Abstract
Magnetic resonance imaging of a 7.5-year-old neutered male Yorkshire terrier with mild generalized ataxia and intermittent neck scratching led to a diagnosis of caudal occipital malformation and syringohydromyelia. Surgical exploration led to a diagnosis of occipital dysplasia with concurrent occipital hypoplasia. Following a dorsal laminectomy of the first cervical vertebra there was no progression or improvement a month later.
Résumé
Hypoplasie occipitale, dysplasie occipitale, syringohydromyélie et hydrocéphalie concomitante chez un Yorkshire Terrier. L’imagerie par résonance magnétique d’un Yorkshire Terrier mâle castré âgé de 7,5 ans présentant une légère ataxie généralisée et un grattage du cou intermittent a permis de poser un diagnostic de malformation occipitale caudale et de syringohydromyélie. L’exploration chirurgicale a permis de poser un diagnostic de dysplasie occipitale avec hypoplasie occipitale. Après une laminectomie dorsale de la première vertèbre cervicale, il n’y avait aucune progression ou amélioration après un mois.
(Traduit par Isabelle Vallières)
A 7.5-year-old neutered male Yorkshire terrier was presented for evaluation of generalized ataxia and intermittent neck scratching of 10 wk duration. Historical findings included a pulmonary coccidioidomycosis infection in 2006 that resolved with fluconazole (US Pharmacopeia, Rockville, Maryland, USA) therapy. Coccidioidomycosis titers were measured at 1:4 about 3 mo before initial presentation, at which time treatment with fluconazole was initiated, and within 2 mo, the titer decreased to < 1:4. At this time, the patient was seen by the referring veterinarian for continued ataxia in all limbs and intermittent neck scratching.
Case description
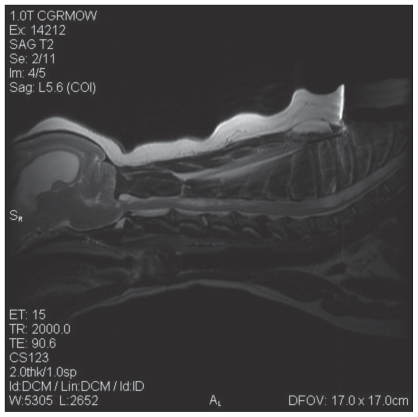
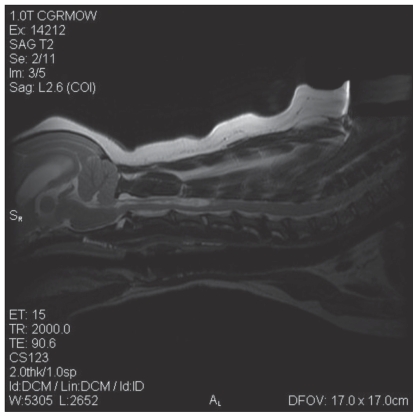
Physical examination revealed generalized ataxia with intermittent dragging of the front feet. Conscious proprioception was mildly decreased on the left and right thoracic limbs, absent on the left pelvic limb, and normal on the right pelvic limb. Hopping and tactile placing of the pelvic and thoracic limbs were mildly decreased on the left side and normal on the right side. The lesion was considered to be either within or rostral to the cervical spinal cord, or multifocal. Radiographs of the spine revealed mild spondylosis deformans at C5-6 and C6-7 and wedge-shaped intervertebral disk spaces at C4-5 and C6-7. Magnetic resonance imaging (MRI) of the spine revealed dilation of the ventricular system with the lateral ventricles most affected (Figure 1); the cerebellum was compressed and protruding through the foramen magnum (Figure 1). Cervical and thoracic spinal parenchymal hyperintensities were also noted on T2 weighted images. The cervical spinal cord hyper-intensity extended from the foramen magnum to C5 with the widest point at C3 and the thoracic spinal cord hyperintensity extended caudal to the first thoracic vertebra (Figure 2). At the widest point of the hyperintensity, 60% to 70% of the spinal cord diameter was affected by syrinx formation. Compression of the spinal cord occurred at C2-3, C4-5, C5-6, and C6-7 due to mild dorsal displacement of the respective intervertebral discs (Figure 2). The MRI diagnosis was moderate hydrocephalus, cervical and thoracic syringohydromyelia, chronic intervertebral disc protrusions at C2-3, C4-5, C5-6, and C6-7 without significant spinal cord compression, as well as caudal occipital bone malformation with cerebellar compression and mild vermis herniation.
Figure 1.
An MRI reveals hydrocephalus, mild cerebellar vermis herniation through the foramen magnum, and cervical and thoracic hyperintensities within the spinal cord indicating syringohydromyelia. Chronic disc protrusions were also noted at C2-3, C4-5, C5-6, and C6-7.
Figure 2.
An MRI reveals mild cerebellar herniation, dilated lateral ventricles, cervical syringohydromyelia, and chronic disc protrusions at C2-3, C4-5, C5-6, C6-7.
On initial presentation to our hospital, physical examination revealed moderate truncal ataxia and delayed conscious proprioception in all limbs with the left limbs more severely affected than the right limbs. On 2 occasions, the patient was seen sitting down and moving his back leg in a scratching motion at the nape of his neck without actually touching his neck (phantom scratching). All other physical examination parameters were within normal limits, including a normal cranial nerve examination. Based on the physical examination and the MRI findings, the clinical signs were attributed to caudal occipital malformation and subsequent syringohydromyelia. Foramen magnum decompression surgery was determined to be the best treatment option. Medical management was not attempted prior to surgery.
Pre-anesthetic complete blood (cell) count and serum biochemistry panel were completed. Abnormal values included thrombocytosis (943 × 109/L, reference range: 175 to 500 × 109/L), increased hematocrit (58%, reference range: 37% to 55%), increased hemoglobin (216 g/L, reference range: 120 to 180 g/L), and elevated ALT (111 U/L, reference range: 10 to 100 U/L). Prior to surgery, the patient was premedicated with buprenorphine (US Pharmacopeia) at 0.01 mg/kg body weight (BW), midazolam (Abbott Laboratories, Abbott Park, Illinois, USA) at 0.25 mg/kg BW, and glycopyrrolate (Fujisawa USA, Deerfield, Illinois, USA) at 0.01 mg/kg BW intramuscularly (IM). Anesthesia was induced with propofol (Hospira, Lake Forest, Illinois, USA) given intravenously to effect (~ 4 mg/kg BW); a 6.0-mm cuffed, wire-guarded endotracheal tube was placed, and anesthesia was maintained with isoflurane (Halocarbon Products corporation, River Edge, New Jersey, USA) delivered in 100% oxygen. Cefazolin (West-Ward Pharmaceutical Company, Eatontown, New Jersey, USA) was given preoperatively at 22 mg/kg BW, IV, which was then given q2h perioperatively. An intravenous 15-mL bolus of hetastarch (BioTime, Berkeley, California, USA) was given and a dopamine (Hospira) constant rate infusion (CRI) was started at 3 μg/kg BW per minute to maintain adequate blood pressure during surgery.
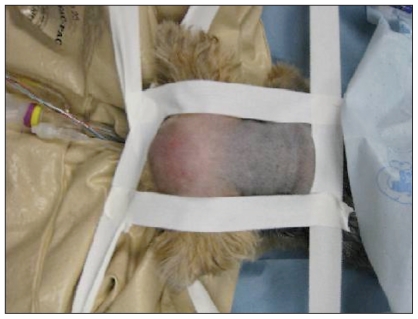
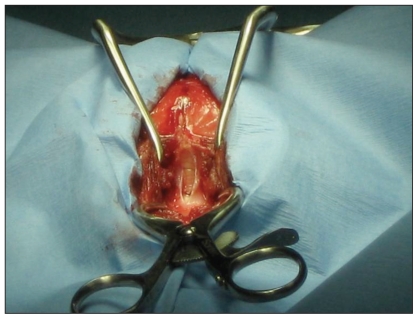
The patient was placed in sternal recumbency with the neck ventroflexed (Figure 3) and the head and dorsal neck were aseptically prepared. A dorsal midline incision was made extending from the occipital protuberance cranially to the level of the 3rd cervical vertebra caudally. Electrocautery was used to incise the median raphe of the superficial dorsal cervical musculature, exposing the underlying biventer cervicis muscles. Dissection continued through the biventer cervicis mucles and the underlying rectus capitis dorsalis muscle with the intent of dissecting down to the level of the occipital bone and the dorsal lamina of the first cervical vertebra. Upon separation of the superficial tissues, a large defect was noted in the occipital bone caudal to the occipital protuberance and laterally to about the level of the nuchal crest (Figure 4). Estimated size of the defect was about 1.5 × 2 cm. With the presence of a large defect in the supraoccipital bone, a diagnosis of concurrent occipital dysplasia and occipital hypoplasia was made. The cerebellum and brainstem were readily visualized as the dura was incised and cerebrospinal fluid (CSF) was collected for analysis. Sharp dissection was used to perform a dorsal durotomy and rongeurs were used to remove the rostral 2/3 of the dorsal lamina of the 1st cervical vertebra. A dorsal durotomy was performed to allow for further decompression of the cerebellum. Arachnoid adhesions were not identified. The dural defect was covered with a single layer of natural biocompatible collagen matrix (Bio-sist, SurgiVet). Epaxial muscles were re-apposed with 2 layers of 2-0 poliglecaprone 25 (Monocryl, Ethicon) in a simple continuous pattern. The subcutaneous tissue was apposed with 3-0 polyglactin 910 (Vicryl, Ethicon) using a simple continuous pattern, and the skin was secured with 4-0 polyamide (Nylon, Ethicon) in a cruciate pattern. A soft, padded cervical bandage extending to the mid-thorax was placed upon recovery to reduce neck movement after surgery. Dyspnea and hyperthermia were noted after surgery and resolved with removal of the bandage. Instead a flexible plastic and foam collar (Bite-not-collar) was placed around the neck to prevent excessive cervical movement.
Figure 3.
Patient was placed in ventral recumbency and secured properly for a foramen magnum decompression surgery.
Figure 4.
Caudal to the occipital protuberance, the occipital bone was absent and a large defect was noted in the supraoccipital bone. Dura overlying the cerebrum and cerebellum was incised and the cerebrum and cerebellum are clearly seen. Foramen magnum decompression surgery involving the occipital bone was not needed due to the large defect in the occipital bone.
Pain management after surgery included fentanyl at 4 μg/kg BW per hour (Cedarburg Pharmaceuticals, Grafton, Wisconsin, USA) and lidocaine at 20 μg/kg BW/min (American Veterinary Products, Fort Collins, Colorado, USA) delivered as an intravenous CRI, and a fentanyl patch (25 μg/h) was placed over the dorsal lumbar region. The patient was walking on all limbs, 1 d after surgery, but generalized ataxia was similar to initial presentation. Dexamethasone SP (Steris Laboratories, Phoenix, Arizona, USA) was given once at 0.2 mg/kg BW, IV. Cefazolin was continued post-operatively q6h for 24 h. The fentanyl/lidocaine CRI was discontinued and pain was judged to be well controlled with the fentanyl patch. Fluconazaole was continued at 3.6 mg/kg BW, q12h, and maintenance IV fluids were continued until discharge. The patient was discharged 1 d post-craniotomy with instructions for confinement and exercise restriction for 4 wk. Instructions were given to maintain a neck brace at all times. A recheck examination was scheduled for 1 wk to reevaluate ataxia, mentation, and the surgical site. Follow-up evaluations were performed monthly thereafter.
Cerebrospinal fluid analysis revealed a lymphocytic pleocytosis characterized by mild elevation in leukocytes (8 cells/μL, reference range: 0 to 5 cells/μL) and elevated erythrocytes (150 cells/per μL, reference range: 0 to 0 cells/μL). Differential count revealed 76% small lymphocytes and 24% monocytes with no monocyte vacuolation or erythrophagia. No evidence of neoplasia or an etiologic agent was observed. Scattered erythrocytes were noted in the background.
The 1-mo post-operative recheck revealed no progression or improvement in clinical signs. The patient remained ambulatory on all limbs with mild to moderate generalized ataxia. No hyperesthesia was noted and conscious proprioceptive deficits were noted in the left thoracic and pelvic limbs. Over the past month, activity has been restricted to cage rest and limited leash walks. Exercise will be gradually increased over the next months. Prognosis for a static recovery is good.
Discussion
Caudal occipital malformation syndrome (COMS) is a congenital anomaly of the caudal occipital region of the skull, which results in overcrowding of the caudal fossa and compression of the cervicomedullary junction at the level of the foramen magnum (1). This syndrome is also referred to as occipital bone hypoplasia and Chiari-like malformation, but it is different from Arnold Chiari malformation, a human disorder involving herniation of the cerebellar tonsils, which are not present in animals (2,3). Occipital bone hypoplasia is defined as the shortening of the basilar part of the occipital bone and possibly the supraoccipital bone resulting in a reduced volume of the caudal fossa (4). Caudal occipital malformation syndrome is predominantly found in small breed dogs, with cavalier King Charles spaniels (CKCS) having a high incidence of the disorder (5). An estimated 95% of CKCS have caudal occipital malformation while an estimated 50% have concurrent syringohydromyelia (2). Breeds affected often have shortened foreskulls; this includes brachycephalics and miniaturized breeds. This syndrome has been reported in CKCS, Brussels griffons, Yorkshire terriers, Maltese terriers, Chihuahuas, minature dachshunds, miniature/toy poodles, bichon frisés, pugs, Shih tzus, pomeranians, Staffordshire bull terriers, Boston terriers, French bulldogs, pekingese, and miniature pinschers (2). Clinical signs of COMS and syringomyelia (SM) are highly variable, but include cervical pain, phantom scratching of the head, neck, shoulder, or sternum, and increased pain associated with rising, temperature extremes, excitement, or posture (6). Other clinical signs include thoracic-limb weakness and muscle atrophy due to ventral horn cell damage, pelvic limb ataxia and weakness from white matter damage and lumbar spine syringohydromyelia, facial nerve paralysis, deafness, and cervical scoliosis (6). Onset of clinical signs most commonly occurs between 6 mo and 3 y of age with more severely affected dogs presenting before 2 y of age (5,6).
Caudal occipital malformation syndrome results in clinical signs due to the direct compression of the malformed occipital bone and progressive meningeal hypertrophy resulting in syringohydromyelia (1). Syringomyelia is defined as a fluid-filled cavity within the spinal cord parenchyma, while hydromyelia is defined as a dilation of the central canal (3,6). Syringomyelia and hydromyelia typically occur together and often communicate with each other; therefore, the term syringohydromyelia is more commonly used (6). Syringohydromyelia is typically thought to be associated with COMS; however, syringohydromyelia may be seen without COMS and many dogs affected with COMS have no evidence of syringohydromyelia. Other causes of syringohydromyelia include a tethered spinal cord, trauma, arachnoiditis, and tumors (3,7).
Syringohydromyelia caused by caudal occipital malformation is believed to result from changes in the CSF flow dynamics (1,6). Normally, cerebrospinal fluid moves across the foramen magnum from the intracranial subarachnoid space to the cervical spinal subarachnoid space in a pulsatile flow following systole and diastole (1). During the cardiac cycle, the intracranial arteries expand and contract causing this rapid influx and efflux of CSF within the subarachnoid space (6). Many theories exist on the pathophysiology of fluid accumulation within the spinal cord parenchyma and central canal. The malformed occipital bone in COMS results in decreased caudal fossa volume and therefore causes cerebellar herniation through the foramen magnum. Cerebellar herniation obstructs the subarachnoid space resulting in a higher-pressure pulsatile flow and turbulence of CSF; this is known as the water-hammer theory (1,3,6), which suggests that when an obstruction blocks systolic CSF flow through the foramen magnum and 4th ventricle, CSF will be forced into the central canal with each arterial pulse (6). Alternatively, the suck theory proposes that with an obstruction of the foramen magnum, CSF is sucked into the central canal region when intra-abdominal or intrathoracic pressure is increased, such as with coughing, sneezing, barking, or exercising (1,6). Multiple other theories have been hypothesized in the formation of syringohydromyelia, with the underlying cause being obstruction to CSF flow (1). The most recent and accepted theory is the intramedullary pulse pressure theory. The intramedullary pulse pressure theory describes a general pathophysiology for syringomyelia (8–10) and suggests that syringomyelia can be attributed to repeated mechanical distension of the spinal cord and increased extracellular fluid caused by the high-pressure system in the microcirculation of the spinal cord (6).
Occipital dysplasia is a separate disease entity defined as an abnormally large foramen magnum caused by incomplete ossification of the ventromedial supraoccipital bone during gestation (4). Occipital dysplasia can be caused by mesodermal insufficiency within the embryo, or from the premature closure of the growth plates of the supraoccipital bone (synostosis). This defect is typically covered by fibrous tissue. Occipital dysplasia does not typically result in compression of the cerebellum because the caudal fossa remains unchanged (4), but it does result in a decreased volume of the caudal fossa, resulting in syringohydromyelia (4). Concurrent occipital dysplasia and occipital hypoplasia have been infrequently reported in the literature and may be related to the late onset of clinical signs in this case (4). Even with a reduced caudal fossa volume, it is possible the syringohydromyelia developed slowly due to less obstruction to CSF flow because of the concurrent occipital dysplasia. Another possibility for the development of syringohydromyelia in this case may be the chronic intervertebral disc protrusions of the cervical spine. Syrinx formation was noted from the foramen magnum to C5 and from T1 caudally. Even though significant spinal cord compression was not observed, chronic inflammation and subsequent stenosis of the subarachnoid space may have altered CSF flow, contributing to the syringohydromyelia. Concurrent occipital dysplasia and occipital hypoplasia should be considered in mature presentations of syringohydromyelia.
Reported surgical techniques for COMS and syringohydromyelia include: foramen magnum decompression (FMD), FMD with C1 laminectomy, FMD with C1 laminectomy and graft, and syringosubarachnoid shunting. A long-term study evaluating the outcome of FMD in 15 dogs with COMS and SM concluded that despite improvement in clinical signs, syrinx formation was not reversed after surgery (11). This suggests that foramen magnum decompression surgery may not adequately improve CSF flow through the foramen magnum (11). Foramen magnum decompression with cranioplasty to limit the potential for scar formation following a decompression surgery is also described (12). Scar formation results in compression of the cerebellum after surgery and may contribute to the recurrence of clinical signs. This technique involves placing titanium screws as anchors for a skull plate constructed from titanium mesh and polymethylmethacrylate around the foramen magnum (12). This surgical technique was tolerated well in 17 cases and resulted in sustained clinical improvement, but further studies and follow-up are needed before this approach is determined to be more successful than FMD alone (12). A bio-compatible collagen matrix (Vet Bio-SIST) has been used to decrease scar formation after surgery. Another technique involving syringosubarachnoid shunting has been reported in 1 dog that resulted in static syringomyelia, but improved clinical signs (13). Although foramen magnum decompression surgery has been the surgical standard, recent evidence suggests that its success in veterinary patients is not equivalent to human patients and alternative surgical techniques may need to be developed. Recent studies have also indicated the malformation caused by occipital hypoplasia may actually involve an overall undersized intracranial compartment instead of only affecting the caudal occipital region (12,14). Inconsistent results with FMD, therefore, may be related to the lack of appropriate decompression achieved with a sub-occipital surgical approach (12). Until further clinical studies are performed, FMD is still considered the treatment of choice for COMS resulting in syringohydromyelia.
Acknowledgments
The author acknowledges Drs. Peter Gordon and Abigail Kovarna, The Veterinary Specialty Center of Tucson for their assistance and advice. The author also thanks Dr. Agatha Kisiel, Atlantic Veterinary College for her guidance and editorial assistance. CVJ
Footnotes
Ms. Cagle will receive 50 copies of her article free of charge, courtesy of The Canadian Veterinary Journal.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Dewey CW, Berg JM, Stefanacci JD, et al. Caudal occipital malformation syndrome in dogs. Compend Contin Educ Pract Vet. 2004;26:886–896. [Google Scholar]
- 2.Bonagura JD, Twedt DC. Kirk’s Current Veterinary Therapy. 14th ed. St. Louis, Missouri: Saunders; 2008. pp. 1102–1107. [Google Scholar]
- 3.de Lahunta A, Glass EN. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. St. Louis: Saunders; 2008. p. 47. [Google Scholar]
- 4.Rusbridge C, Knowler SP. Coexistence of occipital dysplasia and occipital hypoplasia/syringomyelia in the cavalier King Charles spaniel. J Small Anim Prac. 2006;47:603–606. doi: 10.1111/j.1748-5827.2006.00048.x. [DOI] [PubMed] [Google Scholar]
- 5.Rusbridge C, Knowler P, Rouleau GA, Minassian BA, Rothuizen J. Inherited occipital hypoplasia/syringomyelia in the cavalier King Charles spaniel: Experiences in setting up a worldwide DNA collection. J Hered. 2005;96:745–749. doi: 10.1093/jhered/esi074. [DOI] [PubMed] [Google Scholar]
- 6.Rusbridge C, Greitz D, Iskandar BJ. Syringomyelia: Current concepts in pathogenesis, diagnosis, and treatment. J Vet Intern Med. 2006;20:469–479. doi: 10.1892/0891-6640(2006)20[469:sccipd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Medow J, Sansone J, Iskandar BJ. Syringomyelia and hydromyelia. In: Albright AL, Pollack AF, Adelson PD, editors. Principles and Practice of Pediatric Neurosurgery. 2nd ed. Stuttgart, New York: Thieme, Med Publ; 2006. pp. 444–473. [Google Scholar]
- 8.Greitz D, Ericson K, Flodmark O. Pathogenesis and mechanics of spinal cord cysts: A new hypothesis based on magnetic resonance studies of cerebrospinal fluid dynamics. Int J Neuroradiol. 1999;5:61–78. [Google Scholar]
- 9.Josephson A, Greitz D, Klason T, et al. A spinal thecal sac constriction model supports the theory that induced pressure gradients in the cord cause edema and cyst formation. Neurosurgery. 2001;48:636–646. doi: 10.1097/00006123-200103000-00039. [DOI] [PubMed] [Google Scholar]
- 10.Greitz D, Flodmark O. Modern concepts of syringohydromyelia. Riv Neuroradiol. 2004;17:360–361. [Google Scholar]
- 11.Rusbridge C. Chiari-like malformation with syringomyelia in the cavalier King Charles spaniel: Long-term outcome after surgical management. Vet Surg. 2007;36:396–405. doi: 10.1111/j.1532-950X.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 12.Dewey CW, Marino DJ, Bailey KS, et al. Foramen magnum decompression with cranioplasty for treatment of caudal occipital malformation syndrome in dogs. Vet Surg. 2007;36:406–415. doi: 10.1111/j.1532-950X.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- 13.Skerritt GC, Hughes D. A syndrome of syringomyelia in the cavalier King Charles spaniel, and its treatment by syringe-subarachnoid shunting. Proceedings from the 12th Annual Symposium of the European Society of Veterinary Neurology; Vienna. 1998. [Google Scholar]
- 14.Scrivani PV, Thompson MS, Winegardner KR, et al. Association between frontal sinus size and syringohydromyelia in small-breed dogs. Am J Vet Res. 2007;68:610–613. doi: 10.2460/ajvr.68.6.610. [DOI] [PubMed] [Google Scholar]
- 15.Rusbridge C, Knowler SP. Inheritance of occipital bone hypoplasia (Chiari type I malformation) in Cavalier King Charles Spaniels. J Vet Intern Med. 2004;18:673–678. doi: 10.1892/0891-6640(2004)18<673:ioobhc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.College of Veterinary Medicine at the University of Minnesota [home-page on the Internet] c2010. Canine head: Sagittal MRI atlas. [Last accessed June 15, 2010]. Available from: http://vanat.cvm.umn.edu/mriHeadAtlas/sagHeadAtlas.html.