2 induced a small but significant number of CD4+LAP+ T cells (2.8 ± 1.4%; range, 1–4%; Fig. 4B). The addition of IL-8 resulted in a significant increase in the generation of CD4+LAP+ T cells (3.6 ± 1.6%; range 1–6.28%; p < 0.0005; Fig. 4B). IL-17 had no effect on CD4+LAP+ T cell differentiation (Fig. 4B).
FIGURE 4.
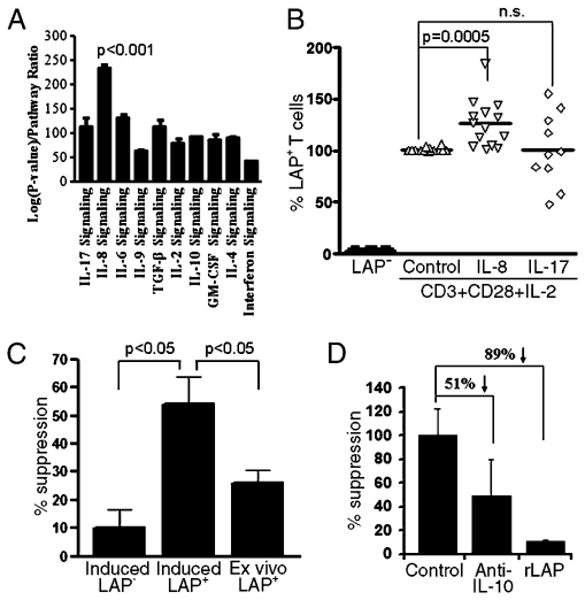
Signaling events in CD4+LAP+ T cells and activation-induced generation of CD4+LAP+ T cells. A, CD4+LAP+ and CD4+LAP− T cells were analyzed by reverse-phase protein array to identify specific cytokine signaling pathways active in CD4+LAP+ T cells. B, CD4+LAP− T cells were cultured in presence of anti-CD3, anti-CD28, and IL-2 in addition to either no cytokine (control) or IL-8 or IL-17 for 6 d. Percentage of CD4+LAP+ T cells post-activation was calculated, assigning the control condition as 100%. C, Activation-induced CD4+LAP+ T cells are more suppressive compared with activation-induced CD4+LAP− T cell population and ex vivo-isolated CD4+LAP+ T cells. Percent suppression for each population is represented as mean (±SD) of three independent experiments. D, Activation-induced CD4+LAP+ T cell suppression is reversed by anti–IL-10 and rLAP. In C and D, a representative experiment of three independent experiments is shown.
To determine whether in vitro-induced CD4+LAP+ T cells are suppressive, in vitro IL-8–differentiated CD4+LAP+ T cells were sorted and cultured in the presence of responder T cells. We found that induced CD4+LAP+ T cells have in vitro-suppressive activity (Fig. 4C). Indeed, the suppressive activity of in vitro IL-8–induced CD4+LAP+ T cells was higher than that observed in freshly isolated CD4+LAP+ T cells from peripheral blood (p < 0.05) (Fig. 4C). The suppressive activity of IL-8–induced CD4+LAP+ T cells could be significantly decreased by rLAP (89%) or anti–IL-10 (51%) (Fig. 4D). Treatment with IL-8 had no significant effect on the suppressive activity of in vitro-induced CD4+LAP+ T cells.
In summary, we have identified a unique population of human Tregs in the peripheral blood, which express LAP on their surface and are different from CD4+Foxp3+CD25high T cells. The induction of this novel subset can be increased in vitro in the presence of IL-8. A number of human autoimmune conditions, including multiple sclerosis, exhibit defects in natural CD4+CD25high Tregs (34). Our findings provide the opportunity to determine if defects in CD4+LAP+ Tregs are also present in human autoimmune conditions and identify CD4+LAP+ Tregs as a therapeutic target for the treatment of autoimmune disorders.
Acknowledgments
This work was supported in part by a grant from the National Multiple Sclerosis Society.
Abbreviations used in this paper
- AAD
aminoactinomycin D
- LAP
latency-associated peptide
- Treg
regulatory T cell
Footnotes
Supplementary Data: http://www.jimmunol.org/cgi/content/full/jimmunol.0903329/D C1
The online version of this article contains supplemental material.
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 6.Savage ND, de Boer T, Walburg KV, Joosten SA, van Meijgaarden K, Geluk A, Ottenhoff TH. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181:2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 8.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 12.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, Weiner HL. CD4+CD25- T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 14.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol. 2008;181:6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 17.Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7327–7337. doi: 10.4049/jimmunol.180.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O'Shea JJ, Shevach EM. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi R, Anderson DE, Weiner HL. Cutting Edge: Immature human dendritic cells express latency-associated peptide and inhibit T cell activation in a TGF-beta-dependent manner. J Immunol. 2007;178:4017–4021. doi: 10.4049/jimmunol.178.7.4017. [DOI] [PubMed] [Google Scholar]
- 21.Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol. 2009;10:958–964. doi: 10.1038/ni.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 26.De Maria R, Cifone MG, Trotta R, Rippo MR, Festuccia C, Santoni A, Testi R. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med. 1994;180:1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Testi R, D'Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 28.Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+ CD4+ CD25− T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Knoops L, Renauld JC. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors. 2004;22:207–215. doi: 10.1080/08977190410001720879. [DOI] [PubMed] [Google Scholar]
- 31.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 33.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci USA. 2009;106:12,885–12,890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]


