Abstract
Neuroadaptations supporting behavioral sensitization to abused drugs are suggested to underlie pathological, excessive motivation toward drugs and drug-associated stimuli. Drug-induced sensitization has also been linked to increased appetitive responses for non-drug, natural reinforcers. The present research investigated whether ethanol (EtOH)-induced neural changes, inferred from psychomotor sensitization, can modify consumption and intake dynamics for the natural reinforcer, sucrose. The effects of EtOH-induced sensitization in mice on the temporal structure of sucrose intake patterns were measured using a lickometer system. Sucrose intake dynamics were measured after sensitization for 1 h daily for 7 days and indicated more rapid initial approach and consumption of sucrose in EtOH-sensitized groups; animals showed a shorter latency to the first intake bout and an increased number of sucrose bottle licks during the initial 15 min of the 1-h sessions. This effect was associated with increased frequency and size of bouts. For the total 1-h session, sucrose intake and bout dynamics were not different between groups, indicating a change in patterns of sucrose intake but not total consumption. When sensitization was prevented by the GABAB receptor agonist baclofen, the increased rate of approach and consumption of sucrose were also prevented. Thus, EtOH-induced sensitization, and not the mere exposure to EtOH, was associated with changes in sucrose intake patterns. These data are consistent with current literature suggesting an enhancing effect of drug-induced sensitization on motivational processes involved in reinforcement.
Keywords: Addiction, Alcohol, Behavioral Sensitization, Motivation, GABAB receptor, Sucrose
Introduction
Progressive, long-lasting enhancements of the behavioral-stimulating effects, as well as significant changes in some neurochemical effects, of abused drugs have been described after repeated drug administration (Heidbreder, et al., 1996; Kalivas and Stewart, 1991; Lessov and Phillips, 1998; Xie and Steketee, 2008). Substantial attention has been focused over the last two decades on the functional relevance and neurobiological basis of this phenomenon known as behavioral sensitization. This interest has been based largely on data showing that drug-induced sensitization is accompanied by an enhancement in drug-seeking and drug-taking behaviors (Nordquist, et al., 2007; Stewart and Badiani, 1993; Vezina, 2004) and in relapse-like behavior (Grimm et al, 2001; Thomas, et al., 2008). Cumulative evidence suggests that neural circuits supporting functions of attribution of biological importance to events and stimuli (i.e., incentive salience), which greatly overlap with those mediating drug-induced behavioral activation, become hypersensitive after repeated drug exposure (Robinson and Berridge, 1993, 2008). Those systems, including mesolimbic dopamine (DA) pathways, are critically influenced by associative learning mechanisms, so that drugs and drug-associated stimuli become increasingly relevant and motivating, which may facilitate pathological drug pursuit and compulsion characteristic of addiction (Robinson and Berridge, 1993, 2008; Vezina, 2004). Drug-induced behavioral sensitization, and especially psychomotor sensitization, has been widely used as an indirect measure of the presence of neural changes thought to be key for the transition from casual to recurrent drug use (Sanchis-Segura and Spanagel, 2006).
In addition to the role of sensitization-related neural alterations in drug addiction, it has been shown that drug-induced sensitization can influence appetitive aspects of behavior directed toward procurement of natural, positive reinforcers. For example, sexual pursuit of male for female rats increased after opiate- or psychostimulant-induced sensitization (Fiorino and Phillips, 1999; Mitchell and Stewart, 1990; Nocjar and Panksepp, 2002); enhanced responding for stimuli associated with water in fluid-deprived rats was found after cocaine-induced sensitization (Taylor and Horger, 1999); a sensitizing regimen of morphine exposure facilitated conditioned feeding of palatable food (Bakshi and Kelley, 1994); and d-amphetamine-induced sensitization was associated with enhanced acquisition of cue-elicited approach to sucrose (Harmer and Phillips, 1998) and with cue-triggered operant responding for stimuli associated with sucrose (Wyvell and Berridge, 2001). These data suggest that neural changes underlying drug-induced sensitization facilitate learning about drug-associated cues and alter motivational processes that support increased and potentially pathological motivation for non-drug reinforcers. This hypothesis has fueled current theories proposing a potential relationship between drug addiction and disorders involving consumption of sweet/caloric food or highly concentrated sugar drinks (Avena, et al., 2008; Carroll, et al., 2008; Holderness, et al., 1994; Kelley, et al., 2005; Lenoir, et al., 2007; Perry et al., 2006).
Ethyl alcohol (ethanol; EtOH) is one of the most widely used addictive drugs (Hines, et al., 2005), and significant relationships between alcoholism and eating disorders as well as sweet preference have been described (Krahn, et al., 2006; Sinha and O’Malley, 2000). Robust psychomotor sensitization to EtOH has been shown using multiple strains of mice (Broadbent, et al., 1999; Didone, et al., 2008; Masur and Boerngen, 1980; Meyer and Phillips, 2007; Lister, 1987; Pastor, et al., 2008; Phillips, et al., 1997), in rats using intracerebroventricular injections (Correa, et al., 2003) and, although rarely studied, also in humans (Newlin and Thomson, 1999). However, the behavioral relevance of EtOH-induced sensitization in the context of natural reinforcers has not been investigated. The use of non-drug reinforcers to evaluate consequences of drug-induced sensitization presents important advantages for the study of motivated behavior, as it allows behavior to be investigated in the absence of interference by drug effects (i.e., motor activation). The present research investigated in mice the effects of EtOH-induced sensitization on 10% sucrose consumption. Due to a lack of studies focusing on dynamics of consummatory aspects of reinforcement after drug-induced sensitization, we examined the effects of EtOH-induced sensitization on the temporal structure of sucrose intake patterns. Patterns and amount of sucrose consumption after the induction of sensitization to EtOH were studied, using a computerized lickometer system. In addition, the importance of EtOH-induced sensitization, as differentiated from EtOH exposure, on patterns of sucrose drinking, was examined using a pharmacological approach. Because prior research showed that the psychomotor sensitizing effects of EtOH could be blocked by the γ-aminobutyric acid (GABA) B receptor agonist (GABAB), baclofen (Broadbent and Harless, 1999), we predicted that mice pretreated with baclofen prior to each EtOH treatment would not exhibit behavioral sensitization, and would show a pattern of sucrose-directed behavior similar to that of mice with no EtOH history.
Materials and Methods
Animals
Genetically heterogeneous WSC-1 male mice (total N = 85; n = 29 in Experiment 1 and n = 56 in Experiment 2) obtained from the Portland Alcohol Research Center colonies were used in these studies. This line was derived from an 8-way cross of genetically diverse inbred strains: A/J, AKR/J, BALB/cJ, C3H/HeJ, C57BL/6J, DBA/2J, Is/Bi, and HS/IBG, and originally used as a non-selected control line for two selected lines (Crabbe, et al., 1985). Mice were 51–70 days old and weighed between 24 and 32 g at study initiation. They had free access to food and water in their home cages (3–4 mice per cage) on a 12:12 h light/dark cycle. Food and water were not available during 1-h sucrose drinking sessions, when mice were singly housed in lickometer chambers. Consumption of water and food in the home cage was recorded daily (per cage) during the entire study; all animals within a cage were assigned to the same treatment group to avoid interaction of mice receiving repeated EtOH treatments with non-EtOH treated mice. Experimental sessions occurred between 10:00 and 16:00 h (lights on at 7:00). Procedures were approved by the Portland VA animal care and use committee and conformed to guidelines specified in the NIH Guidelines for the Care and Use of Laboratory Animals.
Drugs and Drinking Solutions
EtOH was prepared fresh daily by dilution of a 200 proof (100% EtOH v/v) stock (Pharmco Products, Brookfield, CT) to a 20% v/v solution in physiological saline (0.9%, Baxter Healthcare Corporation, Deerfield, IL). Sucrose solution was prepared by dissolving ≥ 99.5% sucrose (Sigma-Aldrich, St. Louis, MO) in tap water to a 10% solution (freshly prepared at least every 3 days). Baclofen (Sigma-Aldrich) was prepared fresh daily at a concentration of 5 mg/10 ml of saline. EtOH, baclofen and control saline injections were administered intraperitoneally (i.p.); saline injection volumes were matched to baclofen or EtOH injection volumes as appropriate. EtOH dosage for induction of sensitization (2 g/kg) followed previous research using WSC mice (Lessov and Phillips, 2003). The concentration of 10% sucrose was based on studies investigating the relationship between sugar intake and drug-induced sensitization (Avena and Hoebel, 2003a,b; Harmer and Phillips, 1998). Baclofen dose (5 mg/kg) for prevention of EtOH-induced sensitization followed the work of Broadbent and Harless (1999).
Experimental Procedures
Experiment 1 included three phases: (i) repeated EtOH or saline treatment for locomotor activity and sensitization acquisition, (ii) sucrose drinking, and (iii) locomotor activity determination after EtOH or saline challenge for a test of the expression of sensitization and of basal activity. For both sucrose drinking and locomotor activity tests, animals were moved from their colony room to the procedure room at least 45 min before study initiation to allow for acclimation. Animals were divided into two groups: EtOH (E) and saline (S). These groups underwent the behavioral sensitization procedure, phase (i), detailed below (Days 1–12). Then, from days 13–19, phase (ii), animals of both groups were offered sucrose in 1-h limited access sessions, as described under Sucrose drinking below. Finally, in phase (iii), locomotor activity after 2 g/kg of EtOH (day 20), and then after saline (day 21), was assessed in all animals. Please see Supplementary Materials for methods of a study in which sucrose drinking was also measured prior to the induction of EtOH-induced sensitization.
Experiment 2 studied the effects of baclofen (5 mg/kg) on EtOH-induced sensitization and on subsequent sucrose drinking. This experiment included four groups: saline-saline (S-S), saline-EtOH (S-E), baclofen-saline (B5-S) or baclofen-EtOH (B5-E), and followed the same general procedures as Experiment 1. However, on days 1–12, saline or baclofen was administered 30 min prior to saline or EtOH (2 g/kg). Baclofen has been shown to reduce EtOH-induced stimulation or sensitization when administered 5–30 min prior to EtOH (Arias, et al., 2009; Broadbent and Harless, 1999; Cott, et al., 1976; Humeniuk, et al., 1993; Knapp, et al., 2007; Shen, et al., 1998). We used a 30-min pretreatment as the longer pretreatment interval allowed time for the animals to calm down from handling prior to treatment with saline or EtOH. Following this induction phase, all animals were exposed to sucrose drinking in lickometer cages as described for phase (ii) in Experiment 1. After this one-week sucrose drinking phase, all animals received saline 30 min before EtOH (2 g/kg), and were then tested for locomotor activity. On the following day, all animals received saline-saline injections (spaced by 30 min) before locomotor activity measurement.
Sucrose drinking
Intake sessions were conducted using 8 custom-made lickometer chambers similar to those used in previous studies (Ford, et al., 2008). These four-walled transparent Plexiglas boxes had an inner floor area of 17.8 x 10.2 cm and a height of 10.2 cm. A stainless steel wire floor insert (VWR, Tualatin, OR) was placed on the chamber floor. The cages had two small portholes located along the back wall to permit access to metal sipper tubes. A single tube per cage was used; the extra hole that was not used was blocked with transparent tape to reduce exploratory behavior directed toward this aperture that might compete with sucrose-directed behavior. A perforated Plexiglas lid attached to the top of each chamber by a Plexiglas hinge allowed for sufficient ventilation. Stainless steel sippers (Ancare, Bellmore, NY) were adjoined to polystyrene serological pipettes (10 ml reservoir; VWR, Tualatin, OR) that were mounted on one outside wall. These drinking tubes permitted volume measurements to the nearest 0.05 ml. Volumes were measured at the time of tube placement and at the end of 1-h drinking sessions.
The wire chamber floor and the metal sipper formed an open electrical circuit that was attached to a lickometer device (MED Associates, Inc., St. Albans, VT). The animal closed the circuit by simultaneously contacting the floor and drinking spout (licking), which permitted registration of cumulative lick records by MED-PC IV software (MED Associates, Inc.) via an interface between an IBM-compatible computer and the lickometers. Independent cumulative sipper contact (lick) records were generated by MED-PC IV software and compiled by a custom data analysis program that calculated the following consumption pattern traits: total sipper contacts (licks), number of bouts, bout size (licks), bout length (min), inter-bout interval (IBI; min), bout lick rates (licks/min), and latency to first bout (min). Based upon previous work in mice examining self-administration patterns of a 10% EtOH solution (Ford et al., 2005, 2008), and our own pilot studies with 10% sucrose, a bout was defined as a series of at least 20 licks with less than 1 min separating each of them. Animals that failed to complete at least one bout during the entire 1-h session on any sucrose drinking day were removed from the study (n = 2/87).
Behavioral Sensitization
The acquisition and expression of psychomotor sensitization to EtOH was investigated using a 21-day protocol (Meyer and Phillips, 2003; Mitchell, et al., 2006). Mice were designated to be in a chronic saline or EtOH group. On days 1–2, all mice received saline before being placed in the activity chambers for 15 min; this served to familiarize the mice with the test procedures and provided a measure of baseline activity. On Days 3–12, mice were injected with saline or 2 g/kg EtOH. On Days 3, 6, 9, and 12, animals were placed in the activity chamber, immediately following the injection, and locomotor activity was measured (15 min). These tests provided measures of sensitization development. On days 4, 5, 7, 8, 10 and 11, activity was not tested; animals were returned to their home cages following acclimation and injections in the testing room. From days 13–19, animals were not injected or tested for locomotor activity but were exposed to sucrose drinking in the lickometer cages. On day 20, all animals received 2 g/kg EtOH to assess the expression of sensitization. Finally, on day 21, all animals were evaluated for locomotor activity after saline to permit comparison of drug-free activity levels before and after repeated EtOH.
Statistical Analyses and Data Treatment
Results were analyzed with one-, two- or three-way ANOVAs with repeated measures when appropriate. Significant interactions were examined for simple effects, and Newman-Keuls tests were used for mean comparisons. Statistica 6.1 (StatSoft, Inc., Tulsa, OK), SoftCR™ 4.0 Cumulative Record Graphical Software (MED Associates, Inc.) and the “R” Project for Statistical Computing (Free Software under the Foundation’s GNU General Public License) software packages were used.
Results
Experiment 1
EtOH-induced locomotor sensitization (Fig 1)
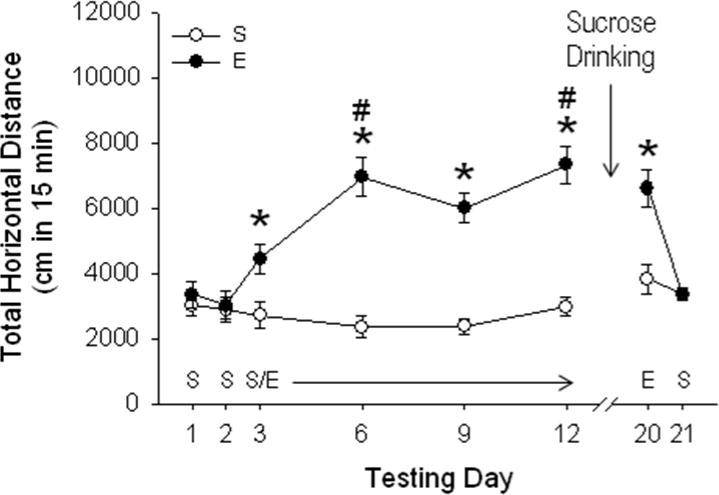
Figure 1.
EtOH produces sensitization to its initial locomotor activating effects; the expression of locomotor sensitization to EtOH is not altered by sucrose drinking. Horizontal distance traveled (cm; mean ± SEM) after saline (S) or 2 g/kg EtOH (E) is shown in mice without previous sucrose drinking experience. Testing occurred on the days listed along the x-axis after treatment with S or E (n=14–15 per group). On days 1–2, the habituation phase, all animals received S. Mice were injected daily with saline or E on days 3–12, which served as the sensitization acquisition phase. On days 13–19, all mice were offered access to sucrose in 1-h sessions. They were all then tested for locomotor activity after E (day 20) and S (day 21) for evaluation of expression of sensitization and basal levels of activity, respectively. Mice showed significant sensitization (increased response to E on days 6–12, compared to day 3). Seven days after the last E administration, animals continued to express sensitization, and sucrose drinking did not alter this expression of E sensitization (day 20). There were no significant effects of sucrose drinking or prior E exposure on final locomotor response after saline treatment (day 21). *p < 0.01 for the difference between E and S groups (simple effect analysis). # p < 0.01 for the difference between E treated mice on day 6 and 12 vs. day 3, indicating significant sensitization (Newman-Keuls mean comparisons).
A repeated measures 2-way ANOVA (EtOH history x test day) for day 1 and 2 data identified no differences between the groups in baseline activity level prior to the EtOH sensitization phase. EtOH produced significant acute stimulation and sensitization to its initial locomotor-stimulating response. Behavioral data registered during days 3–12 (the acquisition of sensitization phase), were analyzed using a two-way ANOVA with repeated measures (EtOH dose x test day), which identified an EtOH dose x test day interaction [F3, 81 = 5.3; p < 0.01]. There was a significant acute stimulant response on day 3, and significant sensitization was present as early as the second EtOH test day (see Fig 1). Statistical analysis of the day 20 data to determine whether EtOH sensitization was expressed identified a main effect of previous repeated EtOH administration [F1, 26 = 7.63; p < 0.05]. This indicates that sensitization to EtOH persisted for at least 7 days after the last EtOH administration. In addition, the similarity of the stimulant responses of the EtOH group on days 12 and 20 indicate that sucrose drinking did not alter the expression of sensitization. Further, the similarity of the acute EtOH stimulant responses of the EtOH group on day 3 and of the saline group on day 20 indicate that sucrose drinking did not cross-sensitize the mice to EtOH. Finally, locomotor activity measured after saline at the conclusion of all testing (day 21) showed no differences between groups, indicating no effects of sucrose or EtOH exposure on levels of baseline activity. EtOH stimulation and sensitization results in animals that had previous sucrose experience were similar and are described in the Supplementary Materials (Fig S1), along with a discussion of previous investigations of the effects of natural reinforcers on drug-induced sensitization.
Consumption of sucrose: Total 1-h period (Fig 2)
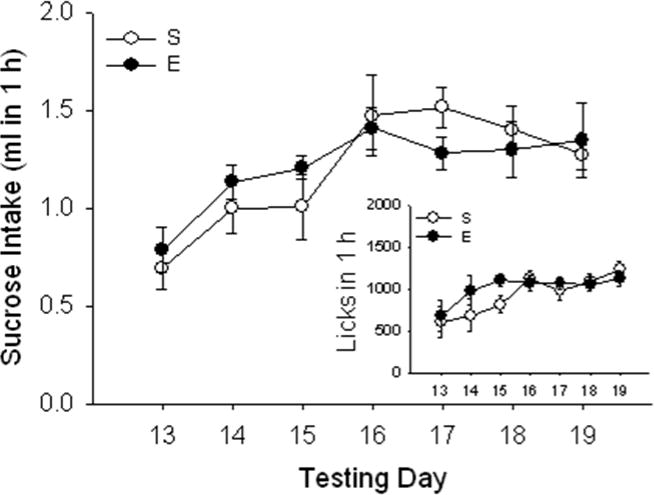
Figure 2.
Total sucrose intake in 1 h is not altered by EtOH-induced sensitization. Sucrose intake (ml; mean ± SEM) after saline (S) or EtOH (E) administration for sensitization induction in the same animals described for Fig. 1. There was a main effect of time but no effect of E. Comparable results were found for number of licks (mean ± SEM; Fig 2 inset). A strong positive correlation was found between ml and licks.
Sucrose consumption increased with experience across days; there was a significant effect of test day from a two-way EtOH dose x test day ANOVA [F6, 162 = 3.47; p < 0.01], but no saline vs. EtOH group difference or group by day interaction for total sucrose consumption. Statistical results were comparable for number of licks (see Fig 2, inset), which strongly correlated with ml of sucrose consumed (r2 = 0.97, p < 0.01 across all days of the study).
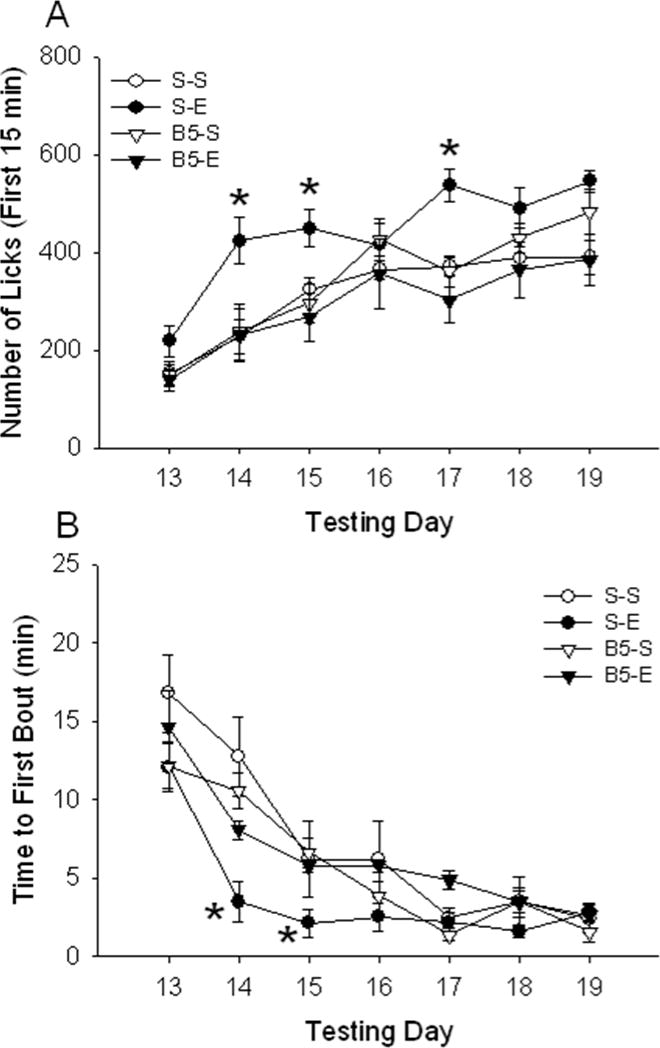
Consumption of sucrose: First 15 min (Fig 3A)
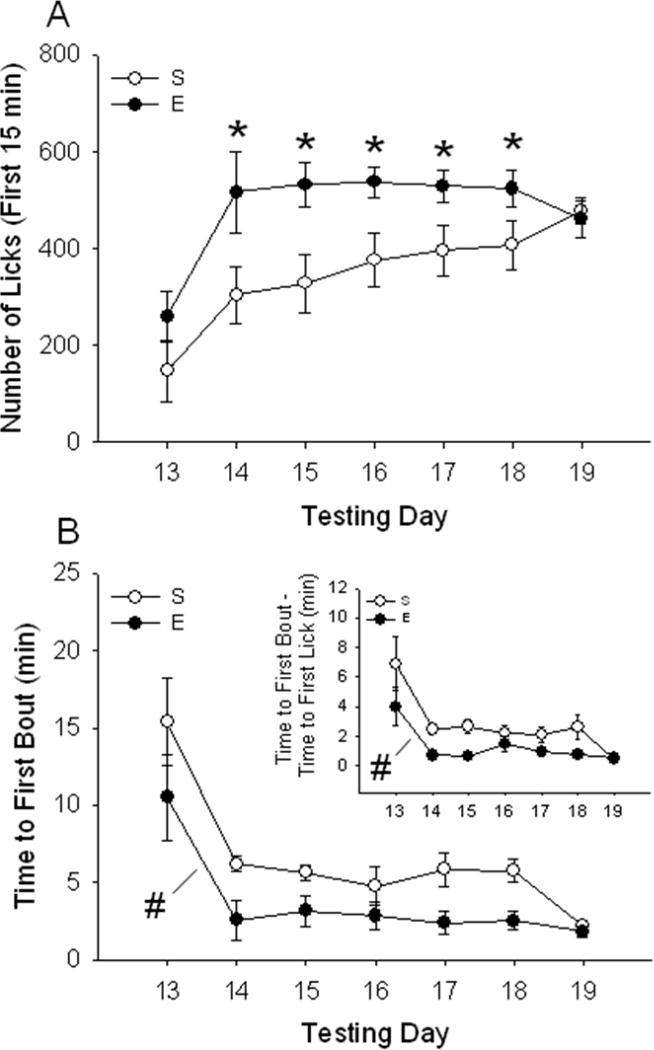
Figure 3.
Consumption of sucrose, indexed as number of licks from the sucrose bottle, is increased in EtOH sensitized mice during the initial 15 min of the session. Sucrose was offered on the days listed along the x-axis. The top panel (A) shows number of sucrose licks (mean ± SEM) during the first 15 min of the 1-h test. *p < 0.05: significantly different from saline on that particular day (simple effect analysis). The bottom panel (B) shows latency (in min) to start the first sucrose bout (mean ± SEM) after EtOH sensitization; EtOH sensitized mice presented a reduced latency to initiate the first bout of sucrose drinking (#p < 0.05 for the main effect of EtOH). Time elapsed between the first lick and the first lick that initiated the first bout is shown in Fig 3B inset. Time (mean ± SEM) was found to be significantly reduced in EtOH-treated animals (#p < 0.05 for the main effect of EtOH). E: EtOH, S: Saline.
Analysis of the total 1-h sucrose consumption data suggested that there was no effect of prior EtOH treatment and sensitization on sucrose consumption (or licks). However, when data were scrutinized for time-dependent effects, a different conclusion was reached. Fig 3A shows the number of licks accumulated during the first 15 min of the total 1-h session. A two-way ANOVA with repeated measures (EtOH dose x test day) revealed a significant interaction of previous repeated saline vs. EtOH administration and test day [F6, 162 = 2.38; p < 0.05]. Significant increases in sucrose drinking in animals that had been previously exposed to EtOH compared to those that had received saline were found on days 14–18. No significant effect of EtOH dose or interaction of EtOH dose and test day was found for the time periods (data not shown).
Latency to the first bout of sucrose consumption (Fig 3B)
The increase in number of licks during early time periods of the drinking sessions led us to speculate that EtOH-exposed mice might be approaching the sucrose sipper, or initiating the first drinking bout, more rapidly. When time to initiate the first bout of sucrose drinking was examined, it was found to be shorter in EtOH-sensitized animals (Fig 3B). A repeated measures two-way ANOVA (EtOH dose x test day) showed main effects of EtOH dose [F1, 27 = 13.96; p < 0.01] and test day [F6, 162 = 18.46; p < 0.01], but no interaction of these two factors. We also found that time between the first lick of the session and the first lick that was part of the first bout (Fig 3B, inset) was significantly shorter in EtOH-treated animals [F1, 27 = 13.91; p < 0.01]. General effects of EtOH treatment on the microstructure of sucrose drinking could be seen by comparing the cumulative lick patterns of saline- vs. EtOH-treated animals; the different patterns for two animals with nearly identical numbers of total licks are shown in Fig S4 of the Supplementary Materials.
Other changes in patterns of sucrose drinking after EtOH-induced sensitization (Table 1)
Table 1.
Bout dynamics of sucrose drinking after EtOH-induced sensitization: total 1 h and first 15-min period.
| Day | Group | Number | Size | Length | IBI | Rate | Group | Number | Size | Length | IBI | Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 1 h; | 4.7 ± 0.9 | 41.2 ± 9.3 | 1.3 ± 0.1 | 4.1 ± 0.4 | 112 ± 39 | 15 min; | 1.7 ± 0.9 | 57.2 ± 9.3 | 2.1 ± 0.2 | 6.2 ± 0.7 | 102 ± 49 |
| 14 | S | 7.6 ± 1.1 | 50.1 ± 9.0 | 0.9 ± 0.2 | 4.5 ± 0.4 | 128 ± 37 | S | 2.6 ± 0.7 | 50.1 ± 9.0 | 1.2 ± 0.2 | 5.2 ± 0.3 | 108 ± 37 |
| 15 | 8.6 ± 1.0 | 67.8 ± 6.4 | 0.9 ± 0.2 | 4.2 ± 0.6 | 235 ± 48 | 3.3 ± 1.2 | 57.8 ± 6.4 | 1.2 ± 0.2 | 5.1 ± 0.5 | 261 ± 68 | ||
| 16 | 10.7 ± 1.2 | 75.8 ± 6.1 | 0.9 ± 0.3 | 2.8 ± 0.2 | 251 ± 41 | 3.7 ± 1.0 | 60.4 ± 4.1 | 0.9 ± 0.3 | 4.1 ± 0.2 | 222 ± 49 | ||
| 17 | 11.7 ± 1.1 | 87.1 ± 5.9 | 1.3 ± 0.2 | 3.6 ± 0.5 | 289 ± 29 | 4.7 ± 1.1 | 67.1 ± 5.9 | 1.1 ± 0.2 | 3.6 ± 0.5 | 289 ± 29 | ||
| 18 | 12.9 ± 1.0 | 84.8 ± 6.6 | 1.1 ± 0.2 | 3.1 ± 0.2 | 322 ± 37 | 5.3 ± 0.5 | 64.8 ± 6.6 | 1.2 ± 0.1 | 4.1 ± 0.3 | 317 ± 42 | ||
| 19 | 14.2 ± 1.1 | 85.1 ± 7.4 | 1.1 ± 0.1 | 3.1 ± 0.3 | 348 ± 40 | 5.5 ± 0.3 | 75.1 ± 7.4 | 1.1 ± 0.3 | 4.1 ± 0.2 | 319 ± 40 | ||
| 13 | 1 h; | 8.9 ± 1.3 | 59.1 ± 7.1 | 1.1 ± 0.2 | 2.8 ± 0.3 | 187 ± 41 | 15 min; | 2.4 ± 0.9 | 49.1 ± 8.1 | 1.8 ± 0.1 | 5.8 ± 0.3 | 188 ± 53 |
| 14 | E | 9.7 ± 1.7 | 69.7 ± 7.9 | 1.3 ± 0.3 | 2.9 ± 0.5 | 319 ± 40 | E | *4.7 ± 0.5 | *90.7 ± 9.9 | 1.9 ± 0.1 | 4.9 ± 0.5 | 219 ± 47 |
| 15 | 13.8 ± 1.4 | 78.8 ± 8.7 | 0.9 ± 0.2 | 3.1 ± 0.3 | 235 ± 49 | *4.9 ± 0.4 | *99.8 ± 6.3 | 1.6 ± 0.3 | 4.1 ± 0.3 | 265 ± 49 | ||
| 16 | 10.1 ± 1.1 | 85.8 ± 6.1 | 1.1 ± 0.1 | 3.1 ± 0.3 | 251 ± 44 | *4.4 ± 0.9 | *86.8 ± 6.1 | 1.1 ± 0.2 | 3.7 ± 0.3 | 283 ± 44 | ||
| 17 | 14.4 ± 0.9 | 74.1 ± 7.7 | 1.1 ± 0.3 | 2.6 ± 0.3 | 289 ± 37 | *5.3 ± 0.3 | 84.7 ± 4.9 | 1.1 ± 0.3 | 3.9 ± 0.3 | 328 ± 37 | ||
| 18 | 13.2 ± 1.0 | 78.9 ± 6.9 | 1.3 ± 0.2 | 3.0 ± 0.3 | 322 ± 42 | 5.1 ± 1.0 | 76.7 ± 5.3 | 1.2 ± 0.1 | 3.3 ± 0.3 | 312 ± 45 | ||
| 19 | 14.1 ± 1.1 | 75.1 ± 7.9 | 1.3 ± 0.2 | 2.6 ± 0.2 | 348 ± 41 | 6.2 ± 0.7 | 76.8 ± 7.4 | 1.2 ± 0.1 | 3.1 ± 0.1 | 309 ± 62 |
Bout variables: number, size (number of licks), length (min), inter-bout-interval (IBI; min), and rate (licks/min). Animals were offered sucrose for seven consecutive days (1 h, daily) after a repeated saline (S) or 2 g/kg EtOH (E) treatment phase to induce psychomotor sensitization.
Significantly different from its respective S control group on the same day (p < 0.05).
Results for additional drinking pattern variables are included in Table 1, which summarizes data collected during the post-EtOH drinking phase (days 13–19) for the entire 1 h and the first 15 min of each session. There were no statistically significant group differences for these drinking pattern variables, when the entire 1-h period was considered, although there was a trend for a main effect of EtOH (p = 0.067) for number of bouts. However, similar to the results for sucrose consumption, effects of prior EtOH-induced sensitization on bout dynamics were found for the initial 15-min period of the sucrose drinking sessions. During this time, an increase in the size and number of bouts were found in the EtOH-sensitized mice (see Table 1). Two-way ANOVAs with repeated measures (EtOH dose x test day) identified a significant interaction of EtOH dose and test day for size [F6, 162 = 9.33; p < 0.05] and number of bouts [F6, 162 = 16.18; p < 0.01]. Other bout dynamic variables were not found to differ between groups in this first 15-min period. During the second 15-min period (data not shown), a significant EtOH dose x test day interaction was found only for number of bouts [F6, 162 = 6.37; p < 0.05]; there was an increased frequency of bouts in EtOH sensitized animals on days 15 and 17 (p < 0.05). There were no significant group differences or interactions with day for the remaining time periods.
Experiment 2
Effect of baclofen on the development of EtOH-induced psychomotor sensitization (Fig 4)
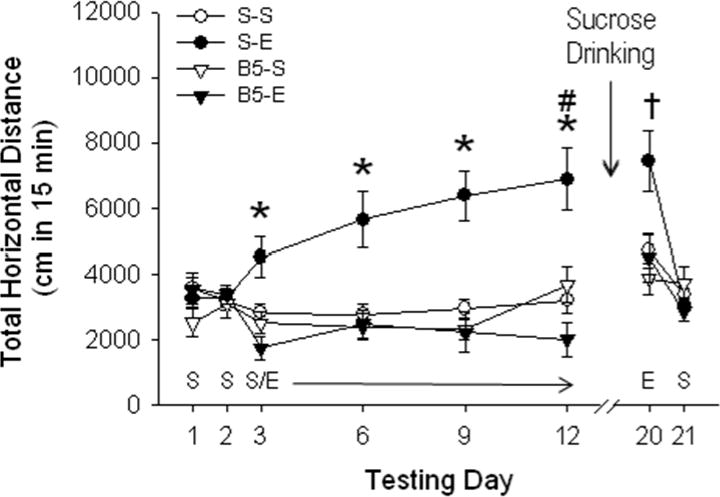
Figure 4.
Baclofen blocks the acute locomotor stimulant response, as well as acquisition of locomotor sensitization to EtOH. Distance traveled (cm; mean ± SEM) after saline (S) or 2 g/kg EtOH (E) is shown following pretreatment with saline or 5 mg/kg baclofen (B5) 30 min prior to activity tests on days 1–12 (n=13–15 per group). * p < 0.01 for the comparison of S-E vs. B5-E on days 3, 6, 9 and 12, # p < 0.01 for the comparison of S-E day 12 vs. day 3. † p < 0.01 for the comparison of S-E vs. the rest of the groups on day 20.
The administration of 5 mg/kg of baclofen prior to each EtOH treatment prevented acquisition of EtOH-induced locomotor sensitization. A three-way ANOVA with repeated measures (EtOH dose x baclofen dose x test day) for data from the baclofen pre-treatment/EtOH sensitization phase (days 3–12) revealed a significant 3-way interaction [F3, 129 = 3.6; p < 0.01]. Further analyses revealed significant EtOH dose x baclofen dose interactions on each of the treatment phase days (3, 6, 9 and 12). Mean comparisons showed that the S-E group had significantly greater activity levels compared to the B5-E group across the entire treatment phase. No significant effect of baclofen pretreatment was found in the B5-S compared to the S-S group. The blocking effect of baclofen on EtOH-induced psychomotor sensitization was also present in the post-sucrose drinking expression test (day 20), again indicating that baclofen blocked the acquisition of sensitization to EtOH. A two-way ANOVA (EtOH dose x baclofen dose) identified a significant interaction effect [F1, 43 = 4.1; p < 0.05], and pairwise comparisons confirmed significant sensitization in the S-E group, compared to the other three treatment groups. No differences among groups were found on saline test day 21.
Effects of the prevention of EtOH-induced sensitization by baclofen on the consumption of sucrose: Total 1 h period
Similar to what was found in our initial study (Fig 2), animals showed stable sucrose intake when it was offered after the sensitization phase (sucrose consumption data not shown). This intake followed a time-dependent course, showing an average (mean of all groups) of 0.4 ± 0.08 ml in 1 h on the first sucrose drinking day and of 1.2 ± 0.1, 1.4 ± 0.09 and 1.3 ± 0.09 ml in 1 h on the last three days of sucrose intake. Also consistent with our initial study, previous EtOH exposure had no significant effect on total sucrose consumption. There was no effect of previous blaclofen on total sucrose consumption either. Analysis of this data set with a 3-way ANOVA with repeated measures (previous EtOH dose x previous baclofen x test day) only showed a significant effect of test day [F6,264 = 6.2; p < 0.01]. These results are similar to those of the initial study for the accumulated 1-h session data. Bout pattern traits of sucrose drinking after EtOH and baclofen treatments are shown in Table S2 of the Supplementary Materials.
Effects of the prevention of EtOH-induced sensitization by baclofen on the consumption of sucrose: First 15 min (Fig 5A)
Figure 5.
Baclofen prevents the increase in sucrose consumption and decreased latency to initiate sucrose consumption seen in EtOH-sensitized mice. Panel A: number of licks (mean ± SEM) during the initial 15 min of the 1-h sucrose session in animals sensitized to EtOH (E) or injected with saline (S), with or without baclofen 5 mg/kg (B5) pretreatment for the same animals described for Fig 4. Baclofen prevented the increased number of sucrose licks seen in S-E mice on some test days. * p < 0.05 for the comparison of the S-E and B5-E groups on days 14, 15 and 17 (simple effect analysis). Panel B: Time (min) to first bout (mean ± SEM) of sucrose drinking. Baclofen prevented the reduced latency to initiate the first bout of sucrose drinking seen in S-E mice on days 14 and 15. * p < 0.05 for the comparison of the S-E and B5-E groups on the indicated days (simple effect analysis).
When data for the first 15 min of the 1-h session were examined, a different outcome was obtained. EtOH sensitized mice again showed an increase in sucrose intake during this time period and prior baclofen treatment prevented that increase. A 3-way ANOVA with repeated measures (baclofen dose x EtOH dose x test day) revealed a significant 3-way interaction [F6,258 = 3.7; p < 0.01]. Further analyses explored the baclofen dose x EtOH dose interaction on each day. This interaction was significant on days 14 [F1,43 = 4.2; p < 0.05], 15 [F1,43 = 3.7; p < 0.05] and 17 [F1,43 = 11.4; p < 0.01]. On those days, the S-E group had a higher number of sucrose tube licks compared to the S-S group, but mice treated with baclofen prior to EtOH treatment did not show this elevation in sucrose drinking.
Effects of the prevention of EtOH-induced sensitization by baclofen on latency to the first bout of sucrose drinking (Fig 5B)
Prior baclofen treatment also prevented the reduction in latency to initiate the first sucrose bout seen in EtOH-sensitized mice that had not received baclofen. A 3-way ANOVA with repeated measures (baclofen dose x EtOH dose x test day) detected a significant 3-way interaction [F6,258 = 2.2; p < 0.05]. Additional analyses that focused on drug effects on each day identified a baclofen dose x EtOH dose interaction on day 14 [F1,43 = 5.5; p < 0.05] and day 15 [F1,43 = 4.7; p < 0.05]. On both days, a significant difference was found between the S-E and the B5-E groups, with baclofen preventing the reduction in time to first bout associated with previous EtOH treatment. Time elapsed between the first lick of the session and the first lick that was part of the first bout (data not shown) was also significantly shorter in EtOH-treated animals, compared to all other groups including the B5-E group.
Discussion
The present experiments show that repeated injections of EtOH, resulting in psychomotor sensitization, modified sugar (10% sucrose) intake patterns, as indicated by an increase in the number and amplitude of drinking bouts, and a reduced latency to initiate the first drinking bout. These effects were most profound during the initial 15-min period of 1-h sucrose drinking sessions. Interestingly, prior induction of sensitization to EtOH did not affect subsequent total sucrose intake, indicating a change in the dynamics of sucrose drinking, and a temporal reorganization of that drinking, rather than an overall increase in intake.
We hypothesized that any change in sucrose intake or intake patterns found after repeated EtOH treatment would be a consequence of sensitization-related neural changes, rather than a general effect of a history of exposure to repeated EtOH. The effects of baclofen treatment found here strongly support this hypothesis. Baclofen was able to prevent the development of sensitization to EtOH and also prevented the changes in sucrose drinking patterns that were associated with EtOH-induced sensitization. Together with previous research (Boehm, et al., 2002; Broadbent and Harless, 1999; Holstein, et al., 2009), these results indicate that GABAB receptors are critically involved in EtOH-induced stimulation and sensitization, and that sensitization might be necessary for the changes in sucrose drinking pattern seen here. It is noteworthy to mention that these effects of baclofen were found in the absence of an effect of baclofen on locomotor activity in saline-treated animals. Also, no effects of prior baclofen treatment alone on later sucrose intake were found. That baclofen was able to prevent both EtOH-induced sensitization and the effect of EtOH exposure on sucrose drinking dynamics rules out certain alternative explanations. For instance, it could be speculated that EtOH had dehydrating actions or effects on sugar metabolism (Wiese, et al., 2000) that might alter motivation for sucrose intake in the direction of the results found here. If that was the case, however, all EtOH-treated animals should have shown similar effects on sucrose drinking regardless of baclofen administration. Also, the records of home cage water and food intake (data not shown) during the EtOH sensitization phase did not show any significant effects of EtOH on these variables. We did find a small, insignificant reduction (15%) in home cage water intake during the sucrose drinking phases that was seen regardless of the treatment condition, probably as a result of the consumption of water in the sucrose solution.
Mice did not require previous experience with sucrose to show post-EtOH changes in sucrose drinking. Visual inspection of the data suggests that previous sucrose consumption may have facilitated re-acquisition of sucrose intake (see Supplementary Materials Figs S2 and S3); however, a robust effect of sensitization to EtOH on sucrose drinking patterns was found regardless of previous sucrose drinking. A memory of the experience with the reinforcer, then, does not seem to be a requirement for EtOH to produce changes in sucrose drinking patterns. It seems reasonable to hypothesize, therefore, that EtOH-induced sensitization altered the experience of drinking sucrose in a way that promoted the changes in consumption patterns seen here. As discussed in a recent review (Anselme, 2009), there are several theories about the effect of drug exposure on the processing of natural rewards, including increased DA function, allostatic mechanisms, and incentive-sensitization views. A critical factor that might explain our results is a sensitization-related increase in salience or in sensitivity to the motivational effects of sucrose. Previous studies evaluating motivation with operant responding procedures have found that prior drug-induced sensitization increased pursuit of both natural reinforcers and their associated stimuli (Bakshi and Kelley, 1994; Fiorino and Phillips, 1999; Harmer and Phillips, 1998; Mitchell and Stewart, 1990; Nocjar and Panksepp, 2002; Taylor and Horger, 1999; Wyvell and Berridge, 2001). Our study did not include an operant task; however, the temporal reorganization of drinking dynamics found after sensitization suggests an enhanced motivational value of sucrose due to sensitization-related neural changes. We found that EtOH-sensitized animals showed a reduced latency to start the first sucrose drinking bout. EtOH sensitization also resulted in increases in frequency and amplitude of bouts during the initial part of the 1-h session. In addition, when we examined the time interval between the first lick of the session and the first lick that was part of the first bout, we found that sensitized mice invested an average of 40% less time between those two licks than did saline treated animals; once sucrose was experienced, drinking behavior was more rapidly engaged in sensitized animals (see cumulative licking records in Fig S4). This increased motivation toward sucrose drinking in EtOH-sensitized animals may more effectively compete with other biologically relevant, time consuming activities such as exploration-associated behaviors. However, consistent with our previous observations (Phillips, et al., 1997; Pastor, et al, 2008), sensitized and saline treated animals showed comparable levels of locomotor activity, when tested in a drug-free state (see day 21, Fig 1); thus, effects of sensitization on sucrose drinking are not likely due to altered general activity levels. In addition, we tested a set of animals (n = 7 sensitized, n = 6 non-sensitized; data not shown) in the lickometer cages with water tubes instead of sucrose for 5 days post-sensitization. Animals consumed very little water under these conditions (mean = 0.2 ± 0.05 ml for the 5 d of the study; likely because they were not fluid deprived), and we found no differences between these two groups in any measure of volume, dynamics of drinking, or timing for the occurrence of first licks, suggesting that exploration of the drinking tube was not different after sensitization. Therefore, increased motivation, and not an enhancement in general exploratory behavior due to sensitization, is a more likely explanation for our current results.
The effects of EtOH-induced sensitization on sucrose drinking were limited to the first 15 min of the 1-h test sessions. The animals were not fluid- or food-deprived, so satiation factors (i.e., a ceiling effect) might have contributed to the timing of the EtOH effect. Increased avidity for sucrose in sensitized animals may have lasted only until satiation factors inhibited drinking. The transient nature of sensitization-induced effects was also seen across sessions, as experience with sucrose was gained. Sensitized and non-sensitized animals showed comparable dynamics of drinking by the end of the 7-day sucrose exposure. We cannot definitively say that these differences in sucrose drinking patterns across days are due to different learning curves between the two groups. However, if the motivational impact of sucrose was amplified by EtOH-induced sensitization, memories about the increased salience of sucrose and its properties could have increased rate of initial approach and consumption of sucrose. Others have argued that reinforcers change the probability of behavior by facilitating acquisition and retention of information and by promoting increased motivation toward them (White and Milner, 1992). Whether or not sensitization facilitated learning about sucrose as a reinforcer is a question that will need to be explored with additional research. However, sensitization-induced increases in motivation for sucrose have been seen previously. Some studies have indicated that drug-induced sensitization does not change the hedonic impact of sweet taste reinforcers, as measured by systematic examinations of positive facial “liking” reactions (Berridge, et al., 2009; Wyvell and Berridge, 2001), but does increase motivation for them. In this regard, it may also be important that, although associative conditioning has been shown to impact drug-induced sensitization, the results of the present study indicate that sensitization can impact behavior toward reinforcers that are not conditioned or associated with the drug itself. This has also been seen in humans. Chronic prodopaminergic drug medication given to some patients, for instance, has been seen to produce sensitized ventral striatal DA neurotransmission and enhanced behavioral manifestations such as food bingeing, compulsive sexual behavior, gambling and increased drug taking (Evans, et al., 2006). The answer to whether or not sensitization-associated neural changes contribute to the explanation for comorbidity between drug abuse and disorders involving consumption of sweet/caloric food or highly concentrated sugar drinks (Avena, et al., 2008; Carroll, et al., 2008; Holderness, et al., 1994; Kelley, et al., 2005; Lenoir, et al., 2007; Perry et al., 2006) will require further research.
A logical direction for the current research would be to expand investigation of the neurochemical factors underlying EtOH sensitization-induced effects on behavior directed toward natural reinforcers. Our results with baclofen suggest a role for GABAB receptor-mediated processes, but their role should be more specifically explored. Strong evidence supports the involvement of striatal DA in drug-induced effects on learning/memory and, particularly, in motivational processes (Berke and Hyman, 2000; Berridge, et al., 2009; Everitt, et al., 2001; Salamone, 1992, 2005). Repeated EtOH treatment, resulting in psychomotor sensitization and cross-sensitization with other drugs, is associated with increased dopaminergic activity in both dorsal and ventral striatal areas (Nestby, et al., 1997; Szumlinski, et al., 2005). DA signaling, especially of mesolimbic structures such as the nucleus accumbens, has been shown to be critical in providing salience (i.e., attractiveness) to reinforcers and stimuli associated with them, as well as in supporting energized and guided behavior toward reinforcers (Berridge, et al., 2009; Salamone, et al., 2005). This analysis of the role of mesolimbic DA function and its enhancement after sensitization might be consistent with our findings and with previous data showing increased pursuit for both natural reinforcers and associated stimuli after drug sensitization (Bakshi and Kelley, 1994; Fiorino and Phillips, 1999; Harmer and Phillips, 1998; Mitchell and Stewart, 1990; Nocjar and Panksepp, 2002; Taylor and Horger, 1999; Wyvell and Berridge, 2001). In this regard, evidence indicates that EtOH-induced GABAB receptor-mediated signaling influences striatal DA activity (Federici, et al., 2009). In the current work, it is possible that baclofen prevented EtOH-induced neuroadaptations in striatal regions, and thus prevented the DA system sensitization necessary to support increased motivation towards sucrose.
In conclusion, evidence from the current research indicates that psychomotor sensitization to EtOH can produce changes in the temporal structure of sucrose intake patterns. GABAB receptor-mediated processes appear to play a role in EtOH sensitization-induced facilitation of initial approach toward and consumption of sucrose. These results are speculatively interpreted as a consequence of sensitization-induced enhancement in motivational processes.
Supplementary Material
Acknowledgments
Supported by a grant from the Department of Veterans Affairs and by NIH/NIAAA grant P60 AA010760. We thank Dr. Sarah E. Holstein, Na Li and Sue Burkhart-Kasch for their technical assistance, and Dr. Amanda L. Sharpe for assistance with the lickometer apparatus.
References
- Anselme P. The effect of exposure to drugs on the processing of natural rewards. Neurosci Biobehav Rev. 2009;33:314–335. doi: 10.1016/j.neubiorev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol’s locomotor-activating effects in preweanling Sprague-Dawley rats. Behav Neurosci. 2009;123:172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross- sensitization to a low dose of amphetamine. Neuroscience. 2003a;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003b;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Sensitization and conditioning of feeding following multiple morphine microinjections into the nucleus accumbens. Brain Res. 1994;648:342–346. doi: 10.1016/0006-8993(94)91139-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115:185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Harless WE. Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice. Psychopharmacology. 1999;141:197–205. doi: 10.1007/s002130050825. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Locomotor stimulant effects of intraventricular injections of low doses of ethanol in rats: acute and repeated administration. Psychopharmacology. 2003;170:368–375. doi: 10.1007/s00213-003-1557-0. [DOI] [PubMed] [Google Scholar]
- Cott J, Carlsson A, Engel J, Lindqvist M. Suppression of ethanol-induced locomotor stimulation by GABA-like drugs. Naunyn Schmiedebergs Arch Pharmacol. 1976;295:203–209. doi: 10.1007/BF00505087. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav Genet. 1985;15:521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- Didone V, Quoilin C, Tirelli E, Quertemont E. Parametric analysis of the development and expression of ethanol-induced behavioral sensitization in female Swiss mice: effects of dose, injection schedule, and test context. Psychopharmacology. 2008;201:249–260. doi: 10.1007/s00213-008-1266-9. [DOI] [PubMed] [Google Scholar]
- Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59:852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Federici M, Nisticò R, Giustizieri M, Bernardi G, Mercuri NB. Ethanol enhances GABAB-mediated inhibitory postsynaptic transmission on rat midbrain dopaminergic neurons by facilitating GIRK currents. Eur J Neurosci. 2009;29:1369–1377. doi: 10.1111/j.1460-9568.2009.06700.x. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABA(A) receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Beckley EH, Nickel JD, Eddy S, Finn DA. Ethanol intake patterns in female mice: influence of allopregnanolone and the inhibition of its synthesis. Drug Alcohol Depend. 2008;97:73–85. doi: 10.1016/j.drugalcdep.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- Hines LM, Ray L, Hutchison K, Tabakoff B. Alcoholism: the dissection for endophenotypes. Dialogues Clin Neurosci. 2005;7:153–163. doi: 10.31887/DCNS.2005.7.2/lhines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderness CC, Brooks-Gunn J, Warren MP. Comorbidity of eating disorders and substance abuse review of the literature. Int J Eat Disord. 1994;16:1–34. doi: 10.1002/1098-108x(199407)16:1<1::aid-eat2260160102>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Holstein SE, Dobbs L, Phillips TJ. Attenuation of the stimulant response to ethanol is associated with enhanced ataxia for a GABA-A, but not a GABA-B, receptor agonist. Alcohol Clin Exp Res. 2009;33:108–120. doi: 10.1111/j.1530-0277.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeniuk RE, White JM, Ong J. The role of GABAB receptors in mediating the stimulatory effects of ethanol in mice. Psychopharmacology. 1993;111:219–224. doi: 10.1007/BF02245527. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn D, Grossman J, Henk H, Mussey M, Crosby R, Gosnell B. Sweet intake, sweet-liking, urges to eat, and weight change: relationship to alcohol dependence and abstinence. Addict Behav. 2006;31:622–631. doi: 10.1016/j.addbeh.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacology. 1998;135:374–382. doi: 10.1007/s002130050525. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Cross-sensitization between the locomotor stimulant effects of ethanol and those of morphine and cocaine in mice. Alcohol Clin Exp Res. 2003;27:616–627. doi: 10.1097/01.ALC.0000062760.17530.74. [DOI] [PubMed] [Google Scholar]
- Lister RG. The effects of repeated doses of ethanol on exploration and its habituation. Psychopharmacology. 1987;92:78–83. doi: 10.1007/BF00215483. [DOI] [PubMed] [Google Scholar]
- Masur J, Boerngen R. The excitatory component of ethanol in mice: a chronic study. Pharmacol Biochem Behav. 1980;13:777–780. doi: 10.1016/0091-3057(80)90206-3. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav Neurosci. 2003;117:641–649. doi: 10.1037/0735-7044.117.3.641. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Behavioral sensitization to ethanol does not result in cross-sensitization to NMDA receptor antagonists. Psychopharmacology. 2007;195:103–115. doi: 10.1007/s00213-007-0871-3. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Stewart J. Facilitation of sexual behaviors in the male rat in the presence of stimuli previously paired with systemic injections of morphine. Pharmacol Biochem Behav. 1990;35:367–372. doi: 10.1016/0091-3057(90)90171-d. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Nestby P, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH, Schoffelmeer AN. Ethanol, like psychostimulants and morphine, causes long-lasting hyperreactivity of dopamine and acetylcholine neurons of rat nucleus accumbens: possible role in behavioural sensitization. Psychopharmacology. 1997;133:69–76. doi: 10.1007/s002130050373. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol. 1999;7:234–243. doi: 10.1037//1064-1297.7.3.234. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RN, Pennartz CM, Vanderschuren LJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol. 2007;17:532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci USA. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology. 2006;186:235–245. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Roberts AJ, Lessov CN. Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacol Biochem Behav. 1997;57:487–93. doi: 10.1016/s0091-3057(96)00448-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology. 1992;107:160–174. doi: 10.1007/BF02245133. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Shen EH, Dorow J, Harland R, Burkhart-Kasch S, Phillips TJ. Seizure sensitivity and GABAergic modulation of ethanol sensitivity in selectively bred FAST and SLOW mouse lines. J Pharmacol Exp Ther. 1998;287:606–615. [PubMed] [Google Scholar]
- Sinha R, O’Malley SS. Alcohol and eating disorders: implications for alcohol treatment and health services research. Alcohol Clin Exp Res. 2000;24:1312–1319. [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology. 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- White NM, Milner PM. The psychobiology of reinforcers. Annu Rev Psychol. 1992;43:443–471. doi: 10.1146/annurev.ps.43.020192.002303. [DOI] [PubMed] [Google Scholar]
- Wiese JG, Shlipak MG, Browner WS. The alcohol hangover. Ann Intern Med. 2000;132:897–902. doi: 10.7326/0003-4819-132-11-200006060-00008. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Repeated exposure to cocaine alters the modulation of mesocorticolimbic glutamate transmission by medial prefrontal cortex Group II metabotropic glutamate receptors. J Neurochem. 2008;107:186–196. doi: 10.1111/j.1471-4159.2008.05593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.