Abstract
Giardia duodenalis and Cryptosporidium spp. infections, and the patterns of cyst and oocyst shedding, were observed in a herd of dairy calves in Ontario over a period of 3 mo. Cysts and oocysts were detected and enumerated in fecal samples using immunofluorescence microscopy; Giardia and Cryptosporidium DNA was detected using the polymerase chain reaction. The prevalence of G. duodenalis increased during the course of the study, reaching a peak of 93.1% when calves were 43 to 54 d old, and then decreased. Conversely, Cryptosporidium spp. prevalence was highest (75.9%) when calves were 11 to 22 d old, and subsequently decreased. The numbers of cysts and oocysts shed per gram of feces were positively correlated over time with the respective prevalence rates. Along with genotyping data, temporal changes in prevalence and shedding patterns should be considered when testing dairy calves for the presence and concentrations of cysts and oocysts, and when considering the potential for zoonotic transmission.
Résumé
Changements temporels dans la prévalence et les tendances d’excrétion des sporocystes de Giardia duodenalis et des oocystes de Cryptosporidium spp. dans un troupeau de veaux laitiers en Ontario. Les infections à Giardia duodenalis et à Cryptosporidium spp. et les tendances de l’excrétion des sporocystes et oocystes ont été observées dans un troupeau de veaux laitiers en Ontario pendant une période de plus de 3 mois. Les sporocystes et les oocystes ont été détectés et énumérés dans les échantillons fécaux à l’aide de l’immunofluomicroscopie; l’ADN de Giardia et de Cryptosporidium a été détectée en utilisant la réaction d’amplification en chaîne par la polymérase. La prévalence de G. duodenalis a augmenté pendant la durée de l’étude, atteignant un sommet de 93,1 % lorsque les veaux étaient âgés de 43 à 54 jours, et a diminué par la suite. Inversement, la prévalence de Cryptosporidium spp. était la plus élevée (75,9 %) lorsque les veaux étaient âgés de 11 à 22 jours, et a diminué par la suite. Au fil du temps, il y avait une corrélation positive entre le nombre de sporocystes et d’oocystes excrétés par gramme de fèces et les taux de prévalence respectifs. Les changements temporels de la prévalence et des tendances d’excrétion devraient être considérés, avec les données de génotypage, lors des tests auprès des veaux laitiers pour déceler la présence et les concentrations de sporocystes et d’oocystes et lors de la considération du potentiel de la transmission zoonotique.
(Traduit par Isabelle Vallières)
Introduction
Giardia duodenalis and Cryptosporidium spp. are common protozoan parasites that cause diarrhea in humans and other mammals, including livestock, cats, dogs, and wild animals worldwide (1–5). In recent years, these parasites have been the subject of numerous prevalence studies in livestock worldwide. The prevalence of both parasites has been reported to be high in cattle, particularly calves, in which 100% cumulative prevalences have been reported (6–10). In Canada, the prevalence in dairy calves ranges from 28% to 73% for G. duodenalis, and from 15% to 46% for Cryptosporidium spp. (11–14). In a study done in Quebec, 89% of the dairy farms in which calves were sampled were positive for Cryptosporidium spp. (15).
Giardia duodenalis cysts have been reported in calves as young as 4 to 5 d old (6,16). The peak prevalences of giardiasis in dairy calves occur over a wide age range, from approximately 4 to 20 wk (7,17–21). A recent longitudinal study in dairy calves found that the highest prevalence of infection with G. duodenalis occurred in pre-weaned calves, with a peak prevalence at 4 and 5 wk of age (10). Cryptosporidium spp. infections occur in dairy calves as young as 2 to 6 d old (6,16,17,22,23). The majority of studies have reported C. parvum infections to be most prevalent in calves 2 to 4 wk old (7,9,11,22,23), although slightly earlier peaks have also been reported (17,18,24). Conversely, Hamnes et al (21) reported that Cryptosporidium infections peaked in older dairy calves that were ~8 to 12 wk old. Santín et al (25) reported 2 peaks in prevalence; one at 2 wk old and a second smaller peak at 6 mo. A longitudinal study of cryptosporidiosis in dairy cattle demonstrated that the highest prevalence of infection occurred in pre-weaned calves, with a significant decrease with the age of the animals (9). These authors reported peak prevalence at 2 wk old, and a second smaller peak at 18 wk.
Several studies have also reported temporal changes in the fecal counts of cysts and oocysts in dairy calves. The numbers of Giardia cysts shed by calves peaked at 2 wk of age, and high numbers continued to be shed until 7 wk (6). Similarly, in British Columbia, the highest cyst counts were found in dairy calves that were 2 to 4 wk old (11). Conversely, in a study done in Alberta, Giardia cyst counts did not reach a maximum until dairy calves were 89 d old (7). Cryptosporidium oocyst counts generally peak in calves at an earlier age than do Giardia cyst counts. Xiao and Herd (6) reported that oocyst counts peaked at 1 wk of age and then declined to low numbers by 3 wk. Similarly, Huetink et al (17) reported the highest oocyst counts in calves between 9 and 29 d old. In British Columbia, the highest counts were found in dairy calves from 0 to 2 wk old for Cryptosporidium (11), whereas in Alberta Cryptosporidium oocyst counts peaked in dairy calves between 10 and 19 d old, and subsequently declined and remained low (7).
The purpose of the present study was to determine the prevalence and shedding patterns, over time, of Giardia duodenalis and Cryptosporidium spp., in dairy calves housed at an agricultural college in Ontario, Canada, and their implications on animal-to-animal and zoonotic transmission.
Materials and methods
Source and collection of specimens
Male Holstein dairy calves used in this study originated from several farms near Kemptville, Ontario. At the source farms, calves were fed colostrum from their dams or pooled colostrum from the herd, and were removed from their dams within 12 h of birth. They were purchased in the summer of 2005, transported to Kemptville College by 3 d of age, and entered into this study (sampling day 0) at ages ranging from 11 to 22 d. All calves were housed in individual hutches and provided feed and water ad libitum.
From July to October, 2005, 29 calves were sampled 6 times each. Using an individual disposable latex glove, feces were collected directly from the rectum of each animal and transferred into a plastic cup. Cups were capped, labelled with the animal’s ear tag number, and immediately placed into an insulated container packed with ice or cold packs. Specimens were subsequently refrigerated and processed within 1 to 3 d of collection. All procedures involving the calves were approved by the University of Guelph Animal Care Committee.
Cyst and oocyst concentration from feces
A sucrose flotation technique (7) was used with the following modifications. To 5 g of feces, 10 mL of phosphate-buffered saline (PBS), pH 7.4, was added and thoroughly mixed with an applicator. The suspension was passed through 4 layers of gauze (A.R. Medicom, Montreal, Quebec) and layered over 5 mL of 1 M sucrose (Sigma-Aldrich Canada, Oakville, Ontario) solution (specific gravity 1.13) into a clean polypropylene conical Falcon tube (Becton Dickinson, Oakville, Ontario). The samples were centrifuged at 800 × g for 5 min; the interface and the upper layer of liquid were transferred with a disposable pipette to a clean tube and centrifuged at 800 × g for 5 min. The supernatant was decanted, leaving a final pellet volume of 1 mL.
Immunofluorescence microscopy for Giardia duodenalis and Cryptosporidium spp
After cyst and oocyst isolation and concentration, a 20-μL sample of the concentrate was spotted onto a microscope slide (Fisher Scientific, Pittsburgh, Pennsylvania, USA). Fluorescein isothiocyanate (FITC)-labeled monoclonal antibody solutions (20 μL each of Giardi-a-Glo and Crypt-a-Glo, Waterborne, New Orleans, Louisiana, USA) were applied to the slide, which was then incubated in a humid air chamber at room temperature in the dark for 45 min. After incubation, the slide was briefly irrigated with PBS and covered with a 22 × 22 mm glass cover slip. Examinations were performed with a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan) equipped with epifluorescence optics. Giardia duodenalis cysts and Cryptosporidium spp. oocysts were identified and enumerated at 200× magnification. The number of cysts and oocysts per gram of feces was calculated using the following modification of the formula of O’Handley et al (7).
| Equation 1 |
where: N = number of oocysts per gram feces, s = number of oocysts counted on the slide, pv = pellet volume (approximately 0.5 mL), vol = volume of sample examined (0.02 mL), and wt = weight of fecal sample (g).
DNA extraction
Total DNA was extracted from each sucrose flotation concentrated sample using the DNeasy Tissue Kit (Qiagen, Mississauga, Ontario). Concentrated sample (200 μL) was transferred to 1.5 mL microcentrifuge tubes and lysed overnight at 56°C using 180 μL of lysis buffer and 20 μL of Proteinase K (20 mg/mL) supplied with the DNeasy Tissue Kit. The remaining protocol followed manufacturer’s instructions except that the nucleic acid was eluted in 100 μL of elution buffer to increase the quantity of recovered DNA.
Amplification of Giardia 16S rRNA gene fragments by PCR
Fragments of the 16S rRNA gene were amplified using modifications of previously described polymerase chain reaction (PCR) protocols. A nested-PCR protocol was used with first round primers Gia2029 (5′-AAGTGTGGTGCAGACGGACTC-3′) and Gia2150c (5′-CTGCTGCCGTCCTTGGATGT-3′) amplifying a 497-bp product (26); and secondary primers RH11 (5′-CATCCGGTCGATCCTGCC-3′) and RH4 (5′-AGTCGAACCCTGATTCTCCGCCAGG-3′) generating a 292-bp fragment (27). Polymerase chain reactions consisted of 1X PCR buffer containing 1.5 mM MgCl2, 0.2 mM dNTP’s, 5% (v/v) dimethyl sulfoxide, 2 U Taq polymerase, and 0.5 μM of each forward and reverse primer in a reaction volume of 50 μL. Following an initial hot start at 96°C for 2 min, 35 cycles, each consisting of 96°C for 45 s, 58°C for 30 s, and 72°C for 45 s were performed. A final extension step at 72°C for 4 min was included. For the second round of PCR, the PCR mixtures, the number of cycles, temperatures and times were identical except that the annealing temperature was decreased to 55°C for 30 s. Products from PCR were analyzed on 1% (w/v) agarose gels and visualized by ethidium bromide (Bio-Rad Laboratories, Mississauga, Ontario) staining. Sequencing of DNA was done to confirm PCR positives and to determine the genotypes of Giardia duodenalis present. The methods and results were previously reported (14).
Amplification of Cryptosporidium 18S rRNA gene fragments by PCR
To amplify the 18S rRNA gene fragments a nested PCR protocol was followed, using the following primers previously described by Xiao et al (28): 5′-TTCTAGAGCTAATACATGCG-3′ and 5′-CCCTAATCCTTCGAAACAGGA-3′ for primary PCR, and 5′-GGAAGGGTTGTATTTATTAGATAAAG-3′ and 5′-AAGGAGTAAGGAACAACCTCCA-3′ for nested PCR, to generate an 830 bp fragment. Amplification of PCR was performed in 50 μL volumes as per Santín et al (25) with a slight modification. In brief, 5 μL DNA was added to 1× PCR buffer, 4.5 mM MgCl2, 0.2 mM each dNTP, 2.5 U Taq, 2.5 μL fetal BSA (0.1g/10 mL), and 1 μM of each forward and reverse primer. Samples were subjected to 35 cycles of 94°C for 45 s, 59°C for 45 s, and 72°C for 1 min, with an initial hot start at 94°C for 3 min, and a final extension step at 72°C for 7 min. The nested PCR mixture was identical, except that 1 μL DNA and a final concentration of 3 mM MgCl2 were used. A total of 40 cycles, each consisting of 94°C for 30 s, 58°C for 90 s, and 72°C for 2 min, were performed with an initial hot start at 94°C for 3 min, and a final extension step at 72°C for 7 min. Products from PCR were analyzed on 1% (w/v) agarose gels and visualized by ethidium bromide [Bio-Rad Laboratories (Canada), Mississauga, Ontario] staining. Sequencing of DNA was done to confirm PCR positives and to determine the species of Cryptosporidium. The methods and results were previously reported (14).
Data analysis
Statistical analyses of the data were performed using the statistical software package Splus6.2 (Insightful Corporation, Seattle, Washington, USA) and MS Excel Spread Sheet for Windows (Office 2000 version, for t-test). For data analysis of infection over time, the method of estimation for the cumulative probability function applying Weibull distribution was used. For persistence of infection, a chi-squared test for equality of proportions with 4 degrees of freedom was used.
Results
All 29 calves (100%) shed G. duodenalis cysts and Cryptosporidium spp. oocysts during the study period. The youngest calf on sampling day 0 was 11 d old, and was negative for both G. duodenalis cysts and Cryptosporidium spp. oocysts. However, 4 other calves (13.8%), including a 12-day-old animal, shed both G. duodenalis and Cryptosporidium spp. on sampling day 0.
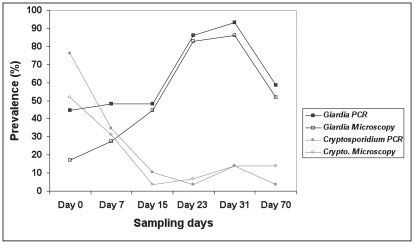
On sampling day 0, 13 of the 29 calves (44.8%) were positive for G. duodenalis based on PCR of a fragment of the 16S rRNA gene, and 5 (17.2%) were positive by microscopy (Figure 1). The percentage of calves shedding G. duodenalis rose steadily with time and reached a peak of 93.1%, by PCR, on sampling day 31 when calves were 43 to 54 d old (Figure 1). By sampling day 31, all 29 calves had shed G. duodenalis cysts on at least 1 occasion. Thereafter, the prevalence of G. duodenalis cysts in the calves began to decrease, but they were still shedding cysts at the end of the study (sampling day 70).
Figure 1.
The prevalence of calves shedding Giardia duodenalis cysts and Cryptosporidium spp. oocysts in relation to sampling day.
The number of calves shedding Cryptosporidium spp. on sampling day 0 was 22 out of 29 (75.8%) based on PCR of a fragment of the 18S rRNA gene, whereas 15 out of 29 (51.7%) calf samples were positive by microscopy (Figure 1). The prevalence of Cryptosporidium spp. was highest on sampling day 0, when the calves were 11 to 22 d of age, and then declined during sampling days 15 to 23. After a small increase on day 31, a drop in the number of positive calves occurred again on sampling day 70, when calves were 81 to 92 d old (Figure 1). Statistical analysis gave a chi-squared value of 22.3 on 5 degrees of freedom, with a P-value of 0.0005, demonstrating a significant variation in the prevalence of cysts and oocysts with respect to sampling days.
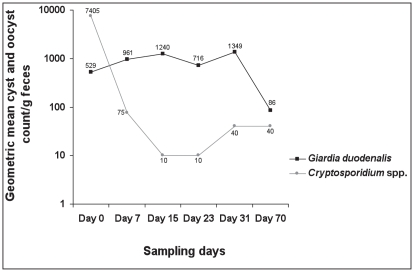
The results of the cyst and oocyst enumerations indicated that, individually, animals shed from 0 to 19,920 G. duodenalis cysts, and from 0 to 75 000 Cryptosporidium spp. oocysts, per gram of feces during the course of this study (results not shown). The geometric mean cyst and oocyst counts over time are shown in Figure 2. On sampling day 0, only 2 (6.9%) calves shed > 1000 G. duodenalis cysts/g of feces, whereas 14 calves (48.3%) shed > 1000 Cryptosporidium spp. oocysts/g, with 3 shedding > 50 000 oocysts/g. During sampling days 7 to 31, the geometric mean number of G. duodenalis cysts and Cryptosporidium spp. oocysts shed/g of feces increased and decreased, respectively (Figure 2), positively correlating with the respective prevalence rates. Statistical analysis based on log counts using ANOVA gave an F-value of 4.65 on 5 and 56 degrees of freedom, with a P-value of 0.0013, demonstrating a significant variation in the number of cysts and oocysts/g of feces with respect to sampling days.
Figure 2.
The geometric mean numbers of Giardia duodenalis cysts and Cryptosporidium spp. oocysts shed by dairy calves over 6 sampling days at Kemptville College, Ontario.
With respect to repeated measurements of the presence and concentration of G. duodenalis and Cryptosporidium spp. in the fecal samples, statistical methods describing the onset of infection (Weibull distribution) and persistence of infection (Pearson chi-squared test) were used. The Weibull distribution estimated that, on or before 40 d of age, an average of 90% of calves will start shedding G. duodenalis cysts. As sampling times were at fixed intervals, no data on the presence of G. duodenalis cysts or Cryptosporidium spp. oocysts in calves were available between collection days, and the estimation procedure took this into account.
Discussion
The present study represents the first temporal study on the prevalence and concentrations of Giardia duodenalis cysts and Cryptosporidium spp. oocysts in dairy calves in eastern Canada, and showed clear trends over time which may be useful in describing the infection dynamics of these pathogens, as well as having implications regarding zoonotic transmission. Both G. duodenalis and Cryptosporidium spp. were detected in each of the 29 calves at some time during the study. This finding is in agreement with earlier studies reporting a 100% cumulative prevalence of these parasites in calves (6–10).
The percentage of the calves shedding G. duodenalis cysts increased over the course of the study with the highest prevalence occurring among calves 43 to 54 d old. Weibull distribution estimated that most calves would be shedding G. duodenalis cysts on or before day 40 of the study. Thereafter, the prevalence of G. duodenalis infections decreased, but approximately half of the calves were still shedding cysts when the animals were 81 to 92 d old. These findings are similar to previously published studies reporting the highest prevalence of G. duodenalis infections in dairy calves approximately 4 to 12 wk old (7,10,18,19,21). Other studies have reported the highest prevalence of G. duodenalis infection in older dairy calves approximately 12 to 20 wk old (17,20). However, in a study in British Columbia, giardiasis was not found to be age dependent as approximately 80% of dairy calves from 2 to 24 wk old were infected (11). These temporal differences in peak prevalence of giardiasis among different studies may be associated with a number of variables including the breed of calves, husbandry practices, and/or the G. duodenalis genotypes involved.
The prevalence patterns over time seen in the present study for Cryptosporidium spp. infections in calves differed from that for G. duodenalis. The highest Cryptosporidium spp. prevalence occurred when the age of the animals ranged from 11 to 22 d. However, since the youngest calf in the study was 11 d old it is possible that the peak shedding period for Cryptosporidium spp. may have been missed in a number of calves. This finding is in agreement with earlier studies (9,11,17,22,23). Subsequently, the prevalence of Cryptosporidium spp. declined and remained low for the duration of the study. A similar decline in prevalence with age has been described in previous studies (18,21,22,24). These results support the conclusion by O’Handley et al (7) that giardiasis may be chronic in dairy calves, whereas cryptosporidiosis may be self-limiting.
Polymerase chain reaction analysis demonstrated a higher prevalence of infection with both G. duodenalis and Cryptosporidium spp. than with immunofluorescence microscopy, but these differences were not statistically significant on any of the sampling days (t-test, P > 0.05). Similarly, PCR was reported to be more sensitive than immunofluorescence microscopy in the detection of Giardia and Cryptosporidium in dairy calves by Santín et al (9,10).
The geometric mean cyst and oocyst counts in the present study were similar to those reported in other Canadian studies (7,11,12). Furthermore, the concentration of cysts and oocysts in the feces over time was positively correlated with the prevalence patterns for both parasites. Other studies have also reported a parallel between the prevalence of infection and the intensity of cyst and oocyst excretion in dairy calves (6,22,23). O’Handley et al (7) reported that the mean number of Giardia cysts in calf feces remained high throughout their study, whereas the number of Cryptosporidium oocysts decreased to low numbers 2 wk after infection.
Becher et al (18) concluded that calf-to-calf contact was the most likely source of transmission of Giardia and Cryptosporidium among dairy calves. In the present study, however, these pathogens were highly prevalent although the calves were housed in individual hutches, and did not have direct contact with other animals. Huetink et al (17) concluded that indirect calf-to-calf transmission by means of vectors is an important route for acquiring infection, at least with Cryptosporidium. Mechanical vectors may include workers or students who frequently handle one calf after another. Other vectors involved in the transmission of G. duodenalis cysts and Cryptosporidium spp. oocysts can include wild mice (29), or, in the summer, houseflies (30), which may contaminate the calves’ feed or water. A further possibility is that cysts and oocysts could have been spread from one hutch to another during run-off following heavy rainfalls in the summer. Hutches may have also been contaminated with cysts and oocysts from previous occupants. The hutch area of Kemptville College has been used for many years, and is often populated with different groups of calves during the spring and summer. In the present study, up to approximately 20 000 G. duodenalis cysts and up to 75 000 Cryptosporidium spp. oocysts/g feces were shed by the calves. Both G. duodenalis cysts and Cryptosporidium spp. oocysts are resistant to adverse environmental conditions, such as temperature extremes, and are capable of remaining infectious for relatively long periods after being passed in feces (1,31). Another important source of infection in calves is cow-to-calf transmission which can occur at birth (17). This source of infection, however, cannot account for the delayed shedding of Cryptosporidium oocysts seen in some calves, nor the subsequent increase in Giardia prevalence.
In addition to the high prevalence of both G. duodenalis and Cryptosporidium spp. in dairy calves in the present study, DNA sequence analyses demonstrated the presence of the zoonotic genotype, G. duodenalis Assemblage B, as well as a predominance of the zoonotic species, C. parvum. We reported these genotyping results in a previous paper (14). A predominance of C. parvum has also been reported in dairy calves in Ontario by Trotz-Williams et al (13,32).
Further studies are warranted to evaluate the public health significance of these findings. Results of this study indicate that infection patterns differ between G. duodenalis and Cryptosporidium spp. and that prevalence rates and intensity of shedding for both parasites depend on the age of the animals. Along with genotyping data, temporal changes in prevalence and shedding patterns should be taken into account when testing dairy calves for the presence and concentrations of cysts and oocysts, and when considering the potential for zoonotic transmission.
Acknowledgments
The authors thank Dennis McKnight, Albert Koekkoek, and Cindy Todd, Kemptville College, University of Guelph, Kemptville, Ontario, for providing access to the calves used in this study. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Fayer R. Cryptosporidium: A water-borne zoonotic parasite. Vet Parasitol. 2004;126:37–56. doi: 10.1016/j.vetpar.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Olson ME, O’Handley RM, Ralston BJ, McAllister TA, Thompson RCA. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 2004;20:185–191. doi: 10.1016/j.pt.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RCA. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet Parasitol. 2004;126:15–35. doi: 10.1016/j.vetpar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Xiao L, Fayer R, Ryan U, Upton SJ. Cryptosporidium taxonomy: Recent advances and implications for public health. Clin Microbiol Rev. 2004;17:72–97. doi: 10.1128/CMR.17.1.72-97.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Handley RM, Olson ME. Giardiasis and cryptosporidiosis in ruminants. Vet Clin North Am Food Anim Prac. 2006;22:623–643. doi: 10.1016/j.cvfa.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Xiao L, Herd RP. Infection patterns of Cryptosporidium and Giardia in calves. Vet Parasitol. 1994;55:257–262. doi: 10.1016/0304-4017(93)00645-f. [DOI] [PubMed] [Google Scholar]
- 7.O’Handley RM, Cockwill C, McAllister TA, Jelinski M, Morck DW, Olson ME. Duration of naturally acquired giardiosis and cryptosporidiosis in dairy calves and their association with diarrhea. J Am Vet Med Assoc. 1999;214:391–396. [PubMed] [Google Scholar]
- 8.O’Handley RM. Giardia in farm animals. In: Olson BE, Olson ME, Wallis PM, editors. Giardia the Cosmopolitan Parasite. New York: CABI Publ; 2002. pp. 97–105. [Google Scholar]
- 9.Santín M, Trout JM, Fayer R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet Parasitol. 2008;155:15–23. doi: 10.1016/j.vetpar.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Santín M, Trout JM, Fayer R. A longitudinal study of Giardia duodenalis genotypes in dairy cows from birth to 2 years of age. Vet Parasitol. 2009;162:40–45. doi: 10.1016/j.vetpar.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Olson ME, Guselle NJ, O’Handley RM, et al. Giardia and Cryptosporidium in dairy calves in British Columbia. Can Vet J. 1997;38:703–706. [PMC free article] [PubMed] [Google Scholar]
- 12.O’Handley RM, Olson ME, Fraser D, Adams P, Thompson RC. Prevalence and genotypic characterization of Giardia in dairy calves from Western Australia and Western Canada. Vet Parasitol. 2000;90:193–200. doi: 10.1016/s0304-4017(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 13.Trotz-Williams LA, Jarvie BD, Martin SW, Leslie KE, Peregrine AS. Prevalence of Cryptosporidium parvum infection in southwestern Ontario and its association with diarrhea in neonatal dairy calves. Can Vet J. 2005;46:349–351. [PMC free article] [PubMed] [Google Scholar]
- 14.Coklin T, Farber J, Parrington L, Dixon B. Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet Parasitol. 2007;150:297–305. doi: 10.1016/j.vetpar.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Ruest N, Faubert GM, Couture Y. Prevalence and geographical distribution of Giardia spp. and Cryptosporidium spp. in dairy farms in Quebec. Can Vet J. 1998;36:697–700. [PMC free article] [PubMed] [Google Scholar]
- 16.Wade SE, Mohammed HO, Schaaf SL. Prevalence of Giardia sp., Cryptosporidium parvum and Cryptosporidium muris (C. andersoni) in 109 dairy herds in five counties of southeastern New York. Vet Parasitol. 2000;93:1–11. doi: 10.1016/s0304-4017(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 17.Huetink RE, van der Giessen JW, Noordhuizen JP, Ploeger HW. Epidemiology of Cryptosporidium spp. and Giardia duodenalis on a dairy farm. Vet Parasitol. 2001;102:53–67. doi: 10.1016/s0304-4017(01)00514-3. [DOI] [PubMed] [Google Scholar]
- 18.Becher KA, Robertson ID, Fraser DM, Palmer DG, Thompson RCA. Molecular epidemiology of Giardia and Cryptosporidium infections in dairy calves originating from three sources in Western Australia. Vet Parasitol. 2004;123:1–9. doi: 10.1016/j.vetpar.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Trout JM, Santín M, Greiner E, Fayer R. Prevalence of Giardia duodenalis genotypes in pre-weaned dairy calves. Vet Parasitol. 2004;124:179–186. doi: 10.1016/j.vetpar.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Trout JM, Santín M, Greiner E, Fayer R. Prevalence and genotypes of Giardia duodenalis in post-weaned dairy calves. Vet Parasitol. 2005;130:177–183. doi: 10.1016/j.vetpar.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Hamnes IS, Gjerde B, Robertson L. Prevalence of Giardia and Cryptosporidium in dairy calves in three areas of Norway. Vet Parasitol. 2006;140:204–216. doi: 10.1016/j.vetpar.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Uga S, Matsuo J, Kono E, et al. Prevalence of Cryptosporidium parvum infection and pattern of oocyst shedding in calves in Japan. Vet Parasitol. 2000;94:27–32. doi: 10.1016/s0304-4017(00)00338-1. [DOI] [PubMed] [Google Scholar]
- 23.Starkey SR, Wade SE, Schaaf S, Mohammed HO. Incidence of Cryptosporidium parvum in the dairy cattle population in a New York City Watershed. Vet Parasitol. 2005;131:197–205. doi: 10.1016/j.vetpar.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 24.Singh BB, Sharma R, Kumar H, et al. Prevalence of Cryptosporidium parvum infection in Punjab (India) and its association with diarrhea in neonatal dairy calves. Vet Parasitol. 2006;140:162–165. doi: 10.1016/j.vetpar.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Santín M, Trout JM, Xiao L, Zhou L, Greiner E, Fayer R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet Parasitol. 2004;122:103–117. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Appelbee AJ, Frederick LM, Heitman TL, Olson ME. Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet Parasitol. 2003;112:289–294. doi: 10.1016/s0304-4017(02)00422-3. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins RM, Meloni BP, Groth DM, Wetherall JD, Reynoldson JA, Thompson RC. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J Parasitol. 1997;83:44–51. [PubMed] [Google Scholar]
- 28.Xiao L, Escalante L, Yang C, et al. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999;65:1578–1583. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klesius PH, Haynes TB, Malo LK. Infectivity of Cryptosporidium sp isolated from wild mice for calves and mice. J Am Vet Med Assoc. 1986;189:192–193. [PubMed] [Google Scholar]
- 30.Graczyk TK, Cranfield MR, Fayer R, Bixler H. House flies (Musca domestica) as transport hosts of Cryptosporidium parvum. Am J Trop Med Hyg. 1999;61:500–504. doi: 10.4269/ajtmh.1999.61.500. [DOI] [PubMed] [Google Scholar]
- 31.Olson ME, Goh J, Phillips M, Guselle N, McAllister TA. Giardia cyst and Cryptosporidium oocyst survival in water, soil, and cattle feces. J Environ Qual. 1999;28:1991–1996. [Google Scholar]
- 32.Trotz-Williams LA, Martin DS, Gatei W, et al. Genotype and subtype analyses of Cryptosporidium isolates from dairy calves and humans in Ontario. Parasitol Res. 2006;99:346–352. doi: 10.1007/s00436-006-0157-4. [DOI] [PubMed] [Google Scholar]