Abstract
Cadherins are a large family of cell-cell adhesion molecules that tether cytoskeletal networks of actin and intermediate filaments to the plasma membrane. This function of cadherins promotes tissue organization and integrity, as demonstrated by numerous disease states that are characterized by the loss of cadherin-based adhesion. However, plasticity in cell adhesion is often required in cellular processes such as tissue patterning during development and epithelial migration during wound healing. Recent work has revealed a pivotal role for various membrane trafficking pathways in regulating cellular transitions between quiescent adhesive states and more dynamic phenotypes. The regulation of cadherins by membrane trafficking is emerging as a key player in this balancing act and studies are beginning to reveal how this process goes awry in the context of disease. This review summarizes the current understanding of how cadherins are routed and how the interface between cadherins and membrane trafficking pathways regulates cell surface adhesive potential. Particular emphasis is placed on the regulation of cadherin trafficking by catenins and the interplay between growth factor signaling pathways and cadherin endocytosis.
Keywords: Endocytosis, adherens junctions, desmosomes, cell-cell adhesion, pemphigus vulgaris, Epithelial Mesenchymal Transition, catenin, growth factors
Cadherins are a family of adhesion molecules that play diverse roles in tissue patterning during development, growth control during tumorigenesis, and in the maintenance of adult tissue architecture. Typically, cadherins mediate calcium dependent and homophilic adhesion, thereby promoting association of cells expressing the same cadherin family members (1). At regions of cell-cell contact, cadherins assemble into large macromolecular complexes such as adherens junctions (1) and desmosomes (2, 3). These structures serve to couple adhesive interactions mediated by cadherins to the actin and intermediate filament cytoskeletons, respectively. An expanding array of human disorders and experiments using a range of genetic model systems solidify the common perception that cadherins are critical for tissue integrity. However, it has also become apparent that the dynamic regulation of cell-cell adhesion is pivotal in a number of developmental processes, including cell motility, cellular differentiation, cell fate decisions, and tissue morphogenesis (4). Likewise, cadherin adhesive activity and cell surface levels are altered during tumorigenesis, wound healing and various pathologies that are characterized by compromised tissue organization. These observations have heightened our appreciation for the conceptual framework that intercellular junctions, and cadherin family adhesion molecules in particular, are highly dynamic entities rather than static rivets that hold cells together.
This review focuses on recently identified molecular interactions that influence cadherin dynamics by selectively routing cadherins through various membrane trafficking pathways. The first section of the review discusses general pathways for cadherin trafficking after biosynthesis, as well as retrieval from the plasma membrane, recycling, and degradation. This section highlights the diversity of the cadherin family and trafficking mechanisms that are employed in their regulation. Following this introduction to the players and pathways, the review addresses cadherin regulation by catenins, comparing and contrasting roles for catenins in different trafficking decisions. We then explore cross talk between cadherins and growth factor receptors, and the apparent co-regulation of adhesion and signaling by endocytic processing of both classes of cell surface receptors. Lastly, we discuss the growing appreciation for the role of cadherins in cancer, pathogen internalization, and other disease processes. Although the field of cadherin dynamics and trafficking is relatively new, the complexity surrounding the regulation of cadherin cell surface expression and internalization is already apparent, as is the key role that these pathways play in the modulation of cadherin function.
General Routing Pathways for Cadherin Trafficking
Early studies revealed that cadherins could maintain adhesion only in the presence of calcium, and that upon calcium depletion cadherins become metabolically unstable. This observation led to the realization that cadherins can be retrieved from the plasma membrane to modulate the adhesive state of the cell surface (5–9). Like other receptors, cadherins are internalized by selective recruitment into specific endocytic pathways. These cadherin entry points include clathrin-mediated endocytosis, caveolae-mediated endocytosis, and internalization routes that are independent of both clathrin and caveolae, including lipid raft-mediated endocytosis and macropinocytosis.
Several classical cadherins, including E-cadherin and VE-cadherin, are internalized via the clathrin-mediated pathway (10–13). Recruitment into the clathrin pathway typically requires an association of the cargo protein, in this case cadherin, with an adaptor complex that couples the cargo to clathrin during coated pit assembly. Thus, a key and emerging issue being addressed in cadherin biology is the identification of adaptors that bind cadherin tails and mediate recruitment of these adhesion molecules to endocytic invaginations for internalization. In the case of VE-cadherin, Gavard and colleagues reported an interaction with β-arrestin upon treatment of endothelial cells with the growth factor VEGF (14). β-arrestin interaction with VE-cadherin is dependent upon the presence of a serine residue in the cadherin tail that is a target for p21-activated kinase (PAK), downstream of Rac activation by VEGF. Furthermore, VE-cadherin endocytosis was shown to be coupled to the regulation of endothelial barrier function in response to VEGF. In addition, our laboratory has uncovered associations between the adaptor complex AP-2 and the VE-cadherin cytoplasmic domain (Chiasson and Kowalczyk, unpublished observation), suggesting that multiple clathrin adaptors may cooperate during VE-cadherin internalization or, alternatively, that different adaptors are engaged selectively depending upon cell signaling activities. Consistent with this latter possibility, E-cadherin also associates with several endocytic adaptors, including disabled-2 (Dab2) and AP-1B (15, 16). Dab2 is implicated in the regulation of epithelial cell polarity by selectively recruiting apical surface E-cadherin into a clathrin-mediated endocytic pathway. In a separate study, E-cadherin was shown to associate with the clathrin adaptor, AP-1B, indirectly through type Iγ phosphatidylinositol phosphate kinase (PIPKIγ) (16). This interaction appears to regulate both E-cadherin endocytosis and recycling. Remarkably, the binding site for PIPKIγ in the E-cadherin tail is mutated in patients suffering from gastric carcinogenesis (16, 17). Collectively, these findings reveal an interface between membrane trafficking pathways and cadherin function in vascular biology, the development and maintenance of epithelial polarity, and in human tumorigenesis.
In addition to clathrin-mediated endocytosis, cadherins are also internalized through engagement of other types of endocytic machinery. For example, E-cadherin is internalized through a caveolae-mediated pathway in A431 human epidermoid carcinoma cells (18). Similarly, Rac activation triggers E-cadherin internalization through a caveolae-mediated pathway in keratinocytes (19). As discussed below, the desmosomal cadherin, desmoglein 3 (Dsg3) can be internalized from keratinocyte cell surfaces through a mechanism independent from clathrin, caveolin, and dynamin, suggesting involvement of a lipid raft-mediated pathway (20). Finally, cadherins are also internalized by macropinocytosis, a pathway that appears to be associated predominantly with the retrieval of non-junctional pools of cadherins (21, 22). Thus, cadherins have been found to undergo endocytic processing by all of the major routes for internalization from the plasma membrane (Table I). As discussed below, it appears that the pathway chosen for internalization is dictated by the cellular environment, particularly with respect to the growth factor milieu and the migratory needs of the cell.
Table I.
Different Routes and Regulators of Cadherin Internalization
| Internalization Route | Cadherin | Adaptors and Regulators |
|---|---|---|
| Clathrin-Mediated | VE-cadherin | p120(11, 75), β-arrestin(14), VEGF(14), Ang1(49), AP-2** |
| E-cadherin | p120(33, 34), Dab2(15), β-catenin(43), PIPKIγ(16), Rac-1/IQGAP1(12), FGF(53), HGF(51) | |
| N-cadherin | NMDAR(44), β-catenin(44) | |
| Caveolae-mediated | E-cadherin | Caveolin-1(19), Rac-1(19) |
| Lipid raft-mediated | Desmoglien 3 | Tyr phosphorylation(20) |
| E-cadherin | Arf6(21, 24, 52) | |
| Macropinocytosis | E-cadherin | EGF(22), Rac-1(22) |
| N-cadherin | β-catenin(47), RAC-1/IQGAP1(47) |
indicates submitted work by C. Chaisson and A. Kowalczyk
Regulation of Cadherin Fate
Once a cadherin is internalized, additional sorting machinery regulates routing of the receptor. Differences in post endocytic fates of the cadherin can have either short or long term consequences on the cell’s adhesive potential, depending on whether the adhesion molecule is sorted to the lysosome for degradation or recycled back to the cell surface. Interestingly, a number of studies have implicated cadherin recycling and degradation in various developmental and morphogenic events. Very little is currently known about how cadherins are selected from sorting endosomes for trafficking to lysosomes. Ubiquitin tagging and association with the hepatocyte growth receptor substrate, Hrs, (23) as well as Rab 5 and 7 GTPases, have been found to be essential in directing E-cadherin to the lysosome upon internalization (24, 25). Conversely, several endosome proteins have been implicated in cadherin recycling, including Rab11 and the sorting nexin, SNX1, with SNX1 implicated in re-routing of E-cadherin back to the plasma membrane for the maintenance of cell-cell adhesion (22, 26).
Selective delivery of newly synthesized membrane proteins is critical to the establishment and maintenance of cell polarity, and sorting of cadherins to the basolateral cell surface plays a central role in the establishment of epithelial morphology. Newly synthesized E-cadherin is processed through recycling endosomes on the way to the plasma membrane, and inhibitory mutants of Rab 11 block normal polarized delivery of E-cadherin to the basolateral cell surface (26). E-cadherin exits the Golgi complex via golgin-97 dependent tubulovesicular carriers, and subsequently fuses with an intermediate recycling endosome, en route to the basolateral plasma membrane (27). E-cadherin sorting to the basolateral surface requires a dileucine motif in the juxtamembrane domain of the cadherin cytoplasmic tail (28), although the adaptor complex that recognizes this motif has not yet been identified. This pathway for E-cadherin trafficking is tightly integrated with other proteins and complexes known to play key roles in epithelial polarity. Loss of function mutants of Drosophila exocyst components, Sec5, 6, and 15 result in an accumulation of DE-cadherin in Rab11 positive recycling endosomes (29). Furthermore, Protein Associated with Lin Seven 1 (PALS), a conserved scaffold protein most closely associated with its role in tight junction protein transport to the plasma membrane, has also been shown to play a role in regulating E-cadherin. Knockdown of PALS caused an accumulation of E-cadherin in the cytoplasm, implicating this protein in the regulation of E-cadherin transport from the Golgi to the plasma membrane (30). Recent studies have also revealed important roles for the recycling endosome in E-cadherin trafficking and epithelial lumen formation (31). Finally, Nejum and Nelson have also observed that at sites of initial cell-cell contact, a basolateral protein, aquaporin 3 (AQP3), but not an apical associated protein, AQP5, accumulates with E-cadherin, suggesting that the cadherins themselves also play an important role in establishing cell polarity (32). These studies are beginning to unravel the molecular mechanisms by which cadherin mediated adhesion and membrane trafficking pathways are integrated to generate polarized tissue architecture.
Catenin Regulation of Cadherin Trafficking
The cytoplasmic tails of cadherins associate with binding partners termed catenins, which function as scaffolds for both cytoskeletal attachments and the recruitment of regulatory molecules which modify cadherin function and cytoskeletal organization. In the adherens junction, β-catenin is an armadillo family protein that binds to classical cadherins such as E-cadherin near the carboxyl terminal region of the cadherin tail (1). In addition, β-catenin associates with α-catenin and other actin binding partners to mediate an indirect linkage of the cadherin to cortical actin networks. Additionally, p120-catenin, another member of the armadillo gene family, also associates with the cadherin tail but in a domain membrane proximal to the β-catenin binding site (1). As discussed below, both β-catenin and p120-catenin appear to play key roles not only in cytoskeletal associations within the adherens junction, but also as traffic cops that influence cadherin routing through cellular organelles.
The clearest example of catenin regulation of cadherin trafficking stems from observations that p120-catenin regulates cadherin turnover. A series of studies from the Reynolds lab showed that loss of p120, either due to genetic alterations in epithelial tumor cell lines (33) or by siRNA knock down of p120 gene expression (34), resulted in increased turnover rates of E-cadherin. Similar results were obtained in our laboratory in microvascular endothelial cells (35). Furthermore, we found that p120 binding to the juxtamembrane domain of VE-cadherin potently inhibits cadherin endocytosis (11). In addition, mutations in the E-cadherin juxtamembrane domain that prevent p120 binding result in increased rates of cadherin endocytosis (36). Collectively, these findings reveal a key role for p120-catenin as a master regulator of cadherin stability, and consequently, of other adherens junction components that are dependent upon cadherin expression for localization at the membrane and for maintenance of steady state expression levels.
The mechanism by which p120 binding to the cadherin tail inhibits endocytosis is currently unclear. An attractive model is that p120 binding functions as a cap on the cadherin juxtamembrane domain and thereby inhibits binding of adaptor complexes that would recruit the cadherin cargo into a coated pit (Figure 1A). Consistent with this possibility, VE-cadherin is internalized in a clathrin dependent manner, and p120 binding to the cadherin juxtamembrane domain is required to prevent internalization (11). Recently, we have found that the VE-cadherin tail associates with the clathrin adaptor complex AP-2, and that p120 prevents the recruitment of VE-cadherin into membrane domains enriched in AP-2 and clathrin (Chiasson and Kowalczyk, submitted). Nonetheless, p120 is remarkably biologically active and also functions as a major cellular rheostat for Rho GTPase activity. Recently, Wildenberg et al proposed that p120 inhibits RhoA locally at the plasma membrane through interactions with p190RhoGAP, and thereby stabilizes cadherin (37). However, we have found that a p120 mutant unable to regulate RhoA is equally potent to wild type p120 in assays measuring VE-cadherin endocytosis (Chiasson and Kowalczyk, submitted). Furthermore, p120 prevents VE-cadherin recruitment into clathrin enriched membrane domains in a Rho independent manner. Thus, it is likely that p120 binding to the cadherin tail prevents adaptor binding, and through this mechanism inhibits cadherin endocytosis and turnover (Figure 1A).
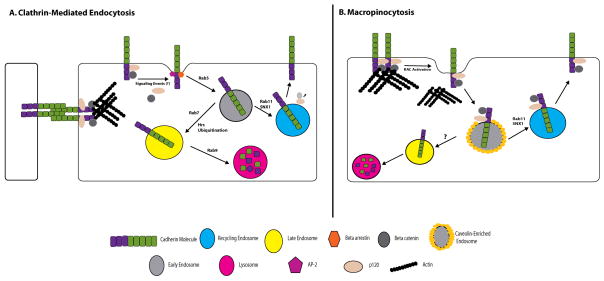
Figure 1. Different Endocytic Pathways Utilized by E-cadherin.
(A) Numerous studies have illustrated that classical cadherins, such as E-cadherin, can undergo various types of endocytic processing. Furthermore, the route taken by E-cadherin seems to be dependent on the presence of its cytoplasmic binding partner, β-catenin or p120. In the case of clathrin-mediated endocytosis, binding of β-catenin and p120 maintains E-cadherin’s association with the actin cytoskeleton network and prevents E-cadherin endocytosis. However, signaling events may cause disassociation between the cadherin and the catenins, thereby allowing adaptors, such as AP-2 and β-arrestin, to interact with the cytoplasmic tail and recruit clathrin and other accessory proteins that promote internalization. Once internalized, E-cadherin enters sorting/early endosomes in which other molecules proteins regulate the fate of the cadherin. GTPases, such as Rabs 5 and 7, as well as post-translational modification of the cadherin cytoplasmic tail (i.e. ubiquitination), promote E-cadherin entry into a lysosomal pathway for degradation. However, Rab 11 and the sorting nexin, SNX1, are essential for routing E-cadherin to recycling endosomes and ultimately back to the plasma membrane. (B) When E-cadherin undergoes clathrin-independent endocytosis, signaling events, such as Rac1 activation, are thought to rearrange the actin cytoskeleton network, allowing for internalization of the complex into early endosomes. This complex is internalized into a caveolin-enriched endosome and does not colocalize with markers for late endosomes or lysosomes, suggesting that the cadherin-catenin complex is then recycled back to the plasma membrane, with the assistance of the sorting nexin, SNX1.
Regardless of the precise mechanism, an important implication of p120 functioning as a set point for cadherin expression levels arises in the context of cells that express multiple cadherin genes. During development and tumorigenic transformation, cells upregulate expression of specific cadherins through a process known as cadherin switching (38). Since p120-catenin levels dictate total cellular cadherin expression levels, upregulation of a new cadherin results in post-translational down-regulation of other cadherins expressed in the cell. Thus, p120 appears to play a key regulatory role in relative cadherin expression levels during both developmental and tumorigenic alterations in cadherin expression profiles (38).
In addition to cadherin endocytosis, several studies have suggested roles for p120 and the cadherin juxtamembrane domain in other aspects of cadherin dynamics. For example, N-cadherin delivery to the plasma membrane for assembly into adherens junctions in fibroblasts requires both an intact microtubule network and the anterograde motor protein kinesin (39). Furthermore, p120 has been shown by several groups to associate with microtubules and kinesin (40, 41), and studies by the Green laboratory suggest that p120 functions as a scaffold to couple N-cadherin to kinesin for delivery to newly forming junctions (42). These findings indicate that, at least for N-cadherin, p120 plays an important role in anterograde trafficking in addition to endocytosis. Nonetheless, it is puzzling that p120 depletion does not cause any change in the rate of delivery of newly synthesized E-cadherin to the plasma membrane (34). One possibility is that p120 plays cell- or cadherin-specific roles in trafficking. Alternatively, p120 may support kinesin mediated delivery to the membrane from recycling endosomes, but may not play an important role in delivery of newly synthesized cadherin to the membrane.
Although p120 has received the bulk of recent attention with respect to cadherin trafficking, a number of studies also suggest that β-catenin contributes to cadherin delivery to the plasma membrane and routing after endocytosis. For example, casein kinase 1 was recently shown to weaken interactions between β-catenin and the E-cadherin cytoplasmic tail, resulting in increased endocytosis of E-cadherin (43). Furthermore, N-cadherin endocytosis at neuronal synapses was found to be regulated by β-catenin (44). In this model system, NMDA-receptor activation inhibited N-cadherin endocytosis by preventing β-catenin tyrosine phosphorylation. Specifically, a Y654F mutant of β-catenin selectively increased surface levels of N-cadherin by preventing N-cadherin internalization, demonstrating a key regulatory role for β-catenin in N-cadherin cell surface levels. Moreover, this inhibition in N-cadherin internalization led to inhibition of long term depression (LTD), implicating the importance of N-cadherin trafficking in regulating synaptic function. Other evidence also suggests that β-catenin may modulate cadherin trafficking. Early studies suggested that E-cadherin mutants that are unable to bindβ-catenin are inefficiently delivered to the plasma membrane (45). More recently, Miyashita and Ozawa reported that E-cadherin mutants lacking the ability to bindβ-catenin accumulate in post golgi compartments, including early endosomes, before being delivered to lysosomes through a sorting mechanism dependent upon a dileucine residue in the cadherin juxtamembrane domain (46). Thus, while p120 appears to be the key cadherin binding partner in regulating endocytosis, emerging evidence suggest that β-catenin also participates in mechanisms that modulate cadherin cell surface availability.
Although β-catenin inhibits N-cadherin endocytosis in some models, Sharma and Henderson demonstrated that β-catenin localizes to membrane ruffles in migrating cells and appears to play an active role in cadherin internalization (47). β-catenin was required for macropinocytosis of N-cadherin at these membrane ruffles through a mechanism that involved IQGAP1, a β-catenin binding partner and down-stream effector of Rac and Cdc42. Consistent with these findings, Rac activation causes internalization of E-cadherin along with β-catenin in a complex that is delivered to caveolin-1 enriched endosomes in keratinocytes (Figure 1B) (19). In contrast to this positive regulatory role in macropinocytosis, Rac and IQGAP1 were found to inhibit clathrin-mediated E-cadherin endocytosis in a cell free model system and in MDCK cells (12, 48). These studies raise questions regarding the role for Rac and β-catenin as positive or negative regulators of cadherin endocytosis. One explanation may be cell type specificity. However, another possibility is that different modes of cadherin internalization from the plasma membrane may engage catenins differentially. For example, during macropinocytosis upon high levels of Rac activation, β-catenin may mediate associations with actin or other cytoplasmic structures necessary for internalization of large complexes. In contrast, clathrin-mediated endocytosis may require disassembly of the cadherin catenin complex, as we and others have suggested previously (Figure 1A) (35, 36). In this scenario, signaling events that stabilize β-catenin and p120 binding to the cadherin tail may prevent clathrin-mediated internalization. Another set of signaling pathways may, in turn, be responsible for loss of catenin association with the cadherin, thereby promoting endocytosis. If this model is correct, a key issue is to identify cellular signaling events that directly modulate the strength of catenin association with the cadherin tail, and to understand how these signaling pathways coordinate globally to regulate the adhesive potential of the cell surface.
Regulation of Cadherin Endocytosis by Growth Factors
Growth factor regulation of adhesion has been recognized as a key mechanism for crosstalk between cell proliferation, migration, and adhesion. Not surprisingly, recent studies have begun to reveal a role for membrane trafficking pathways as a cellular mechanism that drives this cross-regulation. As discussed above, VEGF initiates a signaling cascade leading to phosphorylation and internalization of VE-cadherin (14). The mechanism for VE-cadherin internalization in VEGF treated endothelial cells involves the recruitment of β-arrestin to the cytoplasmic tail of VE-cadherin, leading to clathrin-mediated internalization (14). Interestingly, this pathway is antagonized by angiopoietin-1 (Ang1), a growth factor known to stabilize endothelial cell-cell contacts (49). Importantly, the regulation of cadherins by growth factors is not a one way street. Studies by Lampugnani and colleagues demonstrated that VE-cadherin regulates VEGFR endocytosis (50). Endothelial cells lacking VE-cadherin exhibit increased rates of VEGFR internalization and signaling, indicating that endothelial cell proliferation and responsiveness to growth factors is regulated by cadherin cell surface levels.
Cross regulation of cadherins and growth factor receptors is not limited to endothelial cells. E-cadherin internalization has also been shown to be regulated in MDCK cells by the hepatocyte growth factor (HGF) (51, 52). HGF binding to its receptor, c-Met, causes cell-cell dissociation, which is tightly coupled to endocytosis of both E-cadherin and c-Met. In this model system, E-cadherin endocytosis and adherens junction disassembly in response to HGF is driven in part by the Arf6 GTPase (52). It is important to appreciate that induction of different growth factors/signaling pathways within the same cell can lead to very different fates for the cadherin in question. The Stow group has shown that in MCF-7 cells, the fate of E-cadherin stability lies in the hands of FGF and EGF. FGF-induced endocytosis of E-cadherin leads to its internalization, along with FGFR1, via a classical clathrin-mediated pathway (53). However, EGF stimulation causes Rac-1 mediated internalization of E-cadherin via macropinocytosis (22). Moreover, E-cadherin also colocalizes with the sorting nexin, SNX-1, after internalization in response to EGF. This association prevents E-cadherin from entering a degradative pathway, as SNX-1 mediates recycling of E-cadherin back to the cell surface for maintenance of adherens junctions. Similar to the example above in which VE-cadherin and VEGF signaling are coordinated, N-cadherin in MCF-7 cells increases the stability of FGFR-1 at the cell surface, which thereby enhances signaling through the MAPK pathway, transcription of MMP-9 and increased cell metastasis (54). Collectively, these studies provide clear evidence that cadherins regulate growth factor receptor internalization as part of a functional reciprocity with growth factor signal transduction and the regulation of cell adhesion.
Co-regulation of cadherin and growth factor signaling is particularly prominent in various examples of epithelial to mesenchymal transitions (EMT) and tumorigenesis. TGFβ signaling, in particular, appears to be a key regulator of cadherin turnover during EMT, as TGFβ signaling is required for loss of E-cadherin expression in response to Ras-Raf signaling (55). Given the key role for cadherin down-regulating during EMT, the mechanisms of TGFβ regulation of cadherin internalization represent fertile ground for further molecular analysis of cadherin endocytosis. A recent study by Bremm and colleagues highlights the cross regulation of cadherin and growth factor signaling (56). In this study, a mutation in E-cadherin was shown to reduce interactions between E-cadherin and EGFR, resulting in increased EGFR cell surface motility, dimerization, and enhanced EGFR activation. Furthermore, this mutation in E-cadherin resulted in increased E-cadherin internalization, again underscoring the cross regulation of growth factor and cadherin trafficking. A number of other examples are emerging where membrane trafficking pathways play a key role in growth factor receptor and cadherin interplay, particularly during developmental events. Given the remarkable complexity of cadherin and growth factor receptor expression profiles, this co-regulatory system is likely to play roles in a wide range of developmental and disease processes.
Cadherin Trafficking and Disease
Increased interest in the basic cellular mechanisms of cadherin membrane trafficking has been paralleled by new insights into how these pathways are usurped in human pathologies. A number of disease states are associated with defects in cadherin endocytosis and misregulation of cadherin cell surface levels. In particular, studies have shed light on the role of cadherin destabilization in tumor cell metastasis. One of the hallmarks of the transition from benign overgrowth to a metastatic tumor is the aforementioned Epithelial to Mesenchymal Transition (EMT). EMTs are characterized by loss of epithelial morphology, acquisition of a more migratory phenotype, and loss of E-cadherin expression. This loss can be due to misregulation of E-cadherin on the transcriptional level, as is the case with Snail-mediated repression of cadherin transcription (57, 58), or due to posttranslational changes. v-Src, a Rous sarcoma virus tyrosine kinase and strong activator of EMT (24) has been implicated in phosphorylation of E-cadherin and the subsequent interaction of the cadherin tail with the E3 ubiquitin ligase, Hakai (59). Hakai is thought to mediate ubiquitin tagging of the cadherin tail, leading to endocytosis and degradation in the lysosome. Although Hakai may trigger E-cadherin endocytosis, it appears that a key role for Hakai is in preventing E-cadherin from recycling back to the plasma membrane, thus fating the cadherin for lysosomal degradation (59). MDM-2, another E3 ubiquitin ligase misregulated in numerous metastatic tumors, also downregulates E-cadherin and promotes cell motility and invasiveness in breast cancer cells (60). Similar findings were reported recently in Kaposi’s sarcoma tumors, an endothelial malignancy commonly associated with HIV/AIDS. In this instance, an E3 ubiquitin ligase expressed by the virus that causes Kaposi’s sarcoma tumors targets VE-cadherin for ubiquitination and degradation (61, 62). In addition to down-regulation of classical cadherins, EMT is also associated with alterations in desmosomal cadherins. For example, EGFR activation leads to shedding and internalization of the desmosomal cadherin Dsg2. Furthermore, MMP inhibition and knockdown of ADAM17 inhibits Dsg2 cleavage and endocytosis in invasive squamous cell carcinoma (63, 64). Further integration of signaling pathways in EMT becomes apparent at the level of cadherin gene regulation. Numerous studies have identified Snail and Slug as key regulators of cadherin gene expression in the context of EMT. Interestingly, Ras activation was shown to regulate transcription of Slug, which is required for desmosomal cadherin endocytosis in NBT-II bladder carcinoma cell lines (65, 66). These observations provide further evidence of tight coupling between growth factor receptors and cadherins in the context of tumor progression, and highlight membrane trafficking of cadherins as a key regulatory module in this network.
Cadherin endocytosis in disease is not limited to tumor biology. In the autoimmune disease pemphigus vulgaris, patients exhibit oral erosions and epidermal blistering. Autoantibodies in this disease target the desmosomal cadherin, desmoglein 3 (Dsg), thereby disrupting desmosomes and compromising epithelial cell-cell adhesion strength. Pemphigus vulgaris IgG binding to Dsg3 causes increased internalization of the desmosomal cadherin via a clathrin and dynamin independent pathway, ultimately leading to lysosomal degradation of Dsg3 (20, 67). Adhesion receptors are targeted in a number of other autoimmune disorders, particularly those impacting the epidermis (2, 68, 69). It is not fully understood how tightly coupled adhesion molecule endocytosis is to disease progression, but in the case of pemphigus, growing evidence suggests that the destabilization of desmosomal cadherins may play a direct role in the pathogenesis of the disease. For example, over-expression of Dsg3 counteracts the effects of PV IgG on Dsg3 endocytosis, desmosomal disassembly, and loss of cell-cell adhesion (Kottke, Jennings, and Kowalczyk, unpublished observation). These findings suggest that endocytosis and degradation of Dsg3 play a causative role in PV pathogenesis, and highlight the importance of cadherin stability in maintaining tissue integrity in adult organisms.
Bacteria and other organisms often invade cells to enter the nutrient rich cytoplasm, a haven that allows them to proliferate and subsequently invade neighboring cells and tissues. Cadherins offer one route for internalization of a number of pathogens to gain entry into epithelial cells. Candida albicans is a fungus which causes oropharyngeal candidiasis, or more commonly referred to as thrush. C. albicans invades the lining of the mouth during thrush and further invades the endothelial cell lining of blood vessels when candidiasis is disseminated. Als3, a protein expressed on the cell surface of C. abicans hyphae, was found to induce its internalization into host cells by binding both N- and E-cadherin. Binding to N- or E-cadherin initiates production of pseudopods to facilitate internalization of C. albicans in endothelial and epithelial cells, respectively (70). Because Als3 is thought to be structurally similar to these classical cadherins, Als3 is a potential mimic of cadherin-cadherin binding. These structural and functional features of Als3 play an important role in the internalization, invasion and dissemination of the fungus. An analogous process has been uncovered for the bacterial pathogen Listeria monocytogenes. In this instance, a bacterial surface protein internalin A (InlA) binds E-cadherin and induces recruitment of catenins and other junctional components and the motor protein myosin VIIA. Furthermore, binding to cadherin activates Rac and recruitment of cortactin and Arp2/3, thus mimicking some aspects of adherens junction assembly (71). Recent work by Bonazzi and others illustrate that in addition to adherens junction assembly, E-cadherin internalization plays a pivotal role in the internalization of lnlA. Binding of the bacterial protein to E-cadherin causes tyrosine phosphorylation of the cytoplasmic tail and subsequent ubiquintination of the cadherin by Hakai (72). These post-translational modifications resulted in bacterial internalization through a mechanism dependent on both clathrin and caveolin (72). Collectively, these studies raise interesting questions regarding the commonality in mechanisms utilized for adherens junction assembly and pathogen entry into host cells.
Conclusion
Membrane trafficking pathways provide critical regulatory oversight of cadherin function. Understanding how cadherin adhesion and dynamics are integrated will provide significant insights into a range of developmental processes and disease pathologies associated with alterations in cadherin dynamics. A number of opportunities and challenges are evident in the cadherin and membrane trafficking fields. For example, the general relationship between cadherin trafficking and adhesion requires further clarification. In a series of papers, Troyanovsky and colleagues have manipulated cadherin dimerization and clathrin-mediated endocytosis to demonstrate that blocking endocytosis can stabilize cadherin dimer formation and adhesion (7, 8). An important implication of this work is that changes in the rates of global endocytic activity or recycling may have profound impact on the overall adhesive status of the plasma membrane. Consistent with this possibility, two recent manuscripts identify a key role for cdc42 and the Par6/aPKC polarity pathway in regulating cadherin endocytosis (73, 74). This pathway regulates cadherin internalization by locally modifying actin cytoskeletal dynamics and dynamin activity. When the pathway is inactivated, endocytosis is reduced and adherens junctions become disorganized. Taken together, these studies indicate that cadherin cell surface levels and junction organization are tightly controlled by the activity of endocytic machinery. Either too much or too little endocytosis of cadherin has deleterious effects on the assembly and function of adhesive intercellular junctions.
Another challenge will be to determine not only how global changes in membrane trafficking impact adhesion, but to also reveal the molecular mechanisms that selectively identify cadherins as cargo and alter cadherin dynamics. These types of studies will be needed in order to determine whether alterations in cadherin trafficking play a causal role in a particular biological response, or if these changes are merely a consequence of the process. Addressing this issue directly will require a detailed analysis of cadherin domains and amino-acid determinants that regulate cadherin endocytosis or other trafficking decisions. Such studies will allow for mutations to be introduced that specifically alter cadherin trafficking rather than introducing global changes in membrane dynamics. Some advances have been made, including the identification of residues in VE-cadherin that mediateβ-arrestin binding and endothelial monolayer permeability. Similarly, sequences in E-cadherin required for binding to the AP-1 adaptor complex play a key role in gastric epithelial tumorigenesis. It is also likely that key advances will be realized with the identification of molecules that regulate cadherins in a specific fashion. Two such examples include Hakai and p120-catenin, both of which selectively recognize cadherins and modify cadherin endocytic processing. Additional studies of using these approaches should reveal situations where regulation of cadherin trafficking has a primary role in a developmental or disease process.
Acknowledgments
We would like to thank Ms. Christine Chiasson, for insightful and engaging conversations during the preparation of this manuscript. We also extend thanks to Drs. Kathleen Green and Victor Faundez for their valuable comments and contributions to our ongoing work. The authors acknowledge funding to APK from the NIH (R01AR048266, R01 AR050501). ED was supported by NIH F31CA110278.
References
- 1.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annual review of cell and developmental biology. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 2.Holthofer B, Windoffer R, Troyanovsky S, Leube RE. Structure and function of desmosomes. International review of cytology. 2007;264:65–163. doi: 10.1016/S0074-7696(07)64003-0. [DOI] [PubMed] [Google Scholar]
- 3.Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nature reviews. 2000;1(3):208–216. doi: 10.1038/35043032. [DOI] [PubMed] [Google Scholar]
- 4.Emery G, Knoblich JA. Endosome dynamics during development. Current opinion in cell biology. 2006;18(4):407–415. doi: 10.1016/j.ceb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Mattey DL, Garrod DR. Splitting and internalization of the desmosomes of cultured kidney epithelial cells by reduction in calcium concentration. Journal of cell science. 1986;85:113–124. doi: 10.1242/jcs.85.1.113. [DOI] [PubMed] [Google Scholar]
- 6.Windoffer R, Borchert-Stuhltrager M, Leube RE. Desmosomes: interconnected calcium-dependent structures of remarkable stability with significant integral membrane protein turnover. Journal of cell science. 2002;115(Pt 8):1717–1732. doi: 10.1242/jcs.115.8.1717. [DOI] [PubMed] [Google Scholar]
- 7.Troyanovsky RB, Laur O, Troyanovsky SM. Stable and unstable cadherin dimers: mechanisms of formation and roles in cell adhesion. Molecular biology of the cell. 2007;18(11):4343–4352. doi: 10.1091/mbc.E07-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troyanovsky RB, Sokolov EP, Troyanovsky SM. Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly. Molecular biology of the cell. 2006;17(8):3484–3493. doi: 10.1091/mbc.E06-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kartenbeck J, Schmelz M, Franke WW, Geiger B. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. The Journal of cell biology. 1991;113(4):881–892. doi: 10.1083/jcb.113.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. The Journal of cell biology. 1999;146(1):219–232. [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Molecular biology of the cell. 2005;16(11):5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. The Journal of cell biology. 2004;166(2):237–248. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Molecular biology of the cell. 2004;15(1):176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nature cell biology. 2006;8(11):1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 15.Yang DH, Cai KQ, Roland IH, Smith ER, Xu XX. Disabled-2 is an epithelial surface positioning gene. The Journal of biological chemistry. 2007;282(17):13114–13122. doi: 10.1074/jbc.M611356200. [DOI] [PubMed] [Google Scholar]
- 16.Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA. Type Igamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin. The Journal of cell biology. 2007;176(3):343–353. doi: 10.1083/jcb.200606023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yabuta T, Shinmura K, Tani M, Yamaguchi S, Yoshimura K, Katai H, Nakajima T, Mochiki E, Tsujinaka T, Takami M, Hirose K, Yamaguchi A, Takenoshita S, Yokota J. E-cadherin gene variants in gastric cancer families whose probands are diagnosed with diffuse gastric cancer. International journal of cancer. 2002;101(5):434–441. doi: 10.1002/ijc.10633. [DOI] [PubMed] [Google Scholar]
- 18.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer cell. 2003;4(6):499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Molecular biology of the cell. 2001;12(4):847–862. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delva E, Jennings JM, Calkins CC, Kottke MD, Faundez V, Kowalczyk AP. Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin and dynamin-independent mechanism. The Journal of biological chemistry. 2008 doi: 10.1074/jbc.M710046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. The Journal of biological chemistry. 2003;278(23):21050–21057. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- 22.Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. Journal of cell science. 2007;120(Pt 10):1818–1828. doi: 10.1242/jcs.000653. [DOI] [PubMed] [Google Scholar]
- 23.Ogata S, Morokuma J, Hayata T, Kolle G, Niehrs C, Ueno N, Cho KW. TGF-beta signaling-mediated morphogenesis: modulation of cell adhesion via cadherin endocytosis. Genes & development. 2007;21(14):1817–1831. doi: 10.1101/gad.1541807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palacios F, Tushir JS, Fujita Y, D’Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Molecular and cellular biology. 2005;25(1):389–402. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyoshima M, Tanaka N, Aoki J, Tanaka Y, Murata K, Kyuuma M, Kobayashi H, Ishii N, Yaegashi N, Sugamura K. Inhibition of tumor growth and metastasis by depletion of vesicular sorting protein Hrs: its regulatory role on E-cadherin and beta-catenin. Cancer research. 2007;67(11):5162–5171. doi: 10.1158/0008-5472.CAN-06-2756. [DOI] [PubMed] [Google Scholar]
- 26.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Molecular biology of the cell. 2005;16(4):1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lock JG, Hammond LA, Houghton F, Gleeson PA, Stow JL. E-cadherin transport from the trans-Golgi network in tubulovesicular carriers is selectively regulated by golgin-97. Traffic (Copenhagen, Denmark) 2005;6(12):1142–1156. doi: 10.1111/j.1600-0854.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 28.Miranda KC, Khromykh T, Christy P, Le TL, Gottardi CJ, Yap AS, Stow JL, Teasdale RD. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. The Journal of biological chemistry. 2001;276(25):22565–22572. doi: 10.1074/jbc.M101907200. [DOI] [PubMed] [Google Scholar]
- 29.Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Developmental cell. 2005;9(3):355–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Chen XW, Margolis B. PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Molecular biology of the cell. 2007;18(3):874–885. doi: 10.1091/mbc.E06-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. American journal of physiology. 2008 doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- 32.Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. The Journal of cell biology. 2007;178(2):323–335. doi: 10.1083/jcb.200705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. The Journal of cell biology. 2002;159(3):465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. The Journal of cell biology. 2003;163(3):525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. The Journal of cell biology. 2003;163(3):535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyashita Y, Ozawa M. Increased internalization of p120-uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. The Journal of biological chemistry. 2007;282(15):11540–11548. doi: 10.1074/jbc.M608351200. [DOI] [PubMed] [Google Scholar]
- 37.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127(5):1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 38.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. Journal of cell science. 2008;121(Pt 6):727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 39.Mary S, Charrasse S, Meriane M, Comunale F, Travo P, Blangy A, Gauthier-Rouviere C. Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Molecular biology of the cell. 2002;13(1):285–301. doi: 10.1091/mbc.01-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roczniak-Ferguson A, Reynolds AB. Regulation of p120-catenin nucleocytoplasmic shuttling activity. Journal of cell science. 2003;116(Pt 20):4201–4212. doi: 10.1242/jcs.00724. [DOI] [PubMed] [Google Scholar]
- 41.Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. The Journal of biological chemistry. 2004;279(10):9512–9521. doi: 10.1074/jbc.M310895200. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. The Journal of cell biology. 2003;163(3):547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J, Richardson EC, Fujita Y. Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts. Molecular and cellular biology. 2007;27(10):3804–3816. doi: 10.1128/MCB.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54(5):771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. The Journal of cell biology. 1999;144(4):687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyashita Y, Ozawa M. A dileucine motif in its cytoplasmic domain directs beta-catenin-uncoupled E-cadherin to the lysosome. Journal of cell science. 2007;120(Pt 24):4395–4406. doi: 10.1242/jcs.03489. [DOI] [PubMed] [Google Scholar]
- 47.Sharma M, Henderson BR. IQ-domain GTPase-activating protein 1 regulates beta-catenin at membrane ruffles and its role in macropinocytosis of N-cadherin and adenomatous polyposis coli. The Journal of biological chemistry. 2007;282(11):8545–8556. doi: 10.1074/jbc.M610272200. [DOI] [PubMed] [Google Scholar]
- 48.Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. The Journal of biological chemistry. 2005;280(25):24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- 49.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Developmental cell. 2008;14(1):25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. The Journal of cell biology. 2006;174(4):593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF, Nakano K, Takaishi K, Takai Y. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells--regulation by Rho, Rac and Rab small G proteins. Oncogene. 1999;18(48):6776–6784. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- 52.Palacios F, Price L, Schweitzer J, Collard JG, D’Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. The EMBO journal. 2001;20(17):4973–4986. doi: 10.1093/emboj/20.17.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryant DM, Wylie FG, Stow JL. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Molecular biology of the cell. 2005;16(1):14–23. doi: 10.1091/mbc.E04-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer cell. 2002;2(4):301–314. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 55.Janda E, Nevolo M, Lehmann K, Downward J, Beug H, Grieco M. Raf plus TGFbeta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene. 2006;25(54):7117–7130. doi: 10.1038/sj.onc.1209701. [DOI] [PubMed] [Google Scholar]
- 56.Bremm A, Walch A, Fuchs M, Mages J, Duyster J, Keller G, Hermannstadter C, Becker KF, Rauser S, Langer R, von Weyhern CH, Hofler H, Luber B. Enhanced activation of epidermal growth factor receptor caused by tumor-derived E-cadherin mutations. Cancer research. 2008;68(3):707–714. doi: 10.1158/0008-5472.CAN-07-1588. [DOI] [PubMed] [Google Scholar]
- 57.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature cell biology. 2000;2(2):84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 58.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature cell biology. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 59.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nature cell biology. 2002;4(3):222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 60.Yang JY, Zong CS, Xia W, Wei Y, Ali-Seyed M, Li Z, Broglio K, Berry DA, Hung MC. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Molecular and cellular biology. 2006;26(19):7269–7282. doi: 10.1128/MCB.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansouri M, Rose PP, Moses AV, Fruh K. Remodeling of endothelial adherens junctions by Kaposi’s sarcoma-associated herpesvirus. Journal of virology. 2008;82(19):9615–9628. doi: 10.1128/JVI.02633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian LW, Greene W, Ye F, Gao SJ. Kaposi’s sarcoma-associated herpesvirus disrupts adherens junctions and increases endothelial permeability by inducing degradation of VE-cadherin. Journal of virology. 2008;82(23):11902–11912. doi: 10.1128/JVI.01042-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lorch JH, Klessner J, Park JK, Getsios S, Wu YL, Stack MS, Green KJ. Epidermal growth factor receptor inhibition promotes desmosome assembly and strengthens intercellular adhesion in squamous cell carcinoma cells. The Journal of biological chemistry. 2004;279(35):37191–37200. doi: 10.1074/jbc.M405123200. [DOI] [PubMed] [Google Scholar]
- 64.Klessner JL, Desai BV, Amargo EV, Getsios S, Green KJ. EGFR and ADAMs Cooperate to Regulate Shedding and Endocytic Trafficking of the Desmosomal Cadherin Desmoglein 2. Molecular biology of the cell. 2008 doi: 10.1091/mbc.E08-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thiery JP, Chopin D. Epithelial cell plasticity in development and tumor progression. Cancer metastasis reviews. 1999;18(1):31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- 66.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. The Journal of cell biology. 1997;137(6):1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calkins CC, Setzer SV, Jennings JM, Summers S, Tsunoda K, Amagai M, Kowalczyk AP. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. Journal of Biological Chemistry. 2006;281(11):7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- 68.McMillan JR, Shimizu H. Desmosomes: structure and function in normal and diseased epidermis. The Journal of dermatology. 2001;28(6):291–298. doi: 10.1111/j.1346-8138.2001.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 69.Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. Journal of cell science. 2006;119(Pt 5):797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- 70.Phan QT, Fratti RA, Prasadarao NV, Edwards JE, Jr, Filler SG. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. The Journal of biological chemistry. 2005;280(11):10455–10461. doi: 10.1074/jbc.M412592200. [DOI] [PubMed] [Google Scholar]
- 71.Sousa S, Cabanes D, Bougneres L, Lecuit M, Sansonetti P, Tran-Van-Nhieu G, Cossart P. Src, cortactin and Arp2/3 complex are required for E-cadherin-mediated internalization of Listeria into cells. Cellular microbiology. 2007;9(11):2629–2643. doi: 10.1111/j.1462-5822.2007.00984.x. [DOI] [PubMed] [Google Scholar]
- 72.Bonazzi M, Veiga E, Cerda JP, Cossart P. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalisation of Listeria monocytogenes. Cellular microbiology. 2008 doi: 10.1111/j.1462-5822.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- 73.Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC Regulate Arp2/3-Mediated Endocytosis to Control Local Adherens Junction Stability. Curr Biol. 2008;18(21):1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 74.Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp Define a Branch of the Cdc42-Par6-aPKC Pathway Regulating E-Cadherin Endocytosis. Curr Biol. 2008;18(21):1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 75.Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, Kowalczyk AP. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. The Journal of biological chemistry. 2003;278(21):19199–19208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]