Introduction
As survival in patients with cystic fibrosis (CF) improves it is likely that new conditions will arise in the aging CF population. There has been speculation that the high-fat diet, which patients are encouraged to eat, and the increasing incidence of CF-related diabetes (CFRD) may put patients at risk of ischemic heart disease (IHD),1 but to date this has not been seen.
The current case is that of a 48-year-old man, with CF, pancreatic insufficiency and CFRD, who presented acutely with a symptomatic non-ST elevation myocardial infarction (NSTEMI).
Case report
A 48-year-old Caucasian man presented to his local hospital with throat and left arm discomfort. CF was diagnosed when he was 3 months old due to failure to thrive and malabsorption. He had a positive sweat test and was homozygous ΔF508. He was transferred to our Adult Unit aged 16 years, with pancreatic insufficiency on enzyme and vitamin supplements. He has pseudomonas colonization and is on long-term nebulized colomycin; he very rarely requires intravenous antibiotics. He previously worked as a plant manager in a concrete factory and now is self-employed undertaking car body repairs. He has had a long history of depression, requiring prolonged periods of timeoff work, and has been on long-term antidepressanttherapy. Three years prior to the presentation he developed symptomatic hypoglycaemia. An oral glucose tolerance test was suggestive of diabetes (basal sample 4.9 mmol/L [normal <7 mmol/L] and two-hour sample 12.3 mmol/L [normal <7.8 mmol/L]) with HbA1C 6.0% (normal 4.3–6.1%). He did home glucose monitoring and as his levels after meals were not above 9.0 mmol/L, treatment was not initiated. Over the preceding 10 years his weight had increased from 68 kg to 76 kg. At his annual review a few months prior to this presentation he had a BMI of 25.6 kg/m2 (weight 75.1 kg), FEV1/FVC 3.11 L/min (91%)/4.32 L/min (103%). He did not desaturate with exertion.
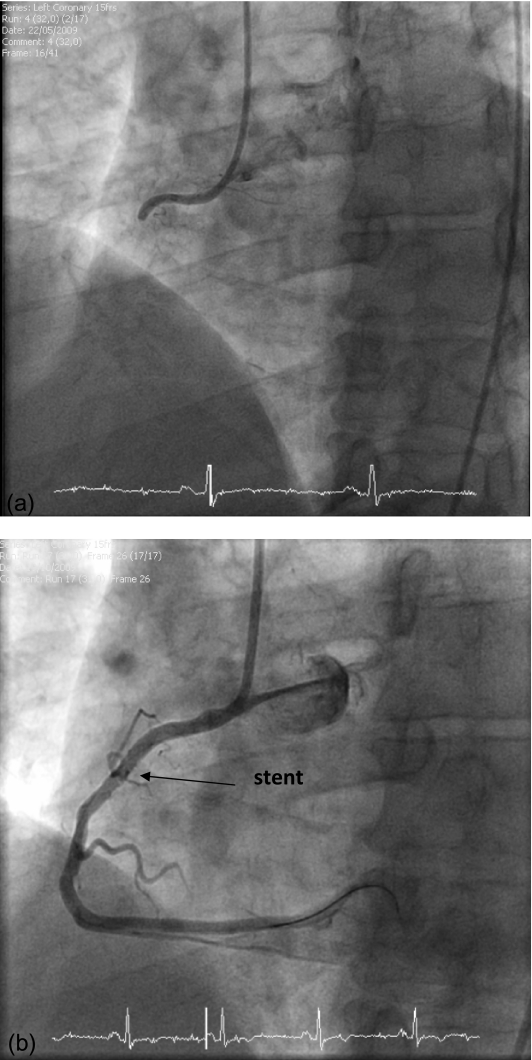
He presented with three episodes of throat pain over the preceding 4 days. On the day of admission the pain lasted several hours and radiated to his jaw and left arm. The discomfort was associated with sweating, dizziness and vomiting. He was a non-smoker. There was a family history of cardiovascular disease – his father had had a CVA and a cousin had had a myocardial infarction at the age of 30 years. On examination he was normotensive (104/56), had oxygen saturations of 91% and no evidence of heart failure. ECG showed sinus rhythm with new T wave inversion in leads III and aVF ( Figure 1). A random troponin level was raised at 0.11 ug/L (normal <0.05 ug/L) and 12-hour level was 0.54 ug/L. He had normal renal function, random glucose 5.9 mmol/L, C-reactive protein (CRP) <2 mg/L (normal <8 mg/L), total cholesterol 4.2 mmol/L (normal ≤5.0 mmol/L) and HDL cholesterol 0.91 mmol/L (normal ≥1.0 mmol/L). He underwent coronary angiography and left ventriculogram, as per protocol following presentation with an NSTEMI. There was complete occlusion of the proximal right coronary artery ( Figure 2a). He underwent percutaneous coronary intervention (PCI), re-canalization and stent insertion with good effect (Figure 2b). Mild hypokinesia was evident in the inferior and inferior-basal regions of the left ventricle. The blood supply to the right ventricle was relatively well-preserved via collateral branches of the left coronary artery circulation (probably preventing more marked evidence of an infarct on ECG).
Figure 1.
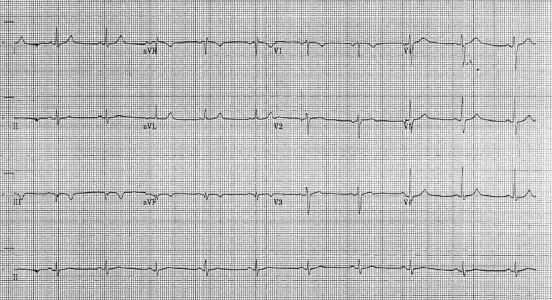
Electrocardiogram showing deep T wave inversion in the inferior leads (III and aVF)
Figure 2.
Coronary angiogram showing the right coronary artery: (a) prior to percutaneous coronary intervention, showing complete occlusion; (b) post-percutaneous coronary intervention, showing artery re-canalization and stenting
He was started on clopidogrel, which he will continue for 12 months, aspirin (lifelong), ramipril and simvastatin. He attended a cardiac rehabilitation programme at his local hospital. He has started repaglinide for the diabetes; our usual practice would have been to start rapid-acting insulin with meals, but given the history of psychological problems it was felt that beginning a regimen with subcutaneous injections was more than he would be able to manage at the time. Insulin will be started in due course. He has spent extensive time with the dietitians and has been advised on lifestyle, weight loss and healthy eating. He has been given a calorie-controlled, reduced-fat diet with no salt restriction, to reach a target weight of 70 kg, which he has achieved.
Discussion
We believe this is the first report of symptomatic myocardial infarction confirmed on angiogram in a patient with CF.
To date, cardiac involvement in CF has generally manifested in patients with severe respiratory disease, as a consequence of progressive hypoxia causing vasoconstriction of the peripheral pulmonary arterial tree, increasing pulmonary artery pressures and subsequent vascular remodeling.2 This presents clinically as pulmonary hypertension, right ventricular dysfunction and ultimately cor pulmonale. The presence of heart failure is associated with an especially poor prognosis,3 although anecdotally may occur less frequently since the introduction of non-invasive ventilation. Hypoxia and disease severity have also been implicated in the finding of right4 and left2 ventricular enlargement with systolic and diastolic dysfunction seen in CF. Additionally arrhythmias have been reported, both ventricular5 and supraventricular tachycardias,6 which are likely to occur due to hypoxia, structural changes, infection or drug side-effects, as well as asymptomatic cardiac arrhythmias that have been identified in 7% of patients as a result of exercise induced hypoxaemia.7
By contrast, IHD has rarely been documented. There are two case reports of incidental asymptomatic coronary artery disease – one in a 41-year-old woman who had CFRD with documented microvascular complications, progressive hypertension due to biopsy proven nephrosclerosis and who had normal cholesterol levels.8 She died of respiratory failure and autopsy revealed generalized atherosclerosis. The second patient was a 40-year-old man, with CFRD, who was taking part in a study of pulmonary hypertension.9 As part of the study Doppler echocardiography, he was noted to have segmental hypokinesis of the left ventricle with reduced ejection fraction. On thallium perfusion scanning, this was found to be due to a previous silent myocardial infarction associated with coronary artery disease. Finally there is a report of a 52-year-old patient with pancreatic insufficiency, who presented with symptomatic exertional dyspnoea out of proportion with his lung function, associated with vague chest discomfort.10 Specific investigation with stress thallium and cardiac catheterisation showed significant occlusion of the left anterior descending artery at several sites and of a branch of the circumflex artery for which he underwent angioplasty and stenting. He had a strong family history of IHD.
There has been speculation that the aging CF population may be at risk of developing IHD. This in part is due to increasing life expectancy, and all the cases reported above, including ours, were in patients aged over 40 years. Additionally, patients with CF are encouraged to eat a high-fat diet and have a high incidence of CFRD, and it is well-known that high cholesterol and diabetes (types 1 and 2) are significant risk factors for IHD, associated with a significant morbidity and mortality in the non-CF population.
Good nutrition has played a key role in improving pulmonary function and long-term survival among CF patients, and central to this has been the recommendation of a high-fat, high-energy diet designed to achieve 120–150% of the recommend daily allowance, with 40% of energy derived from fat.11,12 This compensates for the impaired digestion of dietary fat and malabsorption that occurs in CF due to pancreatic exocrine insufficiency. Several studies show that patients on a high-fat diet nevertheless have normal lipid and normal or low cholesterol levels.13,14 However, a large study of 192 patients (aged 5–51 years) undergoing fasting lipid profiles, showed that patients with CF had higher triglyceride and lower cholesterol concentrations compared to the general population, irrespective of age and diabetic status;15 isolated hypertriglyceridaemia was seen in 16% of patients, which may have implications for atherosclerosis. A very recent study has shown an increase in total cholesterol and triglycerides in CF with increasing age and BMI.16 Moreover, patients with pancreatic sufficiency were more likely to have raised total cholesterol, low-density lipoprotein (LDL) cholesterol and total or high-density lipoprotein (HDL) cholesterol compared to those with pancreatic insufficiency.16 Hence, improving diet may have cardiovascular consequences for all CF patients, but those with pancreatic sufficiency may be particularly at risk of obesity and cardiovascular disease as they get older. It may be prudent to ensure that the high-fat diet is cardioprotective, by encouraging mono- and poly-unsaturated fats.1,17
CFRD and impaired glucose tolerance (IGT) occur increasingly in CF as patients survive longer; recent reports suggest an adult prevalence of 40–50%18 for CFRD and 18–50% for IGT.19 Where a standard diabetic diet advocates restricted carbohydrate and reduced fat intake, patients with CFRD are encouraged to follow more closely a CF diet that is high-energy, with no fat, carbohydrate or salt limitation.12,17,20 CFRD is a distinct entity, but nevertheless shares some features of types 1 and 2 diabetes, and in particular has been associated with microvascular complications21–23 including retinopathy in 10–27%,24–27 nephropathy 13–21%24–26 and neuropathy 3%.26 To date, however, macrovascular disease has not been reported.24,25
The Framingham risk score provides an assessment to identify the risk of IHD over the following 10 years for any individual, based on age, sex, blood pressure, serum total cholesterol, HDL cholesterol and smoking status;28 a risk score of 20% or above is significant and merits primary prevention. We calculated the risk score for our patient, and found it was only 2%, or 8% when using a modified assessment tool for type 2 diabetic patients.29 In other words, despite concerns that a high-fat diet and diabetes might increase the risk of IHD, the case we report was of low-risk stratification. This may be true for CF in general.
Other factors pertinent to CF may be relevant risks for IHD. Patients with CF have chronic inflammation and high CRP levels. CRP is a sensitive, non-specific marker of inflammation that has been associated with traditional cardiovascular risk factors,30 although there is limited evidence linking therapeutic changes in CRP level to primary prevention of IHD events.31 High-sensitivity CRP is more specific and may merit further investigation. It is intriguing to note that ongoing screening studies of potential anti-inflammatory agents for CF lung disease include simvastatin,32 such that it may have a multifactorial role in CF, in lipid lowering and inflammation.
Of particular note in the current case was the fact that the remaining coronary arteries were unaffected, and the cause of the acute coronary artery occlusion is unclear. It may have occurred due to a thrombotic event or sudden plaque rupture. A recent report looking at 50 CF patients has suggested that patients with CF have increased large artery stiffness compared with controls, based on an increased mean augmentation index.33 In the general population increased arterial stiffness is an independent predictor and risk factor for cardiovascular disease, and has been observed in greater frequency in patients with chronic inflammatory disease such as COPD.34 A shear stress factor may have been compounded by stiff arteries resulting in superficial erosion and eventually ruptured plaque. Finally CFTR chloride channels are found in the heart, and in the mouse model have been demonstrated to contribute to cardiac ischaemic preconditioning, a cardioprotective process whereby brief ischaemic episodes protect the heart from sustained ischaemia.35 This may be prevented by the CFTR gene abnormality.
Conclusion
To our knowledge this is the first case of a symptomatic myocardial infarction in a CF patient. He did not have a high-risk stratification score for IHD despite the potential risks of CFRD, diet and family history. Other factors may potentially be implicated, meriting further investigation. Nevertheless IHD may become more common in the ageing CF population, particularly among those who are pancreatic sufficient, diabetic and overweight; these patients should be carefully monitored and their diet managed accordingly.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding None
Ethical approval Not applicable
Guarantor FMRP
Contributorship Both authors contributed equally
Acknowledgements
I would like to acknowledge Milly Dack for her role in looking after the patient, and Khin Gyi and Diana Bilton for their comments regarding the manuscript
References
- 1.Hodson ME, Geddes D, Bush A. Cystic Fibrosis. Third edn London: Hodder Arnold; 2007 [Google Scholar]
- 2.Bright-Thomas RJ, Webb AK. The heart in cystic fibrosis. J R Soc Med 2002;95 Suppl. 41:2–10 [PMC free article] [PubMed] [Google Scholar]
- 3.Stern RC, Borkat G, Hirschfeld SS, et al. Heart failure in cystic fibrosis. Treatment and prognosis of cor pulmonale with failure of the right side of the heart. Am J Dis Child 1980;134:267–72 [DOI] [PubMed] [Google Scholar]
- 4.Florea VG, Florea ND, Sharma R, et al. Right ventricular dysfunction in adult severe cystic fibrosis. Chest 2000;118:1063–8 [DOI] [PubMed] [Google Scholar]
- 5.Cheron G, Paradis K, Steru D, Demay G, Lenoir G. Cardiac involvement in cystic fibrosis revealed by a ventricular arrhythmia. Acta Paediatr Scand 1984;73:697–700 [DOI] [PubMed] [Google Scholar]
- 6.Sullivan MM, Moss RB, Hindi RD, Lewiston NJ. Supraventricular tachycardia in patients with cystic fibrosis. Chest 1986;90:239–42 [DOI] [PubMed] [Google Scholar]
- 7.Ruf K, Hebestreit H. Exercise-induced hypoxemia and cardiac arrhythmia in cystic fibrosis. J Cyst Fibros 2009;8:83–90 [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger DM, Holsclaw DS, Fyfe B. Generalized atherosclerosis in an adult with CF and diabetes mellitus [abstract]. Nashville, TN: Eleventh Annual North American Cystic Fibrosis Conference; 1997 [Google Scholar]
- 9.Fraser KL, Tullis DE, Sasson Z, Hyland RH, Thornley KS, Hanly PJ. Pulmonary hypertension and cardiac function in adult cystic fibrosis: role of hypoxemia. Chest 1999;115:1321–8 [DOI] [PubMed] [Google Scholar]
- 10.Onady GM, Farinet CL. An adult cystic fibrosis patient presenting with persistent dyspnea: case report. BMC Pulm Med 2006;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsey BW, Farrell PM, Pencharz P. Nutritional assessment and management in cystic fibrosis: a consensus report. The Consensus Committee. Am J Clin Nutr 1992;55:108–16 [DOI] [PubMed] [Google Scholar]
- 12.UK Cystic Fibrosis Trust Nutrition Working Group Nutritional Management of Cystic Fibrosis. Bromley: Cystic Fibrosis Trust; 2002 [Google Scholar]
- 13.Gordon CM, Anderson EJ, Herlyn K, et al. Nutrient status of adults with cystic fibrosis. J Am Diet Assoc 2007;107:2114–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart C, Wilson DC, Hanna AK, Corey M. Lipid metabolism in adults with cystic fibrosis [abstract]. Nashville, TN: Eleventh North American Cystic Fibrosis Conference; 1997 [Google Scholar]
- 15.Figueroa V, Milla C, Parks EJ, Schwarzenberg SJ, Moran A. Abnormal lipid concentrations in cystic fibrosis. Am J Clin Nutr 2002;75:1005–11 [DOI] [PubMed] [Google Scholar]
- 16.Rhodes B, Nash EF, Tullis E, et al. Prevalence of dyslipidemia in adults with cystic fibrosis. J Cyst Fibros 2010;9:24–8 [DOI] [PubMed] [Google Scholar]
- 17.UK Cystic Fibrosis Trust Diabetes Working Group Management of Cystic Fibrosis Related Diabetes Mellitus. Bromley: Cystic Fibrosis Trust; 2004 [Google Scholar]
- 18.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran A, Hardin D, Rodman D, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract 1999;45:61–73 [DOI] [PubMed] [Google Scholar]
- 20.Wilson DC, Kalnins D, Stewart C, et al. Challenges in the dietary treatment of cystic fibrosis related diabetes mellitus. Clin Nutr 2000;19:87–93 [DOI] [PubMed] [Google Scholar]
- 21.Lanng S, Thorsteinsson B, Lund-Andersen C, Nerup J, Schiotz PO, Koch C. Diabetes mellitus in Danish cystic fibrosis patients: prevalence and late diabetic complications. Acta Paediatr 1994;83:72–77 [DOI] [PubMed] [Google Scholar]
- 22.Rodman HM, Doershuk CF, Roland JM. The interaction of 2 diseases: diabetes mellitus and cystic fibrosis. Medicine (Baltimore) 1986;65:389–97 [DOI] [PubMed] [Google Scholar]
- 23.Sullivan MM, Denning CR. Diabetic microangiopathy in patients with cystic fibrosis. Pediatrics 1989;84:642–7 [PubMed] [Google Scholar]
- 24.Andersen HU, Lanng S, Pressler T, Laugesen CS, Mathiesen ER. Cystic fibrosis-related diabetes: the presence of microvascular diabetes complications. Diabetes Care 2006;29:2660–3 [DOI] [PubMed] [Google Scholar]
- 25.Schwarzenberg SJ, Thomas W, Olsen TW, et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056–61 [DOI] [PubMed] [Google Scholar]
- 26.van den Berg JM, Morton AM, Kok SW, Pijl H, Conway SP, Heijerman HG. Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros 2008;7:515–19 [DOI] [PubMed] [Google Scholar]
- 27.Yung B, Landers A, Mathalone B, Gyi KM, Hodson ME. Diabetic retinopathy in adult patients with cystic fibrosis-related diabetes. Respir Med 1998;92:871–2 [DOI] [PubMed] [Google Scholar]
- 28.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47 [DOI] [PubMed] [Google Scholar]
- 29.UKPDS Risk Engine 2007. See www.dtu.ox.ac.uk/index.php?maindoc=/riskengine/
- 30.Miller M, Zhan M, Havas S. High attributable risk of elevated C-reactive protein level to conventional coronary heart disease risk factors: the Third National Health and Nutrition Examination Survey. Arch Intern Med 2005;165:2063–8 [DOI] [PubMed] [Google Scholar]
- 31.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the US Preventive Services Task Force. Ann Intern Med 2009;151:483–95 [DOI] [PubMed] [Google Scholar]
- 32.Jones AM, Helm JM. Emerging treatments in cystic fibrosis. Drugs 2009;69:1903–10 [DOI] [PubMed] [Google Scholar]
- 33.Hull JH, Garrod R, Ho TB, et al. Increased augmentation index in patients with cystic fibrosis. Eur Respir J 2009;34:1322–8 [DOI] [PubMed] [Google Scholar]
- 34.Mills NL, Miller JJ, Anand A, et al. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular risk. Thorax 2008;63:306–11 [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Liu LL, Ye LL, et al. Targeted inactivation of cystic fibrosis transmembrane conductance regulator chloride channel gene prevents ischemic preconditioning in isolated mouse heart. Circulation 2004;110:700–4 [DOI] [PubMed] [Google Scholar]