Abstract
To determine the role of central serotonin 5-HT3 receptors in respiratory motor control, respiratory motor bursts were recorded from hypoglossal (XII) nerve rootlets on isolated adult turtle brainstems during bath-application of 5-HT3 receptor agonists and antagonists. mCPBG and PBG (5-HT3 receptor agonists) acutely increased XII burst frequency and regularity, and decreased bursts/episode. Tropisetron and MDL72222 (5-HT3 antagonists) increased bursts/episode, suggesting endogenous 5-HT3 receptor activation modulates burst timing in vitro. Tropisetron blocked all mCPBG effects, and the PBG-induced reduction in bursts/episode. Tropisetron application following mCPBG application did not reverse the long-lasting (2 h) mCPBG-induced decrease in bursts/episode. We conclude that endogenous 5-HT3 receptor activation regulates respiratory frequency, regularity, and episodicity in turtles and may induce a form of respiratory plasticity with the long-lasting changes in respiratory regularity.
Keywords: reptile, chelonian, episodic breathing, frequency, regularity, respiratory motor control, serotonin, 5-HT3 receptors, plasticity
1. Introduction
Serotonergic neurons in the brainstem project to the entire central nervous system and exert powerful neuromodulatory influences on motor systems, such as the respiratory control system (Hodges and Richerson, 2008). Serotonin receptor activation induces respiratory neuroplasticity (Feldman et al., 2003; Lovett-Barr et al., 2006), which is a long-lasting change in neural control based on prior experience (Mitchell and Johnson, 2003). Serotonin-dependent neuromodulation and plasticity can occur at the level of rhythm generating circuitry (controlling inspiratory and expiratory timing) or at the level of pattern forming circuitry and motoneurons (controlling respiratory burst shape and amplitude). Although the mechanisms underlying serotonin-dependent neuromodulation and plasticity of spinal respiratory motor output (e.g., phrenic long-term facilitation) have been extensively studied (Fuller et al., 2000; Mitchell et al., 2001), little is known with respect to acute and long-term serotonin-dependent changes in brainstem respiratory burst timing, such as burst frequency, regularity, and episodicity (i.e., clustering of breaths into episodes separated by periods of apnea).
Increased serotonin neuronal activity is hypothesized to exert a net stimulatory effect on respiratory motor output because of serotonin’s co-localization with other excitatory neurotransmitters (Hodges and Richerson, 2008). However, serotonin receptor activation produces both excitatory and inhibitory effects on respiratory control that appear to be related to experimental preparation, development, species, and route of drug administration. In mammalian preparations, serotonin receptor activation tends to decrease or abolish respiratory burst frequency and ventilation in vivo (Lundberg et al., 1980, Khater-Boidin et al., 1999), and increase frequency, or produce a biphasic frequency response, in vitro (Di Pasquale et al., 1992, Onimaru et al., 1998). In addition, 5-HT2A receptor blockade decreases regularity of respiratory activity in neonatal mice brainstem slices (Peña and Ramirez, 2002), but extensive studies of control and long-lasting changes in regularity in mammalian respiratory rhythm are limited. In isolated brainstems from pre- and postmetamorphic frogs, serotonin receptor activation or raphé neuron stimulation produces complex biphasic, dose-dependent, stage-dependent effects on lung burst frequency (Kinkead et al., 2002; Belzile et al., 2007). In isolated adult turtle brainstems, respiratory burst frequency decreases during bath application of serotonin, but is followed by a long-lasting frequency increase during washout (Johnson et al., 2001). While one report shows serotonin decreasing the number of lung bursts per episode in isolated postmetamorphic frog brainstems (Kinkead et al., 2002), there are no systematic studies as to how central serotonin receptor activation alters respiratory burst episodicity and regularity in ectothermic vertebrates.
Following exposure to intermittent hypoxia, there is a long-lasting (>30 min) increase in respiratory tidal volume and frequency in awake rats (Olson et al., 2001), and a long-lasting (>60 min) increase in phrenic burst amplitude and frequency in anesthetized, paralyzed, pump-ventilated rats (Fuller et al., 2000; Baker-Herman et al., 2008). Since a long-lasting increase in phrenic burst amplitude requires serotonin 5-HT2 receptor activation (Kinkead and Mitchell, 1999), the long-lasting increase in respiratory frequency may also require serotonin receptor activation. In contrast to mammals, 5-HT3 receptors in postmetamorphic frogs are involved in changes in respiratory burst frequency (Belzile et al., 2002). In isolated adult turtle brainstems, 5-HT3 receptor activation acutely increases respiratory burst frequency and appears to elicit a long-lasting increase in frequency (Johnson et al., 2001).
To investigate the role of serotonin 5-HT3 receptors on respiratory motor output, 5-HT3 agonists were applied to isolated adult turtle brainstems. Our goal was to determine if the acute and long-lasting serotonin-dependent changes in respiratory burst frequency were 5-HT3 dependent, and if other features of respiratory burst timing, such as episodicity and regularity, were altered by 5-HT3 receptor activation. In addition, 5-HT3 antagonists were applied to determine if episodicity in turtle brainstems was regulated by endogenous 5-HT3 receptor activation. Preliminary data were published in abstract form (Bartman and Johnson, 2009).
2. Methods
2.1 Procedures
All procedures were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison School of Veterinary Medicine. Adult red-eared slider turtles (Trachemys scripta, n = 311, weight = 722 ± 8 g) were obtained from commercial suppliers and kept in a large open tank where they had access to water for swimming, and heat lamps and dry areas for basking. Room temperature was set to 27–28°C with light 14 h per day. Turtles were fed ReptoMin® floating food sticks (Tetra, Blacksburg, VA) 3–4 times per week.
Turtle brainstems were isolated as described previously (Johnson et al., 1998b). Turtles were intubated and anesthetized with 5% isoflurane (balance O2) until forelimb withdrawal to noxious foot pinch was eliminated. Turtles were rapidly decapitated and decerebrated. Brainstems were removed and pinned down in a recording chamber (13 ml volume) with the ventral surface facing upwards (Fig. 1A). Brainstems were superfused (4–6 ml min−1) with standard solution containing HEPES (N-[2-hydroxyethyl]piperazine-N′-[2-ethane-sulfonic acid]) buffer as follows (in mM): 100 NaCl, 23 NaHCO3, 10 Glucose, 5 HEPES (sodium salt), 5 HEPES (free acid), 2.5 CaCl2, 2.5 MgCl2, 1.0 K2PO4, and 1.0 KCl. Standard solution was bubbled with 5% CO2-95% O2; pH = ~7.35. To record respiratory motor bursts, glass suction electrodes were attached to hypoglossal (XII) nerve rootlets (Fig. 1A). Signals were amplified (10,000×) and band-pass filtered (1.0–500 Hz) using a differential AC amplifier (model 1700, A-M Systems, Everett, WA) before being rectified and integrated (time constant = 200 ms) using a moving averager (MA-821/RSP, CWE, Inc., Ardmore, PA; Figs. 1B, 1C). Analysis was performed using Axoscope software (Axon Instruments, Foster City, CA). After allowing the brainstems to equilibrate for 3–6 h, baseline data were obtained by recording 30 min of spontaneous respiratory motor activity before adding drugs to the reservoir.
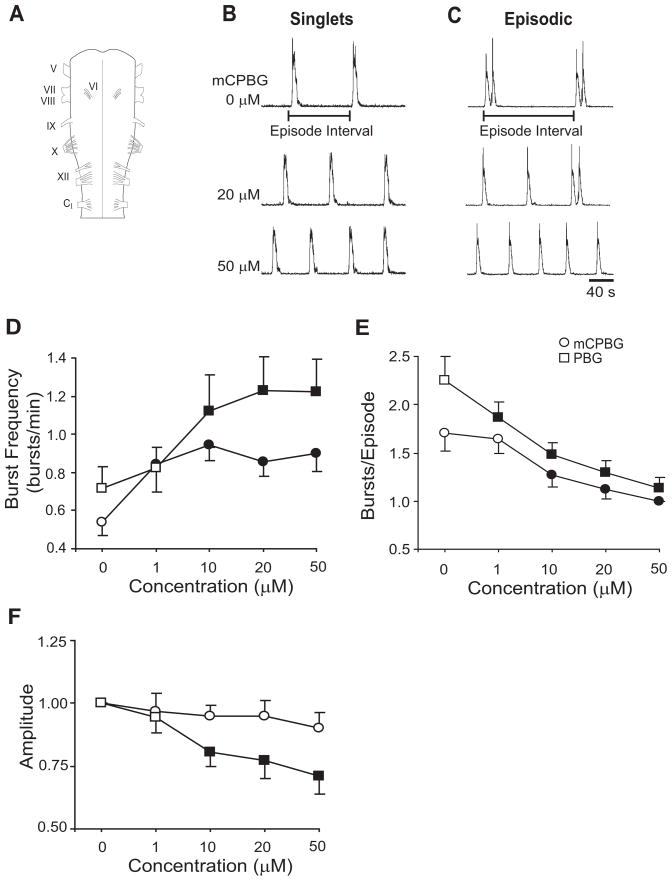
Fig. 1.
5-HT3 receptor activation alters respiratory motor output. (A) Drawing of turtle brainstem with cranial nerves labeled. A suction electrode attached to the hypoglossal (XII) nerve roots records spontaneous respiratory motor output. Integrated and rectified traces of respiratory motor output show mCPBG-dependent (5-HT3 agonist) changes in brainstems producing singlet (B) and episodic (C) respiratory-related bursts of motor activity. Episode interval, which is used to quantify regularity, is measured as time from the start of one episode to the start of the next episode. Singlets are considered an episode with one respiratory burst. Cumulative dose-response experiments show that mCPBG (circle) and PBG (square) increased burst frequency (D) and decreased bursts/episode (E) in a dose-dependent manner. Burst amplitude was decreased by PBG, but not mCPBG (F). Values which differ significantly (P<0.05) from the baseline value are indicated by a solid symbol.
All drugs used in this study were obtained from Sigma/RBI Aldrich (St. Louis, MO): N-phenyl-imidocarbonimidic diamide (1-phenylbiguanide, PBG, 5-HT3 agonist), 1-(m-chlorophenyl)-biguanide (mCPBG, 5-HT3 agonist), 2-Methylserotonin maleate salt (2-methyl-5-HT, 5-HT3 agonist), 3-Tropanyl-indole-3-carboxylate hydrochloride (tropisetron, 5-HT3 antagonist), 3-Tropanyl-3,5-dichlorobenzoate (MDL72222, 5-HT3 antagonist), 1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one hydrocholoride (ondansetron, 5-HT3 antagonist).
2.2 Data Analysis
Respiratory burst variables were measured as described previously (Johnson et al., 1998b). Burst amplitude was measured at the highest point of integrated XII nerve discharge in arbitrary units and normalized to the average amplitude during the baseline period. Burst frequency was calculated as number of bursts/min and burst duration was measured as the time from the onset to the end of XII burst discharge. When two or more bursts were separated by less than twice the average duration of a single burst they were defined as part of the same episode (Fig. 1C). This definition was used to calculate bursts/episode in a 30-min time period (for brainstems producing singlet discharge, episodes were considered to be one burst per episode; Fig. 1B). When peak duration changed significantly during or after drug exposure (e.g., Table 1), a new average peak duration was measured and used to define episodes. Percent time-to-peak was calculated by measuring the time from burst onset to burst amplitude, and dividing by burst duration. Episode interval was the time from the start of one episode to the start of the next episode. To quantify the degree of episode regularity, episode interval coefficient of variation was calculated by dividing episode interval standard deviation by the mean of the episode interval. All measurements were averaged into 30-min bins and reported as the mean ± S.E.M. A two-way ANOVA with repeated measures design was performed using statistical software (Sigma Stat, Jandel Scientific Software, San Rafael, CA). If normality or equal variance assumptions failed, data were ranked before analysis with two-way ANOVA with repeated measures design (e.g., Figs. 2B, 4A, 4B, 4D). Post-hoc comparisons were made using the Student-Newman-Keul’s test. P-values < 0.05 were considered significant.
Table 1.
| 5-HT3 agonists | 5-HT3 antagonists | |||
|---|---|---|---|---|
| mCPBG | PBG | Tropisetron | MDL72222 | |
| Burst Duration (s) | ||||
| Baseline | 12.0 ± 0.8 | 11.8 ± 0.8 | 11.8 ± 1.0 | 11.0 ± 1.2 |
| Drug Application | 9.7 ± 0.8 | 12.2 ± 0.6 | 9.0 ± 1.4 | 7.3 ± 1.0* |
| Washout | 9.2 ± 0.9* | 11.5 ± 0.7 | ||
| Percent Time-to-Peak | ||||
| Baseline | 28 ± 6 | 40 ± 2 | 24 ± 4 | 19 ± 4 |
| Drug Application | 31 ± 7 | 43 ± 2 | 17 ± 3 | 13 ± 3 |
| Washout | 25 ± 5 | 41 ± 2 | ||
| Amplitude (normalized) | ||||
| Baseline | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Drug Application | 0.94 ± 0.05 | 0.98 ± 0.02 | 0.72 ± 0.05* | 0.80 ± 0.04* |
| Washout | 0.89 ± 0.05 | 0.94 ± 0.02 | ||
Percent time-to-peak is the time-to-peak (s) divided by peak duration (s). Amplitude is normalized to baseline values.
The asterisk indicates p<0.05 compared to baseline.
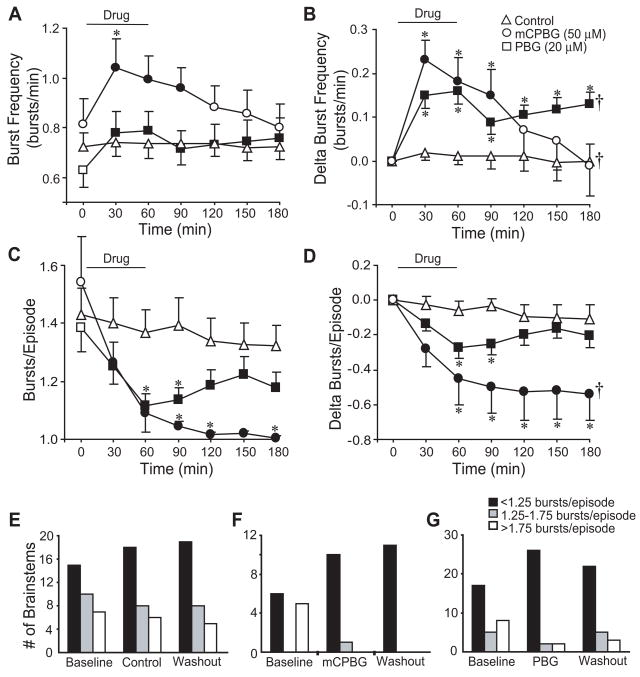
Fig. 2.
5-HT3 agonists modulate respiratory burst discharge. mCPBG (50 μM) and PBG (20 μM) increased burst frequency during drug application with the PBG-dependent increase persisting during washout (A, B). mCPBG and PBG reduced bursts/episode during drug application (C). While bursts/episode returned close to baseline during washout in PBG treated brainstems, the decrease persisted 2 h after mCPBG application (D). Control studies show a slow increase in the number of brainstems producing singlet discharge (<1.25 bursts/episode), and a corresponding decrease in brainstems producing episodic discharge (>1.75 bursts/episode) (E). mCPBG and PBG transformed episodic discharge to singlet discharge (F, G). Symbols: *P<0.05 compared to controls at that time point; †P<0.05 for drug effect; values which differ significantly (P<0.05) from the baseline value are indicated by a solid symbol.
Fig. 4.
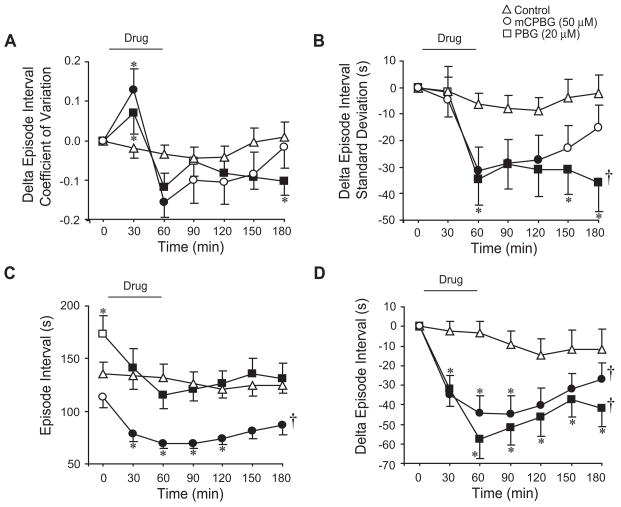
5-HT3 receptor activation increases regularity in brainstems producing singlet discharge. For brainstems producing singlet discharge (<1.25 bursts/episode during baseline), mCPBG and PBG induced acute and long-lasting decreases in episode interval coefficient of variation (A) and episode interval standard deviation (B). While mCPBG and PBG showed no significant change in episode interval (C) due to variability in baseline levels, acute and long-lasting decrease in episode interval were revealed when graphed as the change in episode interval (D). Symbols: *P<0.05 compared to controls at that time point; †P<0.05 for drug effect; values which differ significantly (P<0.05) from the baseline value are indicated by a solid symbol.
3. Results
3.1 Dose-dependent effects of 5-HT3 receptor activation on respiratory burst timing and pattern
To test for dose-dependent effects of 5-HT3 agonists, cumulative dose-response experiments were performed by exposing brainstems to sequentially increasing concentrations (1.0, 10, 20, 50 μM; 40-min exposure to each concentration) of mCPBG, PBG, or 2-methyl-5-HT. At 10–50 μM, mCPBG (n=8) and PBG (n=9) increased burst frequency and decreased bursts/episode in isolated brainstems (P<0.05; Figs. 1D, 1E). PBG, but not mCPBG, decreased burst amplitude by 29% (P<0.05; Fig. 1F). 2-methyl-5-HT produced highly variable effects, such as no change in burst frequency between 1.0–20 μM, and a 3–4 fold decrease in burst frequency at 50 μM (3/8 brainstems nearly stopped bursting; data not shown). Thus, 2-methyl-5-HT was excluded from further studies. Based on the dose-response results and previously published data (see Fig. 5 in Johnson et al., 2001), 50 μM mCPBG and 20 μM PBG were selected for subsequent experiments, as these concentrations appeared to produce robust and consistent changes in burst frequency and episodicity.
3.2 Acute and long-lasting effects of 5-HT3 receptor activation
Although PBG produced acute (during 60-min drug application) and long-lasting (end of 120-min washout period) increases in burst frequency in isolated turtles brainstems (Johnson et al., 2001), the acute and long-lasting effects of 5-HT3 receptor activation on bursts/episode, episode interval coefficient of variation, burst duration, and percent time-to-peak were not previously characterized. To address these questions, mCPBG (50 μM, n=11) or PBG (20 μM, n=32) were bath-applied for 60 min, followed by a 120-min washout period. For control brainstems (n=30), there were no significant changes in burst frequency or bursts/episode during the entire 180-min period (Fig. 2A, 2B).
mCPBG acutely increased burst frequency 29.1 ± 8.4% (P<0.001), an effect that did not persist during washout (Fig. 2A). PBG acutely increased burst frequency 31.8 ± 5.3% (P<0.001), and burst frequency remained elevated by 21.5 ± 4.6% at 120 min post-drug (P<0.001; Fig. 2A). When graphed as the change in burst frequency to eliminate baseline differences, mCPBG and PBG acutely increased burst frequency during the 60-min drug exposure (P<0.001). PBG produced a long-lasting increase in burst frequency (P<0.001 at 120 min post-drug; P<0.001 for drug effect), whereas burst frequency returned to baseline following mCPBG exposure (P=0.009 for drug effect; Fig. 2B).
mCPBG (50 μM) and PBG (20 μM) acutely reduced bursts/episode by 0.45 ± 0.15 and 0.27 ± 0.06, respectively, during the 60-min drug exposure (P<0.001) with the bursts/episode remaining significantly decreased throughout the 120-min washout (Fig. 2C). When graphed as the change in bursts/episode, mCPBG produced both an acute and long-lasting decrease (P=0.002 for drug effect), whereas PBG decreased bursts/episode acutely during the 60-min drug exposure (P<0.001), but returned close to controls during the 120-min washout (Fig. 2D). The distribution of brainstems producing episodic (>1.75 bursts/episode) discharge tended to decrease during control conditions, with a corresponding slow increase in the number of brainstems producing singlet discharge (<1.25 bursts/episode; Fig. 2E). However, mCPBG and PBG rapidly changed this distribution by sharply increasing the number of brainstems producing singlet discharge from 54% to 90% and from 57% to 87%, respectively. At the end of the 120-min washout, all mCPBG-treated brainstems and 73% of PBG-treated brainstems were still producing singlet discharge (Figs. 2F, 2G). With respect to burst shape, mCPBG shortened burst duration from 12.0 ± 0.8 s (baseline) to 9.2 ± 0.9 s after the 120-min washout (P<0.05; Table 1). mCPBG did not alter the percent time-to-peak or burst amplitude, while PBG did not alter burst duration, percent time-to-peak, or burst amplitude.
3.3 5-HT3 receptor activation increases episode regularity
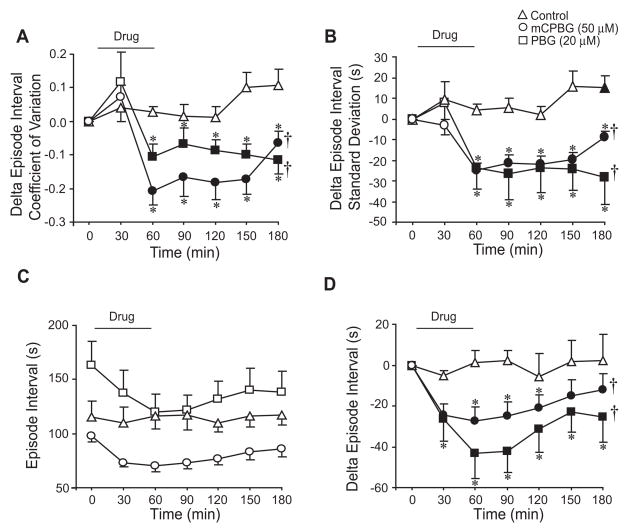
Bath application of mCPBG (50 μM) and PBG (20 μM) acutely produced a biphasic initial increase and then decrease in episode interval coefficient of variation (P=0.035; P=0.003, respectively; Fig. 3A). PBG-treated brainstems also exhibited a long-lasting decrease in episode coefficient of variation (P=0.012; Fig. 3A). To further investigate the variables that determine episode interval coefficient of variation, episode standard deviation and mean episode interval were graphed separately (Figs. 3B–3D). mCPBG and PBG acutely decreased episode standard deviation by 31.3 ± 9.0 s (P<0.001) and 34.8 ± 9.6 s (P=0.036), respectively (Fig. 3B). During the 120-min washout, episode standard deviation remained decreased in PBG-treated brainstems by 36.0 ± 10.6 s (P=0.05 for drug effect), but returned close to baseline levels in mCPBG-treated brainstems (Fig. 3B). Episode interval acutely decreased by 44.4 ± 8.9 s (P=0.003) and 57.9 ± 9.6 s (P<0.001) in mCPBG- and PBG-treated brainstems (Fig. 3C). When graphed as change in episode interval to eliminate baseline differences, episode interval was acutely decreased by mCPBG (P=0.003) and PBG (P<0.001) and remained depressed during the 120-min washout (P=0.036 and P<0.001 for drug effects, respectively). There were no significant changes in these variables for control brainstems (n=30).
Fig. 3.
5-HT3 receptor activation increases episode burst regularity. mCPBG (50 μM) and PBG (20 μM) elicited an increase, than a decrease in episode interval coefficient of variation during drug application, with little change during washout compared to controls (A). mCPBG and PBG application acutely decreased episode interval standard deviation with the decrease induced by PBG persisting for 2 h during washout (B). mCPBG and PBG also induced acute and long-lasting decreases in episode interval (C, D). Symbols: *P<0.05 compared to controls at that time point; †P<0.05 for drug effect; values which differ significantly (P<0.05) from the baseline value are indicated by a solid symbol.
To test whether 5-HT3 receptor activation increased the regularity of singlet XII bursts, data from the above brainstems that discharged during baseline with bursts/episode less than 1.25 were analyzed in a similar manner. Bath application of mCPBG (50 μM; n=6) and PBG (20 μM; n=17) acutely reduced episode interval coefficient of variation during the 60-min drug application (P<0.001 for both drugs) and the 120-min washout (P=0.004 and P=0.002 for drug effects, respectively; Fig. 4A). mCPBG and PBG elicited acute and long-lasting decreases in episode standard deviation (P<0.001 for drug effects; Fig. 4B). There were no significant changes in episode interval for mCBPG- and PBG-treated brainstems (Fig. 4C). However, when data were graphed as change in episode interval to eliminate baseline differences, mCPBG and PBG acutely decreased episode interval (P<0.001 for both), and induced a long-lasting decrease during washout (P=0.032 and P=0.005 for drug effects, respectively; Fig. 4D). Except for a small increase in episode interval standard deviation at the 180-min time point (Fig. 4B), there were no significant changes in these variables for control brainstems (n=15).
3.4 Endogenous 5-HT3 receptor regulation of burst timing
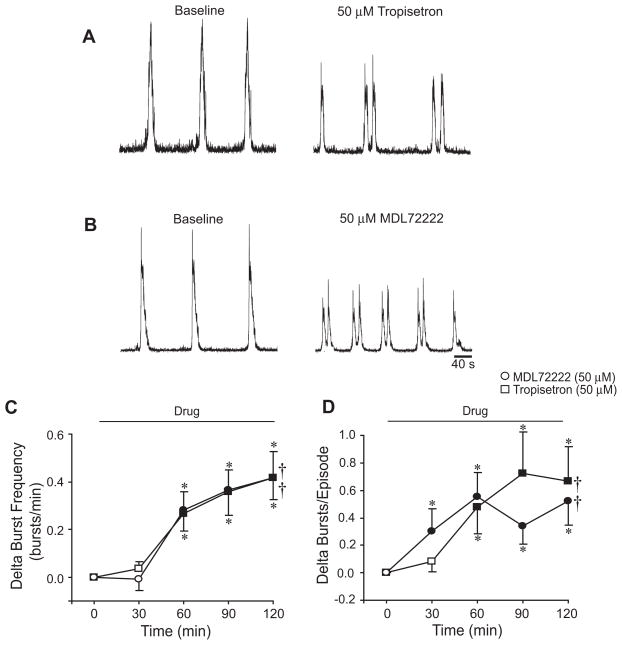
To test whether endogenous 5-HT3 receptor activation modulates respiratory motor pattern, tropisetron, MDL72222, or ondansetron (5-HT3 antagonists) were bath-applied to the isolated brainstems for 120 min. At the end of the drug exposure, tropisetron (50 μM; n=14) and MDL72222 (50 μM; n=11) increased burst frequency by 0.42 ± 0.11 and 0.42 ± 0.09 bursts/min, respectively (P<0.001 for drug effect; Figs. 5A, 5B, 5C). Tropisetron and MDL72222 also increased bursts/episode by a maximum of 0.6 ± 0.2 and 0.5 ± 0.2 from baseline values of 1.3 ± 0.1 and 1.4 ± 0.2 bursts/episode, respectively (P<0.001; Fig. 5D). With respect to XII burst shape, tropisetron did not alter burst duration or percent time-to-peak, but did decrease amplitude by 28.3 ± 5.4% during application (P<0.05; Table 1). MDL72222 decreased burst duration from 11.0 ± 1.2 s (baseline) to 7.3 ± 1.0 s (P=0.026) and amplitude by 20.5 ± 4.5% (P<0.05), but did not alter percent time-to-peak (Table 1). Ondansetron (1.0–20 μM; n=17) did not alter bursts/episode or burst frequency, but also did not appear to block the acute effects of mCPBG or PBG in pilot studies. Thus, ondansetron was excluded from further studies.
Fig. 5.
5-HT3 receptor antagonists increase frequency and episodicity. Integrated traces of XII respiratory motor output are shown before (left) and after (right) bath-application of tropisetron (50 μM) (A) or MDL72222 (50 μM) (B). Bath-application of tropisetron or MDL72222 for 120 min increased burst frequency (C) and bursts/episode (D). Symbols: *P<0.05 compared to controls (shown in Fig. 2B, 2D) at that time point; †P<0.05 for drug effect; values which differ significantly (P<0.05) from the baseline value are indicated by a solid symbol.
3.5 Blockade of mCPBG and PBG effects by tropisetron
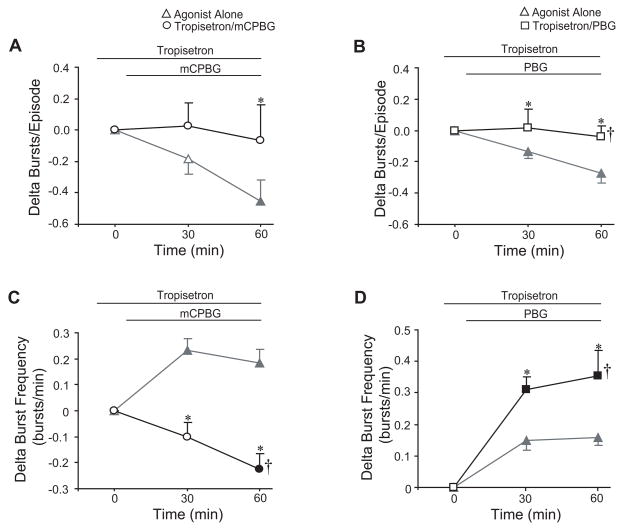
To test whether mCPBG or PBG acted via 5-HT3 receptors, tropisetron (50 μM) was bath-applied alone for 30 min prior to a 60-min co-application of tropisetron with mCPBG (50 μM; n=8) or PBG (20 μM; n=14). Tropisetron blocked the acute mCPBG- and PBG-dependent decreases in bursts/episode (P=0.025 and P=0.001 respectively; Figs. 6A, 6B). Tropisetron also blocked the mCPBG-dependent increase in burst frequency; instead, burst frequency decreased by 0.23 ± 0.06 bursts/min (P<0.001 for drug effect; Fig. 6C). In contrast, tropisetron augmented the PBG-dependent increase in frequency, causing an increase of 0.35 ± 0.08 compared to 0.16 ± 0.03 bursts/min with PBG treatment alone (P=0.003 for drug effect; Fig. 6D).
Fig. 6.
5-HT3-dependent changes are blocked by a 5-HT3 antagonist. Tropisetron (50 μM) blocked the acute mCPBG (50 μM) and PBG-dependent (20 μM) decreases in bursts/episode (A, B). While tropisetron blocked the acute increase in frequency (C) normally caused by mCPBG application, tropisetron did not block, but actually augmented the PBG-dependent increase in frequency (D). Data were graphed as change from tropisetron steady-state levels. For comparison, data from bath-applied mCPBG or PBG from Fig. 2 are shown in gray triangles in each graph. Symbols: *P<0.05 compared to controls at that time point; †P<0.05 for drug effect; values which differ significantly (P<0.05) from the baseline value are indicated by a solid symbol.
3.6 Maintenance of long-lasting decrease in bursts/episode does not require 5-HT3 receptor activation
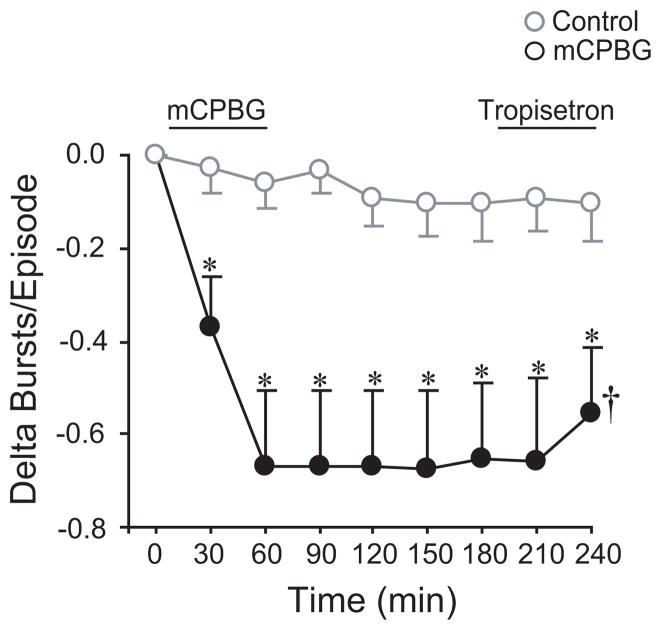
To test 5-HT3 receptor activation was necessary for the maintenance of the mCPBG-dependent, long-lasting decrease in bursts/episode, the competitive antagonist tropisetron (50 μM) was applied at the end of the 2-h washout period (n=8). mCPBG decreased the bursts/episode from 1.69 ± 0.17 bursts/episode (baseline) to 1.02 ± 0.02 bursts/episode within 60 min (delta bursts/episode = −0.67 ± 0.16 bursts/episode; P<0.001) where it remained during the 2-h washout (Fig. 7). The reduction in bursts/episode originally induced by mCPBG was not immediately reversed by tropisetron (P<0.001 for drug effect). Thus, 5-HT3 receptor activation was not required for the long-lasting decrease in bursts/episode.
Fig. 7.
5-HT3 receptor activation is not necessary for the maintenance of the long-lasting decrease in episodicity. A 60-min mCPBG (50 μM) application was used to induce a long-lasting decrease in bursts/episode prior to a 2-h washout and bath application of tropisetron (50 μM) for 60-min. During the tropisetron application, bursts/episode did not return to baseline levels. Time control data from Fig. 2 are shown in gray circles. Symbols: *P<0.05 compared to controls at that time point; †P<0.05 for drug effect; values which differ significantly (P<0.05) from the baseline value are indicated by a solid symbol.
3.7 Episodic discharge can be rapidly switched
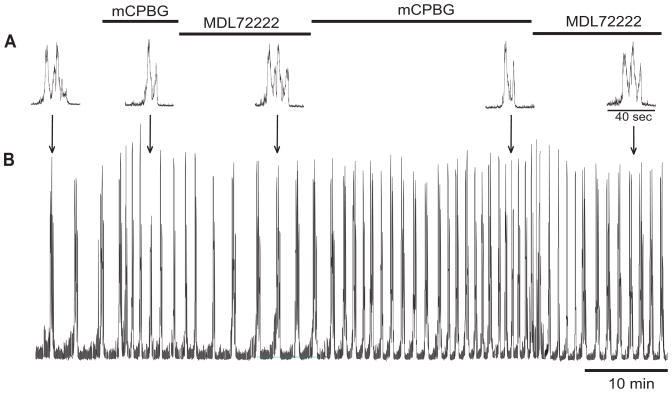
To test whether 5-HT3 receptor agonists and antagonists could rapidly switch the burst pattern from episodes to singlets, a brainstem producing an episodic discharge was exposed to mCPBG (50 μM) until singlet discharge was observed (Fig. 8). The bath solution was then switched to MDL72222 (50 μM) until episodic discharge was observed. The same pattern of drug application was repeated over the next 30 min with similar results showing that mCPBG induced singlets and MDL72222 induced episodes.
Fig. 8.
Rapid switching from episodic to singlet burst patterns. Integrated traces of XII root respiratory motor output from a brainstem producing episodic discharge. Bath-applied mCBPG (50 μM) rapidly converted episodic discharge to singlet discharge, and bath-applied MDL72222 (50 μM) switched the singlet discharge back to episodic bursting. This was repeated once more to illustrate the capacity for rapidly altering episodic breathing in an isolated adult turtle brainstem.
4. Discussion
The main findings were that 5-HT3 receptor activation acutely converted episodic respiratory discharge to singlet discharge and increased episode regularity. Bath-application of 5-HT3 receptor antagonists had the opposite effect (i.e., increased the number of bursts/episode), suggesting endogenous 5-HT3 receptor activation contributes to regulation of respiratory burst timing. Finally, 5-HT3 receptor activation was not required for the long-lasting decrease in bursts/episode, which is consistent with the hypothesis that this represents a form of respiratory neuroplasticity.
4.1 Acute effects of 5-HT3 receptor activation in respiratory motor control
Serotonin 5-HT3 receptors are ligand-gated, cation-permeable ion channels expressed throughout the CNS, including the brainstem (Barnes et al., 1990; Doucet et al., 2000). 5-HT3 receptors have varying degrees of conductance and permeability to Na+, K+, and Ca2+ ions depending on subunit composition (Barnes et al., 2009). Peripherally, 5-HT3 receptor activation in mammals is associated with a reduction in sleep apnea (Carley et al., 2001, Fenik et al., 2001), and modulation of serotonin-induced pulmonary chemoreflexes (Kopczyńska, 2004). However, there are no mammalian studies regarding the effects of central 5-HT3 receptor activation on respiratory rhythm generation.
In ectotherms, activation of peripheral 5-HT3 receptors in eels increases ventilatory frequency and amplitude, an effect blocked by the 5-HT3 antagonist MDL72222 (Janvier et al., 1996). Electrical stimulation of the raphé nucleus in isolated pre-metamorphic tadpole brainstems increases lung respiratory frequency by 1300% over baseline via a 5-HT3-dependent mechanism; however bursts/episode and regularity were not studied (Belzile et al., 2007). In isolated turtle brainstems, 5-HT3 receptor activation via PBG increases burst frequency by ~100% during drug application (Johnson et al., 2001). Thus, central and peripheral 5-HT3 receptor activation appears to increase ventilation in ectothermic vertebrates. In this study, 5-HT3 receptor activation via mCPBG or PBG application to turtle brainstems acutely increased burst frequency and decreased bursts/episode. 5-HT3 receptor activation also increased the regularity of episodes regardless of whether the episodes contained multiple bursts or singlet bursts. This is the first study to demonstrate that central 5-HT3 receptor activation modulates clustering of respiratory bursts into episodes and the regularity of the episodes.
4.2 5-HT3 receptor activation may induce regularity plasticity
Plasticity can be defined as a persistent morphological or functional change in a neural control system based on prior experience (Mitchell and Johnson, 2003). Serotonin release is a well-established, evolutionarily-conserved mechanism for inducing neuroplasticity. With respect to respiratory motor control, 5-HT2 receptor activation is necessary for the induction of plasticity induced by intermittent hypoxia (Ling et al., 2001), hypercapnic exercise (Johnson and Mitchell, 2001), and spinal cord injury (Kinkead et al., 1998). While 5-HT3 receptors were not associated with plasticity in the respiratory control system, they are involved in other forms of neuroplasticity. For example, 5-HT3 receptor activation results in a blockade of hippocampal synaptic long-term potentiation via facilitation of GABAergic interneurons (Maeda et al., 1994; Reznic et al., 1997). In contrast, 5-HT3 receptor activation is necessary for both the induction and maintenance of activity-dependent synaptic long-term potentiation in the superior cervical ganglion, although the mechanism is still unclear (Alkadhi et al., 1996).
With respect to the long-lasting 5-HT3-dependent changes in respiratory burst pattern and frequency in turtle brainstems, this study showed that mCPBG induced a long-lasting decrease in bursts/episode while PBG induced a long-lasting increase in burst frequency. The long-lasting mCPBG-dependent effects were blocked by tropisetron when given prior to, but not following, mCPBG application. Thus, 5-HT3 receptor activation appears to be required for induction, but not maintenance, of the long-lasting decrease in bursts/episode induced by mCPBG application. This is similar to the finding that 5-HT2 receptor activation is required for induction, but not maintenance, of phrenic long-term facilitation following intermittent hypoxia in anesthetized rats (Fuller et al., 2001). Although consistent with the hypothesis that mCPBG induced a form of respiratory neuroplasticity, several caveats need to be considered. First, the location, pharmacological properties, and ion selectivity of turtle (and reptile) 5-HT3 receptors are poorly understood. Second, the binding and dissociation constants for the 5-HT3 agonist and antagonist drugs interacting with turtle 5-HT3 receptors at room temperature are not known. Third, the timecourse and extent to which these 5-HT3-related drugs penetrate and wash out of turtle brainstems is not known. Further detailed studies at the cellular level will be required to determine whether the long-lasting 5-HT3-dependent effects represent respiratory neuroplasticity.
With respect to the long-lasting changes in burst frequency due to PBG application, several points need to be considered. In a previous study on turtle brainstems (Johnson et al., 2001), PBG produced a long-lasting (>2 h) increase in burst frequency referred to as “frequency plasticity” (Johnson et al., 2001). In that same study, tropisetron blocked the 5-HT-dependent increase in burst frequency and anecdotal evidence (n=2 brainstems) showed that tropisetron blocked the PBG-dependent acute and long-lasting frequency increase, which suggested that PBG-dependent frequency changes were due to 5-HT3 receptor activation (Johnson et al., 2001). However, in this study, the acute and long-lasting PBG-dependent frequency increases were not blocked by tropisetron (Fig. 6D). This suggests that PBG acts via 5-HT3 receptors to elicit acute and long-lasting decreases in bursts/episode and episode interval coefficient of variation, but PBG may also be interacting with other neurotransmitter receptors to acutely increase burst frequency, such as catecholamine receptors (see discussion below).
4.3 Endogenous activation of 5-HT3 receptors determines episodic breathing pattern
Episodic breathing is found in mammals under conditions of hibernation or sleep, and is the normal breathing pattern for many ectothermic vertebrates (Milsom, 1991, Fong et al., 2009). In amphibians, episodic breathing can be pharmacologically altered while maintaining a constant ventilatory drive, i.e., the number of breaths/episode can be changed without changing the total number of breaths per unit of time (Harris et al., 2002). For example, baclofen and nitric oxide change episodic bursts to singlet bursts without changing ventilatory drive during drug application in isolated tadpole brainstems (Straus et al., 2000; Harris et al., 2002). In contrast, olfactory and pulmonary CO2 receptors modulate both ventilatory drive and episodic breathing pattern in intact bullfrogs (Kinkead and Milsom, 1996). In turtles, 5-HT3 receptor activation via mCPBG acutely increased ventilatory drive (burst frequency) and decreased bursts/episode. However after the 2-h washout, burst frequency returned to baseline while the reduction in bursts/episode was maintained, thereby showing that episodic breathing pattern could be uncoupled from ventilatory drive. The uncoupling of episodic breathing from ventilatory drive is similar to the effects of baclofen and nitric oxide in amphibians except that no drug is present in the turtle brainstem experiments. Finally, tropisetron application to turtle brainstems increased bursts/episode, which suggests that serotonin endogenously modulates breathing pattern in intact turtles. Variability in the degree of endogenous 5-HT3 receptor activation would account for the differences in baseline episodicity in isolated turtle brainstems; i.e., 25% of brainstems produce episodic discharge (>2.0 bursts/episode) while 56% of brainstems produce singlet discharge (<1.25 bursts/episode; Johnson and Creighton, 2005).
The ability to rapidly and reversibly switch back and forth from episodes to singlets in turtle brainstems suggests that turtles may use this mechanism to optimize their breathing pattern to accommodate changes in their environment. We hypothesize that semi-aquatic turtles switch from a primarily episodic breathing pattern while in water (to maximize gas exchange prior to diving) to a primarily singlet pattern while on land. This hypothesis is supported by studies showing that terrestrial chelonians tend to breathe in singlets while aquatic chelonians tend to breathe episodically (McCutcheon, 1943; Gaunt and Gans, 1967). For example, the terrestrial tortoise (Testudo pardalis) breathes in singlets while the aquatic turtle (Pelomedusa subrufa) breathes episodically (Burggren et al., 1977). Likewise, the terrestrial tortoise (Testudo graeca) exhibits both singlet and episodic breathing, but the singlet breathing pattern is dominant (Gans and Hughes, 1967). For intact, semi-aquatic, red-eared slider turtles (Trachemys scripta) placed in water-filled tanks, the breathing pattern is mostly episodic with occasional singlets (Johnson and Creighton, 2005; Sladky et al., 2007; Johnson et al., 2008). To our knowledge, the breathing pattern of any chelonian on land versus in water has not been systematically studied, nor is it known whether 5-HT3 receptor activation modulates breathing pattern in terrestrial or aquatic chelonians. Red-eared slider turtles might be an ideal species for testing this hypothesis because they spend significant time on land and in water. Alternatively, it’s possible that the 5-HT3-dependent mechanism for altering breathing pattern is unique only to semi-aquatic turtles.
4.4 Differences between 5-HT3 receptor drugs
The three 5-HT3 agonists and antagonists used in this study produced inconsistent results. For example, 2-methyl-5-HT acutely increased bursts/episode and episode interval coefficient of variation, while mCPBG and PBG had the opposite effects. With respect to 5-HT3 antagonists, ondansetron did not alter breathing pattern, but reproducible increases in bursts/episode were obtained with tropisetron and MDL72222. One explanation is that different drug responses observed in this study were due to species differences. For example, mCPBG has 100× greater affinity for rat versus human 5-HT3 receptors (Edwards et al., 1996), and 2-methyl-5-HT has different affinities among humans, mice, and dogs (Miyake et al., 1995; Jensen et al., 2006). Similar species and tissue differences in binding are well documented for ondansetron (Wolf, 2000). In addition, drugs developed for use in mammals may have altered affinity and efficacy under the conditions used in our in vitro experiments (e.g., low temperature), which are physiologically relevant to turtles. Thus, the systematic evaluation of three 5-HT3 receptor agonists and antagonists in this study revealed that mCPBG was the most consistent and reliable 5-HT3 receptor agonist because the acute and long-lasting effects of mCPBG were expressed in all brainstems, and these effects were completely abolished by tropisetron.
Our data also showed that mCPBG produced more robust and consistent acute and long-lasting decreases in bursts/episode than PBG. In addition, PBG application resulted in long-lasting increase in frequency that was not blocked by tropisetron. It is possible that PBG caused dopamine release via a 5-HT3-independent (Schmidt and Black, 1989) or 5-HT3-dependent mechanism (Chen et al., 1991) because bath-applied dopamine increases burst frequency in isolated turtle brainstems (Johnson et al., 1998a). However, since dopamine application does not produce frequency plasticity (Johnson et al., 1998a), co-activation of 5-HT3 and some other catecholamine receptor may be required to induce frequency plasticity in turtle brainstems.
4.5 5-HT3 receptor activation and burst shape
In this study, mCPBG and PBG did not alter respiratory burst amplitude. This is consistent with other findings that local 5-HT3 receptor activation does not alter XII motoneuron excitability in sleeping bulldogs (Veasey et al., 2001), anesthetized rats (Fenik et al., 2001), or neonatal rat brainstem slices (Ladewig et al., 2004). mCPBG reduced burst duration during washout without changing percent time-to-peak, whereas PBG had no effect on these aspects of burst shape. In contrast, tropisetron and MDL72222 steadily decreased respiratory burst amplitude by 20–30% during the 2-h application period, and MDL72222 decreased burst duration. The mechanism for the antagonist-dependent decrease in amplitude or burst duration is not clear. It is possible that tropisetron and MDL72222 were acting non-specifically on receptors expressed on XII motoneurons or interneurons projecting to XII motoneurons. For example, tropisetron and MDL72222 block nicotinic cholinergic receptor subtypes (Rothlin et al., 2003; Papke et al., 2005) that mediate the amplitude increase produced by local injection of nicotine into the XII nucleus of rhythmically active slices (Shao, 2001).
4.6 Summary and conclusions
In isolated adult turtle brainstems, 5-HT3 receptor activation acutely increased respiratory burst frequency, regularity, and singlet bursts, whereas 5-HT3 receptor blockade increased episodicity. Under in vitro conditions, 5-HT3 receptor activation and blockade rapidly and reversibly changed the respiratory burst pattern from singlet to episodic bursting. In addition, long-lasting increases in singlet burst pattern and episode regularity were induced by 5-HT3 receptor activation. These data suggest that 5-HT3 receptor activation plays an important role in modulating respiratory pattern in turtles, perhaps to optimize breathing on land versus in water. In addition, 5-HT3-dependent modulation of turtle respiratory motor pattern in vitro provides a new powerful experimental model for identifying neurons involved in regulating episodic breathing and breathing regularity.
Acknowledgments
This work was supported by a National Science Foundation grant (IOB 0517302). Michelle Bartman was supported by a National Heart Lung Blood Institute grant (T32 HL07654).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkadhi KA, Salgado-Commissariat D, Hogan YH, Akpaudo SB. Induction and maintenance of ganglionic long-term potentiation require activation of 5-hydroxytryptamine (5-HT3) receptors. J Physiol. 1996;496:479–89. doi: 10.1113/jphysiol.1996.sp021700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol. 2008;162:8–17. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Deakin JF, Ironside JW, Kilpatrick GJ, Naylor RJ, Rudd JA, Simpson MD, Slater P. Identification and distribution of 5-HT3 recognition sites within the human brainstem. Neurosci Lett. 1990;111:80–86. doi: 10.1016/0304-3940(90)90348-d. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor-the relationship between structure and function. Neuropharm. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman ME, Johnson SM. Episodic respiratory motor output is decreased by 5-HT3 receptor agonists and increased by 5-HT3 receptor antagonists in isolated adult turtle brainstems. Experimental Biology. 2009 abstracts. #960.8. [Google Scholar]
- Belzile O, Gulemetova R, Kinkead R. Role of 5-HT2A/C receptors in serotonergic modulation of respiratory motor output during tadpole development. Respir Physiol Neurobiol. 2002;133:277–282. doi: 10.1016/s1569-9048(02)00169-6. [DOI] [PubMed] [Google Scholar]
- Belzile O, Gulemetova R, Kinkead R. Effects of medullary Raphé stimulation on fictive lung ventilation during development in Rana Catesbeiana. J Exp Bio. 2007;210:2046–2056. doi: 10.1242/jeb.003202. [DOI] [PubMed] [Google Scholar]
- Burggren WW, Glass ML, Johansen K. Pulmonary ventilation: perfusion relationships in terrestrial and aquatic chelonian reptiles. 1977;55:2024–2034. doi: 10.1139/z77-263. [DOI] [PubMed] [Google Scholar]
- Carley DW, Depoortere H, Radulovacki M. R-zacopride, a 5-HT3 antagonist/5-HT4 agonist, reduces sleep apneas in rats. Pharm Biochem and Behav. 2001;69:283–289. doi: 10.1016/s0091-3057(01)00535-4. [DOI] [PubMed] [Google Scholar]
- Chen JP, van Praag HM, Gardner EL. Activation of 5-HT3 receptor by 1-phenylbiguanide increases dopamine release in the rat nucleus accumbens. Brain Res. 1991;543:354–357. doi: 10.1016/0006-8993(91)90050-6. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;142:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Doucet E, Miquel MC, Nosjean A, Verge D, Hamon M, Emerit MB. Immunolabeling of the rat central nervous system with antibodies partially selective of the short form of the 5-HT3 receptor. Neurosci. 2000;95:881–892. doi: 10.1016/s0306-4522(99)00494-7. [DOI] [PubMed] [Google Scholar]
- Edwards E, Hampton E, Ashby CR, Zhang J, Wang RY. 5-HT3-like receptors in the rat medial prefrontal cortex: further pharmacological characterization. Brain Res. 1996;733:21–30. doi: 10.1016/0006-8993(96)00529-x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik P, Ogawa H, Veasey SC. Hypoglossal nerve response to 5-HT3 drugs injected into the XII nucleus and vena cava in the rat. Sleep. 2001;24:871–878. doi: 10.1093/sleep/24.8.871. [DOI] [PubMed] [Google Scholar]
- Fong AY, Zimmer MB, Milsom WK. The conditional nature of the “Central Rhythm Generator” and the production of episodic breathing. Respir Physiol Neurobiol. 2009;168:179–187. doi: 10.1016/j.resp.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Gans C, Hughes GM. The mechanism of lung ventilation in the tortoise Testudo graeca Linné. J Exp Biol. 1967;47:1–20. doi: 10.1242/jeb.47.1.1. [DOI] [PubMed] [Google Scholar]
- Gaunt AS, Gans C. Mechanics of respiration in the snapping turtle, Chelydra serpentina (Linné) J Morph. 1967;128:195–226. [Google Scholar]
- Harris MB, Wilson RJA, Vasilakos K, Taylor BE, Remmers JE. Central respiratory activity of the tadpole in vitro brain stem is modulated diversely by nitric oxide. Am J Physiol Reg Integr Comp Physiol. 2002;283:R417–R428. doi: 10.1152/ajpregu.00513.2001. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Resp Physiol Neurobio. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier JJ, Peyraud-Waïtzenegger M, Soulier P. Mediation of serotonin-induced hyperventilation via 5-HT3-receptor in European eel Anguilla anguilla. J Comp Physiol B. 1996;165:640–646. doi: 10.1007/BF00301132. [DOI] [PubMed] [Google Scholar]
- Jensen TN, Nielsen J, Frederiksen K, Ebert B. Molecular cloning and pharmacological characterization of serotonin 5-HT(3A) receptor subtype in dog. Eur J Pharmacol. 2006;538:23–31. doi: 10.1016/j.ejphar.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Johnson SM, Mitchell GS. Catecholaminergic modulation of respiratory rhythm in an in vitro turtle brain stem preparation. J Appl Physiol. 1998a;85:105–114. doi: 10.1152/jappl.1998.85.1.105. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Mitchell GS. p-Chlorophenylalanine eliminates long-term modulation of the exercise ventilatory response in goats. Respir Physiol. 2001;128:161–169. doi: 10.1016/s0034-5687(01)00256-0. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Johnson RA, Mitchell GS. Hypoxia, temperature, and pH/CO2 effects on respiratory discharge from a turtle brain stem preparation. J Appl Physiol. 1998b;84:649–660. doi: 10.1152/jappl.1998.84.2.649. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JER, Henderson DR, Wenninger MR, Mitchell GS. Serotonin elicits long-lasting enhancement of rhythmic respiratory activity in turtle brain stems in vitro. J Appl Physiol. 2001;91:2703–2712. doi: 10.1152/jappl.2001.91.6.2703. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Creighton RJ. Spinal cord injury-induced changes in breathing are not due to supraspinal plasticity in turtles (Pseudemys scripta) Am J Physiol Integr Comp Physiol. 2005;289:R1550–R1561. doi: 10.1152/ajpregu.00397.2005. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Kinney ME, Wiegel LM. Inhibitory and excitatory effects of micro-, delta-, and kappa-opioid receptor activation on breathing in awake turtles, Trachemys scripta. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1599–1612. doi: 10.1152/ajpregu.00020.2008. [DOI] [PubMed] [Google Scholar]
- Khater-Boidin J, Rose D, Glérant JC, Duron B. Central effects of 5-HT on respiratory rhythm in newborn rats in vivo. Eur J Neurosci. 1999;11:3433–3340. doi: 10.1046/j.1460-9568.1999.00762.x. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Milsom WK. CO2-sensitive olfactory and pulmonary receptor modulation of episodic breathing in bullfrogs. Am J Physiol Reg Integr Comp Physiol. 1996;270:R134–R144. doi: 10.1152/ajpregu.1996.270.1.R134. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol. 1999;277:R658–R666. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Belzile O, Gulemetova R. Serotonergic modulation of respiratory motor output during tadpole development. J Appl Physiol. 2002;93:936–946. doi: 10.1152/japplphysiol.00104.2002. [DOI] [PubMed] [Google Scholar]
- Kopczyńska B, Szereda-Przestaszewska M. 5HT2 and 5HT3 receptors’ contribution to modeling of post-serotonin respiratory pattern in cats. Life Sci. 2004;75:2281–2290. doi: 10.1016/j.lfs.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Ladewig T, Lalley PM, Keller BU. Serotonergic modulation of intracellular calcium dynamics in neonatal hypoglossal motoneurons from mouse. Brain Res. 2004;1001:1–12. doi: 10.1016/j.brainres.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neurosci. 2006;142:885–892. doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Lundberg DB, Mueller RA, Breese GR. An evaluation of the mechanism by which serotonergic activation depresses respiration. J Pharmacol Exp Ther. 1980;212:397–404. [PubMed] [Google Scholar]
- Maeda T, Kaneko S, Satoh M. Inhibitory influence via 5-HT3 receptors on the induction of LTP in mossy fiber-CA3 system of guinea-pig hippocampal slices. Neurosci Rev. 1994;18:277–282. doi: 10.1016/0168-0102(94)90163-5. [DOI] [PubMed] [Google Scholar]
- McCutcheon FH. The respiratory mechanism in turtles. Physiol Zool. 1943;3:255–269. [Google Scholar]
- Milsom WK. Intermittent breathing in vertebrates. Annu Rev Physiol. 1991;53:87–105. doi: 10.1146/annurev.ph.53.030191.000511. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Invited review: Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol. 1995;48:407–416. [PubMed] [Google Scholar]
- Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchell GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol. 2001;91:709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch. 1998;435:485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- Papke RL, Schiff HC, Jack BA, Horenstein NA. Molecular dissection of tropisetron, an alpha7 nicotinic acetylcholine receptor-selective partial agonist. Neurosci Lett. 2005;378:140–144. doi: 10.1016/j.neulet.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;24:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznic J, Staubli U. Effects of 5-HT3 receptor antagonism on hippocampal cellular activity in the freely moving rat. J Neurophysiol. 1997;77:517–521. doi: 10.1152/jn.1997.77.1.517. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Lioudyno MI, Silbering AF, Plazas PV, Casati ME, Katz E, Guth PS, Elgoyhen AB. Direct interaction of serotonin type 3 receptor ligands with recombinant and native alpha 9 alpha 10-containing nicotinic cholinergic receptors. Mol Pharmacol. 2003;63:1067–1074. doi: 10.1124/mol.63.5.1067. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Black CK. The putative 5-HT3 agonist phenylbiguanide induces carrier-mediated release of [3H]dopamine. Eur J Pharmcol. 1989;167:309–310. doi: 10.1016/0014-2999(89)90595-5. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Mechanisms underlying regulation of respiratory pattern by nicotine in preBötzinger complex. J Neurophysiol. 2001;85:2461–2467. doi: 10.1152/jn.2001.85.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky KK, Miletic V, Paul-Murphy J, Kinney ME, Dallwig RK, Johnson SM. Analgesic efficacy and respiratory effects of butorphanol and morphine in turtles. J Am Vet Med Assoc. 2007;230:1356–1362. doi: 10.2460/javma.230.9.1356. [DOI] [PubMed] [Google Scholar]
- Straus C, Wilson RJA, Tezenas du Montcel S, Remmers JE. Baclofen eliminates cluster lung breathing of the tadpole brainstem, in vitro. Neurosci Lett. 2000;292:13–16. doi: 10.1016/s0304-3940(00)01422-1. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Chachkes J, Fenik P, Hendricks JC. The effects of ondansetron on sleep-disordered breathing in the English Bulldog. Sleep. 2001;24:155–160. doi: 10.1093/sleep/24.2.155. [DOI] [PubMed] [Google Scholar]
- Wolf H. Preclinical and clinical pharmacology of the 5-HT3 receptor antagonists. Scand J Rheumatol. 2000;113:37–45. doi: 10.1080/030097400446625. [DOI] [PubMed] [Google Scholar]