Synopsis
This article reviews the current surgical management of patients with secondary and tertiary hyperparathyroidism. The focus is on innovative surgical strategies that have improved the care of these patients over the past 10 to 15 years. Modalities such as intraoperative parathyroid hormone monitoring and radioguided probe utilization are discussed.
Keywords: Secondary hyperparathyroidism, Tertiary hyperparathyroidism, radioguided, parathyroidectomy, parathyroid hormone monitoring
Secondary and tertiary hyperparathyroidism (HPT) usually result from parathyroid gland hyperplasia that produces excess parathyroid hormone (PTH). Collectively, secondary and tertiary HPT comprise a minority of the patients diagnosed with HPT. Due to the relative rarity of these conditions and common underlying disease pathology, they are frequently discussed and researched together. While the disease processes are related, secondary and tertiary HPT are two distinct and separate entities.
Etiology
Secondary HPT occurs most commonly “secondary” to chronic renal failure (CRF). For this reason, secondary HPT is frequently referred to as renal HPT. Estimates report that as many as 90% of patients with CRF develop this disease by the time hemodialysis is initiated.24 Other causes of secondary HPT include osteomalacia, rickets, and malabsorption. The pathophysiology of secondary hyperparathyroidism results from the relationship between CRF and parathyroid hyperplasia. Abnormalities in the renal tubular absorption of phosphate lead to reduced phosphate excretion and hyperphosphatemia. Impaired renal conversion of 25-hydroxycholecalciferal to 1,25-dihydroxycholecalciferol (vitamin D, calcitriol) also causes a decrease in the intestinal absorption of calcium. In combination, elevated serum phosphate levels and reduced vitamin D production result in decreases in serum calcium levels or hypocalcemia. Hyperphoshatemia and low Vitamin D also cause elevated PTH levels. As a consequence of prolonged hypocalcemia, parathyroid chief cell hyperplasia occurs and PTH secretion increases. Skeletal resistance to PTH results in persistently and frequently extremely elevated PTH levels and renal osteopathy.
Tertiary HPT occurs most commonly in the setting of renal transplant where patients with secondary HPT continue to have elevated PTH levels after receiving a renal allograft. This disease is observed in up to 30% of kidney transplant recipients and was first described in the early 1960’s.18 After undergoing transplantation, these patients have persistent or recurrent (secondary) HPT after an initial period of resolution. However, tertiary HPT can develop after any long-standing period of hypocalcemia such as those seen with chronic dialysis or gastrointestinal malabsorption. In these patients, prolonged hypocalcemia also causes parathyroid chief cell hyperplasia and excess PTH. After correction of the primary disorder (CRF) by renal transplant, the hypertrophied parathyroid tissue fails to resolute and continues to over-secrete PTH. Consequently, serum calcium levels are normal or even elevated in these patients because the hyperplastic glands function autonomously despite withdrawal of calcium and calcitriol therapy. Tertiary HPT is classically caused by hyperplasia of all four glands, though some reports indicate that over 20% of patients may have single or double adenomas as the underlying pathology.18,19 Whether these cases represent post-transplant patients in whom sporadic primary HPT (adenomas) has developed or resolution of autonomous function in all but one or two glands is unclear. The etiology of tertiary HPT in this subset of patients may also result from asymmetric hyperplasia.18
Diagnosis
The diagnosis and work-up of patients with secondary and tertiary HPT combines both clinical and laboratory investigation. Many patients with these diseases are asymptomatic and will only have abnormalities detectable by laboratory and radiographic studies. In patients with secondary HPT, labs may reveal hypocalcemia or normocalcemia and hyperphosphatemia. In addition, patients with secondary HPT have extremely elevated intact PTH levels and decreased vitamin D levels. In contrast, patients with tertiary HPT will have normal or elevated serum calcium concentrations in combination with moderately elevated intact PTH levels. Labs in patients with tertiary HPT may also reveal decreased vitamin D (1,25-dihydroxycholecalciferol) and phosphate levels and elevated alkaline phosphatase. Patients with both diseases also can become symptomatic. Untreated secondary HPT leads to progressive bone disease, osteitis fibrosa cystica, and soft-tissue calcifications. Patients with tertiary HPT may experience bone pain or fractures, pruritis, nephrolithiasis, pancreatitis, soft tissue or vascular calcifications, and mental status changes. Plain X-ray films or bone density studies can show changes consistent with osteopenia or osteoporosis. Imaging of the neck is normally unnecessary, since secondary and tertiary HPT primarily result from four gland hyperplasia. However, preoperative imaging may be useful in facilitating surgery, especially in the reoperative setting and if one of the glands is in an ectopic position.
Medical Treatment of Secondary and Tertiary Hyperparathyroidism
Management of patients with secondary HPT is predominantly medical, while treatment of patients with tertiary HPT is surgical. Supplementation of calcium using oral calcitriol and vitamin D is usually sufficient to manage PTH levels in patients with CRF and secondary HPT. To treat patients with secondary HPT who become refractory to replacement of calcium and vitamin D, several alternative therapies have become available over the last 10 to 15 years. These treatment options include calcimimetics, such as cinacalcet, new phosphate binders, and vitamin D analogues that are less likely to result in hypercalcemia.3,23,36 These agents are designed to bridge patients to renal transplant, the optimal treatment for secondary HPT.
In patients with tertiary HPT, medical treatment is not curative and, generally, not indicated. In contrast to patients with secondary HPT, these patients are not routinely given oral calcium and phosphate binders since they are typically normocalcemic or hypercalcemic and concurrently hypophosphatemic. Vitamin D (calcitriol) supplementation can be prescribed, but often only delays surgical intervention since patients typically become refractory to vitamin D replacement. Supplementing phosphate levels with oral agents can lead to nephrocalcinosis and hyperphosphaturia and should be performed sparingly.
Indications for Surgical Management
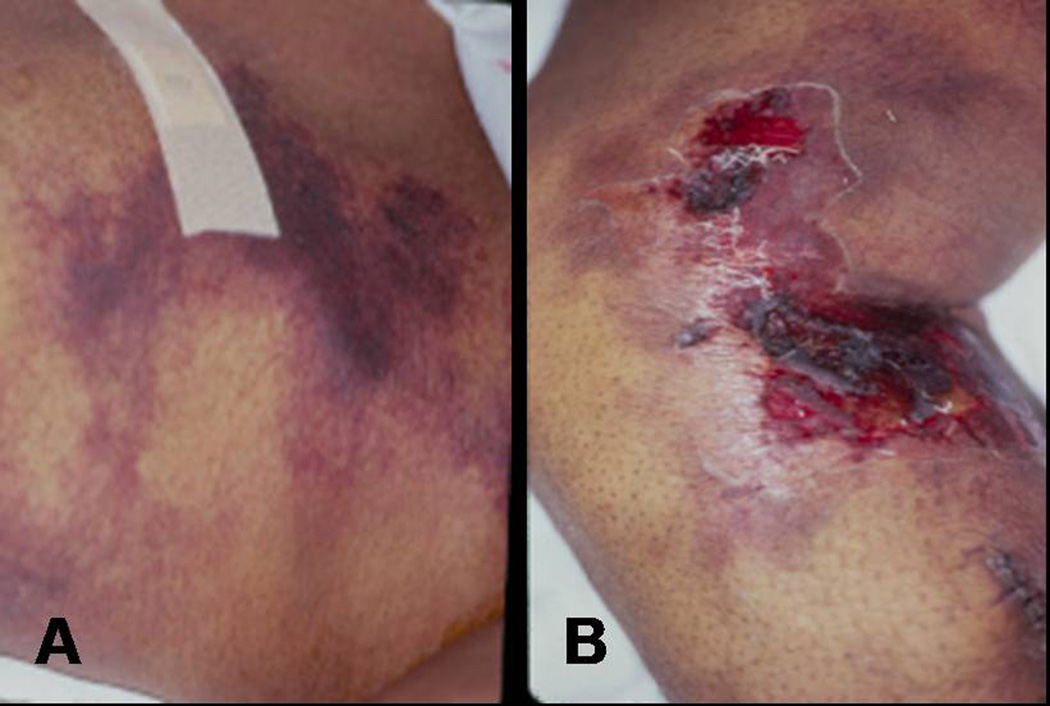
Although novel methods for medically treating patients with secondary HPT have been introduced, surgical intervention is still necessary at times. About 1–2% of patients with secondary HPT require parathyroidectomy each year.37 In the early 1960s, the first operations for patients with renal HPT were performed. The indications for parathyroidectomy in secondary HPT are listed in Table 1. The most life threatening indication for parathyroidectomy in patients with secondary HPT is calciphylaxis which occurs in only 4% of patients undergoing surgery.1 Calciphylaxis results in expanding, painful, cutaneous, purpuritic lesions that cause tissue calcification and ischemic necrosis which lead to dry gangrene if untreated (Fig.1). Overwhelming sepsis and wound breakdown cause significant mortality, as high as 87% in one report.7 In patients with calciphylaxis who are urgently treated with total parathyroidectomy, wound healing is enhanced and median survival is prolonged.11 In addition, surgery has been shown to help avoid amputation, decrease pain, and reduce the use of narcotics.25
Table 1.
Indications for Parathyroidectomy in Patients with Secondary HPT
| Calciphylaxis |
| Patient preference |
| Medical observation not possible |
| Failure of maximal medical management with: |
| Hypercalcemia |
| Hypercalcuria |
| PTH >800 pg/mL |
| Hyperphosphatemia (with calcium×phosphorus > 70) |
| Osteoporosis |
| Symptoms: pruritis, pathologic bone fracture, ectopic soft tissue calcifications, severe vascular calcifications, or bone pain |
Figure 1.
Photographs of calciphylaxis showing cutaneous purpuritic lesions and ischemic necrosis on the torso (A) and medial leg (B) of a patient with secondary HPT.
Unlike patients with secondary HPT, the mainstay of treatment for tertiary HPT is surgery. The development of tertiary HPT requiring surgical intervention occurs in 1–5% of patients with HPT after undergoing kidney transplant.14,19,37 Indications for parathyroidectomy in patients with tertiary HPT are listed in Table 2. Additional minor indications that have been proposed include renal phosphorus wasting (including hypophosphatemia) and parathyroid gland weight >500 mg on ultrasound evaluation.37 Unfortunately, evidence-based guidelines on the selection criteria for parathyroidectomy in patients with tertiary HPT are inconsistent.
Table 2.
Indications for Parathyroidectomy in Patients with Tertiary HPT
| Severe hypercalcemia (serum calcium > 11.5 or 12 mg/dL) |
| Persistent hypercalcemia (serum calcium > 10.2 mg/dL more than three months to one year after surgery) |
| Severe Osteopenia (low bone mineral density) |
| Symptomatic hyperparathyroidism |
| Fatigue |
| Pruritis |
| Bone pain or pathologic bone fracture |
| Peptic ulcer disease |
| Mental status changes |
| History of renal calculi |
Pre-operative Localization Studies
After the decision to proceed with surgery has been made, pre-operative localization studies are not always routinely performed in patients with secondary or tertiary HPT for many reasons. However, it is very useful in terms of identifying otherwise unsuspected ectopic glands and facilitating the operation in reoperative cases. First, the diseases result in hyperplasia of all four parathyroid glands. The sensitivity of imaging modalities such as 99mTc-sestimibi scintigraphy and ultrasound is poor in patients with multiple gland disease, regardless of the etiology of HPT.12,30,33 In a study that examined patients with both secondary and tertiary HPT, 99mTc-sestamibi scintigraphy failed to identify all hyperfunctioning parathyroids in every case.25 In another investigation, the reported sensitivities of preoperative localization with 99mTc-sestimibi and ultrasound in patients operated on for an initial diagnosis of tertiary HPT were 9% and 67%, respectively.17 Other techniques such as CT and MRI have been described, but in very few cases.17
In patients with primary HPT and single adenomas, localizing studies are advantageous since they allow identification of parathyroid glands in ectopic locations. In patients with secondary and tertiary HPT, the ability of imaging techniques to identify ectopic parathyroids is limited. Ectopic mediastinal glands were identified in only 38% of patients with secondary and tertiary HPT who were discovered to harbor mediastinal parathyroids at surgery.25 In an analysis of patients with only tertiary HPT, localization of ectopic glands with 99mTc-sestimibi scintigraphy, ultrasound, or MRI was not demonstrated in any case despite 32% of the study subjects having ectopic parathyroids at the time of surgery.17 Another rationale for not performing preoperative imaging is that the surgical approach is unlikely to change; bilateral neck exploration is the standard of care for all patients with secondary and tertiary HPT. Even in series that have investigated single and double adenomas as the underlying etiology of tertiary HPT, thorough exploration of the neck is still the recommended procedure.14,17,19,41 As a result, routine pre-operative imaging is not recommended before a primary resection for secondary or tertiary HPT. The use of imaging in cases of recurrent or persistent HPT prior to a re-exploration is strongly encouraged and is discussed later in this review.
Operative Approaches
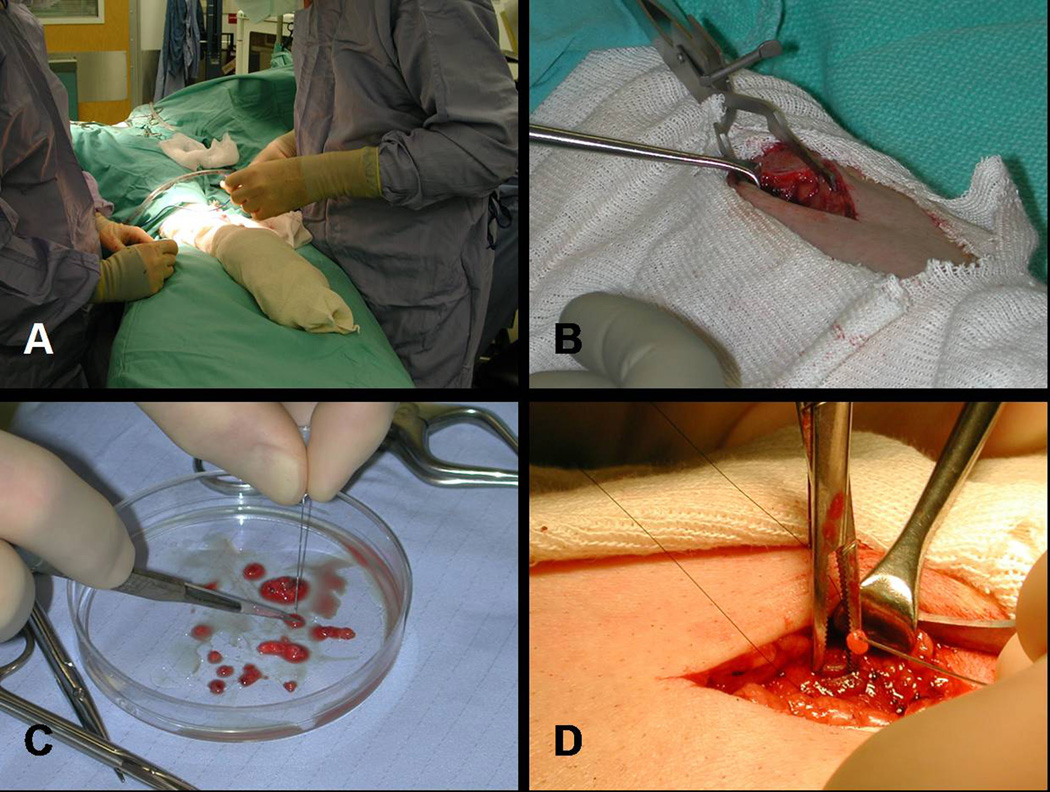
The surgical approaches described for patients with secondary and tertiary HPT are similar. For patients with secondary HPT who meet the indications for operative management, three different surgical procedures utilizing a bilateral neck exploration have been described. These surgeries include subtotal parathyroidectomy (removal of 3 and 1/ 2 glands leaving a remnant in situ), total parathyroidectomy (four gland resection) with autotransplantation, and total parathyroidectomy without autotransplantation. Surgical management of tertiary HPT can be performed using either one of two accepted approaches: subtotal parathyroidectomy or total parathyroidectomy with autotransplantation. In all cases, cryopreservation of resected tissue is important to address possible complications of post-operative hypoparathyroidism where the autograft or remnant fails to function. The surgical procedure performed is often due to surgeon preference. At our institution, we favor total parathyroidectomy with autotransplantation and cryopreservation of the remaining parathyroid tissue for treatment of patients with secondary HPT (Fig. 2). When performing this procedure, marking the re-implanted tissue in the forearm with non-absorbable silk suture and a hemoclip is important. If persistent or recurrent HPT occurs after total parathyroidectomy with autotransplantation, reoperation in these patients can be performed under local anesthesia only, unless the persistence or recurrence is due to missed parathyroid glands at the initial operation. In patients with tertiary HPT, we prefer subtotal parathyroidectomy that leaves the native blood supply to half of a parathyroid intact and, theoretically, is associated with a reduced risk of hypocalcemia. Advocates of this procedure recommend biopsying and marking (with a clip or suture) the most normal appearing gland first. If the tissue remains viable, the remaining 3 glands can be excised, and the biopsied parathyroid is left in situ. In the event that reoperation in the neck is necessary after subtotal parathyroidectomy, marking the parathyroid remnant is very important to facilitate dissection.
Figure 2.
Photographs of total parathyroidectomy and implantation of parathyroid tissue into the non-dominant forearm. Prior to surgery the non-dominant forearm should be prepped (A). After a small incision is made (B) and 50 to 100 mg of parathyroid tissue is dissected from a gland (C), the parathyroid tissue is transplanted into the brachioradialis muscle (D).
For patients with tertiary HPT, simultaneous thymectomy can be performed with either subtotal or total parathyroidectomy. Some surgeons routinely resect the thymus bilaterally to reduce the risk of recurrence, since ectopic glands are frequently found within the thymic tissue. However, our approach is to only resect the thymus if an inferior gland cannot be found or when the radioguided probe suggests increased activity within an area of the thymus. Data on thymectomy in these patients are lacking.
In addition to these traditional surgical approaches, limited or less-than-subtotal parathyroid resection in select patients with only one or two diseased gland (s) has been described in patients with tertiary HPT, but remains controversial.13,17–19,26,27,39 Traditionally caused by hyperplasia of all four glands, tertiary HPT also can result from a single or double adenoma, asymmetric hyperplasia, or incomplete resolution of all hyperplastic glands in up to 30% of patients.18,19 Of note, bilateral neck exploration should still be performed in all of these cases with resection of only the diseased glands. IoPTH measurement can aid in identifying these cases with the goal being more than a 50% drop at 10 minutes.13 Studies have shown equivalent (over a 95%) success rates using a limited approach compared to subtotal or total parathyroidectomy based on normocalcemia and symptom improvement post-operatively.19,27 However, Triponez and colleagues reported a 5.2 times greater risk of persistent or recurrent HPT after limited resection for tertiary HPT.39 The difference between the results of these reports may result from the indications for performing a less-than-subtotal resection. If only one or two glands were resected in a patient with tertiary HPT because other glands could not be located, and the patient has four hyperplastic parathyroids, the rate of persistent or recurrent disease should be 100% because an inadequate resection has been performed.
Outcomes after Parathyroidectomy
Surgeon preference is often the main driving force behind the type of operation performed in patients with secondary and tertiary HPT. Overall, the success rates after any form of parathyroidectomy discussed are high. The differences between procedures are related more to risks of recurrence and complications. To date, several retrospective analyses and one randomized trial have attempted to identify the superiority of one surgical approach over another in patients with secondary HPT. However, direct comparisons between surgical approaches have not been made in patients with tertiary HPT.
In a study that randomized 40 patients with secondary HPT to either subtotal parathyroidectomy or total parathyroidectomy with autografting, the cure rate was higher and the recurrence rate lower after total parathyroidectomy.31 Patients undergoing total parathyroidectomy with implantation had significantly better calcium level normalization, improved bone mineral density, and less pruritis and muscle weakness.31 Because re-operation is simpler in the forearm, these authors favor total parathyroidectomy with autotransplantation over subtotal surgery in patients with secondary HPT. Despite these findings, a retrospective review of the same two procedures performed for secondary HPT found no difference in the rates of failure or recurrence.10 The results of this review do support the findings that patients treated with total parathyroidectomy and autotransplantation have significantly higher calcium levels and bone remineralization after surgery.10 In these two studies, 100% of patients with secondary HPT who underwent total parathyroidectomy and autotransplantation were considered cured compared to 90% of patients after subtotal parathyroidectomy.10,31 A variation of subtotal surgery described as “near-total” parathyroidectomy, where the parathyroid remnant is made more precisely, has a cure rate of 96%.25
While subtotal parathyroidectomy or total parathyroidectomy with autotransplantation have been the standard procedures of choice for patients with secondary HPT, more recently total parathyroidectomy without autotransplantation has been proposed. In a retrospective analysis of total parathyroidectomy with and without autotransplantation, one group showed a significantly higher rate of recurrence in patients with autografts in the forearm tissue vs. those without autografts (45% vs. 0%).28 Lorenz et al. also revealed a recurrence rate of 0% after total parathyroidectomy without autotransplantation.21 Therefore, these studies favor total parathyroidectomy without autotransplantation since omission of the forearm autograft appears to prevent recurrence.
In patients with tertiary HPT, success rates after surgery also are high. Furthermore, similar outcomes are observed after subtotal parathyroidectomy when compared to total parathyroidectomy with autotransplantation. In several reviews, many with long-term follow-up, over 94% of patients with tertiary HPT achieved biochemical cure on the basis of total serum calcium levels after subtotal parathyroidectomy.17,19,25,30 Although, one study reported that only 71% of patients also had normalized PTH levels.17 In addition, symptoms are improved in over 90% of patients with tertiary HPT treated by subtotal parathyroidectomy.19,30
Persistent or Recurrent Hyperparathyroidism
After operation for either secondary or tertiary HPT, management of persistent or recurrent disease is challenging to the surgeon and radiologist alike. Persistent secondary and tertiary HPT generally results from an inadequate initial resection and/or a missed 5th or ectopic gland and requires reoperation. In patients with secondary HPT, the risk of developing recurrent HPT increases over time and can occur after any of the surgical procedures. Subtotal and total parathyroidectomy with autotransplantation have higher rates of recurrence in patients with secondary HPT (reported range 5–80%) compared to total parathyroidectomy without autotransplantation (reported range 0–4%) due to hyperplasia of the parathyroid remnant.28,35,41 However, in patients with tertiary HPT, recurrence rates are equivalent among the surgical approaches discussed. Rates of recurrence after operation for tertiary HPT range from 0% to 8% and are increased in patients with a nodular variety of hyperplasia, an elevated proliferative index, or redevelopment of CRF.17,19,27,30
In patients with either persistent or recurrent HPT due to residual functioning parathyroid tissue, reoperation is facilitated by pre-operative localization. 99mTc-sestamibi scintigraphy of the neck, mediastinum, or forearm is advantageous in these cases because only a single hyperfunctioning focus remains. Chou and others demonstrated that 99mTc-sestamibi scanning successfully identified the parathyroid remnant in 85% of patients with persistent or recurrent secondary HPT.5 The locations of these remaining glands were in the normal anatomical position, carotid sheath, and mediastinum indicating the usefulness of this test in identifying ectopic parathyroids.5 Patients with persistent or recurrent tertiary HPT benefit from preoperative localization studies as well. The true positive rates of localization for 99mTc-sestimibi scans, ultrasound, and MRI were 100% for each of these modalities in this population.17 If imaging of the neck and forearm fails to identify the location of the active parathyroid, differential PTH sampling of the arms can be performed, with a gradient of 20:1 suggesting forearm graft hyperplasia. Rarely, transplanted tissue is placed into the sternocleidomastoid muscles.
Surgical treatment of persistent or recurrent secondary or tertiary HPT in patients who have undergone total parathyroidectomy with autotransplantation can be performed under local anesthesia and avoids the risks associated with re-exploration of the neck, such as recurrent laryngeal nerve injury. Success rates for reoperation in secondary HPT patients with persistent disease range from 89 to 100%, whereas patients with recurrent secondary HPT have success 70 to 100% of the time.5,32 One study found that the success rate of reoperation for recurrent secondary HPT was significantly better in patients undergoing initial subtotal resection compared to total resection with autotransplantation (87% vs. 70%, P=.02).32 This finding is counterintuitive since re-operation on the forearm is theoretically easier compared to the neck. However, the recurrence was located in the neck or mediastinum in over 50% of the patients initially treated with total parathyroidectomy and forearm grafting.32
Intraoperative PTH Monitoring
Surgical adjuncts that have revolutionized the treatment of patients with primary HPT have been applied to patients with secondary and tertiary HPT as well. In particular, intraoperative PTH (ioPTH) measurement is now being used in patients with both secondary and tertiary HPT. However, unlike patients with primary disease, surgery for either disease still requires bilateral exploration to remove all 4 parathyroid glands. IoPTH monitoring is complicated in this patient population because of impaired renal function and delayed renal clearance of PTH. Conflicting results regarding the usefulness of ioPTH monitoring for secondary HPT have been published.2,6,15,16 Studies have shown that ioPTH testing depends on renal function and PTH assay specificity.2,6,16 However, studies differ in their definition of successful parathyroidectomy. When a 50% drop from the baseline ioPTH is considered predictive of sufficient parathyroid tissue resection, these investigations support the utility of ioPTH monitoring in patients with secondary HPT. Depending upon the specificity of the assay, an adequate drop in ioPTH levels (>50%) may not be seen for up to 30 minutes after curative resection because of CRF and delayed PTH clearance.2,6,16
For both initial and reoperative treatment of patients with tertiary HPT, ioPTH testing also has been shown to be beneficial.13,15,25,38,40 However, the ioPTH criteria that are predictive of cure in these patients are debated. Applying the traditional Miami criteria proposed for patients with primary HPT (>50% ioPTH drop at 10 mins) affords a 94% or greater sensitivity in patients with tertiary HPT.13,38 In patients with primary HPT from hyperplasia, a final ioPTH level of less than 35 pg/mL or a greater than 90% drop from baseline is predictive of cure about 90% of the time, suggesting that a cutoff ioPTH value should be utilized in patients with multigland disease from any etiology.40 In patients with tertiary HPT, Milas and Weber have proposed that a target ioPTH level less than 200 pg/mL predicts curative resections, while others support an ioPTH level less than 65 pg/ml.15,25,40 Lack of consensus guidelines for ioPTH monitoring in patients with tertiary HPT and skepticism over the potential impact on the overall success rate of parathyroidectomy has led some groups to abandon the routine use of this adjunct.38 However, one investigation did report that ioPTH impacted operative management in 16% of cases.13
Radioguided Parathyroidectomy
Another state of the art advance that some believe facilitates intraoperative management of patients with secondary and tertiary HPT is application of radioguided techniques. Patients are injected with 10 mCi of 99mTc-sestamibi approximately one to two hours prior to surgery. A gamma probe is then utilized in the same manner as in primary HPT except that all four glands are explored.4 Therefore, this technique should be applicable to patients with secondary or tertiary HPT.
We have previously shown that radioguidance reduces operative time, length of stay, and the need for frozen section in patients with secondary and tertiary HPT.26 Other reports also support the use of radioguided parathyroidectomy in this patient population.20,22,29 The ability of the gamma probe to locate supernumerary and ectopic parathyroid glands decreases the risk of persistent or recurrent disease and is an advantage associated with using this technology in patients with secondary and tertiary HPT.26 The radioguided probe also is advantageous in patients with recurrent HPT from forearm graft hyperplasia, especially if the parathyroid fragment is unmarked.34 Another benefit of the gamma probe includes the fact that it can confirm the presence of parathyroid tissue and omit the need for frozen section or ioPTH of tissue aspirate.14,26 Thus, radioguided surgery can be a useful adjunct in patient with secondary and tertiary HPT.
Cryopreservation
In any patient undergoing subtotal or total parathyroidectomy, regardless of HPT etiology, cryopreservation of parathyroid tissue should be obligatory. The success rates of cryopreserved autografts are variable throughout the literature, but are always lower than immediate, fresh autografts. In a study of long-term functionality of cryopreserved tissue, about 60% of autografts are functional to some degree; however, less than 50% of patients had fully functioning tissue.9 These data also suggested that the duration of cryopreservation predicted graft failure, and no functioning autografts occurred after 2 years of cryopreservation.9
Complications
When performing any of these operations, the risks of post-operative hypocalcemia must be considered. Accelerated bone remineralization (“hungry bone syndrome”) and delayed forearm graft or cervical remnant function increase the risk of transient hypocalcemia. Thus, supplementation with calcium and vitamin D immediately after surgery helps to prevent hypocalcemia during the post-operative period. Oral calcium carbonate (2–3 g) is recommended with or without calcitriol initiated immediately following parathyroidectomy. Patients with secondary HPT are typically already on calcium and calcitriol and are continued postoperatively.
Because the procedures performed for both secondary and tertiary HPT are the same, complications after subtotal or total parathyroidectomy are common to both diseases. The most frequently reported minor complication after subtotal or total parathyroidectomy is transient hypocalcemia which varies widely, but generally is observed in 15 to 30% of patients.17,19,27 Severe or permanent hypocalcemia requiring either prolonged admission or readmission for intravenous calcium or life-long calcium and vitamin D supplementation also is seen, but much less frequently (0% to 7%).17,19,25,27 In addition, hypoparathyroidism requiring reimplantation of cryopreserved tissue has been reported in up to 7% of patients.11, 30,16, 18 Recurrent laryngeal nerve injuries are frequently discussed, but typically are transient neuropraxias, and permanent injuries occur in only about 1% of cases. 17,19,27,37 Other complications such as wound infection, hematomas, and dehiscence, and death have been described, but are rarely seen. Cardiac dysrhythmias, gout or pseudogout, pancreatitis, and renal failure may develop secondary to hypocalcemia or hypoparathyroidism, though these complications are exceedingly rare.
Conclusion
Despite advances in medical and surgical treatment, the incidence of secondary and tertiary HPT is on the rise in the United States and elsewhere because of the increasing incidence and prevalence of CRF.8 From 1990 to 2001, according to the United States Renal Data System (USRDS), the prevalence of CRF rose over 100%. Because of earlier diagnosis of secondary HPT and new medical treatment options, the incidence of parathyroidectomy in this population has been constant or decreasing throughout the world even though the incidence of secondary HPT has increased.8,22 Nonetheless, cutting edge innovations including ioPTH testing using rapid PTH assays and radioguided gamma probe utilization have kept the surgical treatment of secondary HPT current with the times.
In the face of medical and surgical advances, ongoing controversy remains over the optimal surgical management of patients with secondary HPT largely due to the relatively small number of patients to study. A multi-institutional randomized controlled trial of all three procedures would be ideal, but challenging to organize and conduct. Currently, a trial comparing total parathyroidectomy without autotransplantation or thymectomy to total parathyroidectomy with autotransplantation and thymectomy in patients with secondary HPT is underway.32
Surgical management of secondary and tertiary HPT is safe and effective at correcting bone mineralization and metabolic disturbances. Improved neuropsychiatric symptoms, survival, and quality of life with reduced cardiovascular events also are benefits of parathyroidectomy. The most commonly accepted approaches in these patients are subtotal parathyroidectomy or total parathyroidectomy with autotransplantation of parathyroid tissue into the non-dominant forearm. Techniques such as intraoperative PTH monitoring and radioguided surgery have advanced these surgeries by eliminating the need for frozen section and decreasing operative times. While surgery remains the only cure for patients with tertiary HPT, the treatment of secondary is predominantly medical employing newer calcimimetics, phosphate binders, and vitamin D analogues. Future advances in the surgical management of these patients will likely require the creation of consensus guidelines and multi-institutional collaborative studies.
Contributor Information
Susan C. Pitt, Department of Surgery, University of Wisconsin, 600 Highland Ave., CSC K4/623, Madison, WI 53792-3284, 608-265-3749, 608-263-7652 (fax), pitt@surgery.wisc.edu.
Rebecca S. Sippel, Department of Surgery, University of Wisconsin, 600 Highland Ave., CSC H4/755, Madison, WI 53792-3284, 608-263-1387, 608-263-7652 (fax), sippel@surgery.wisc.edu.
Herbert Chen, Department of Surgery, University of Wisconsin, 600 Highland Ave., CSC H4/722, Madison, WI 53792-3284, 608-263-1387, 608-263-7652 (fax), chen@surgery.wisc.edu.
References
- 1.Angelis M, Wong L, Myers S, et al. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122:1083. doi: 10.1016/s0039-6060(97)90212-9. [DOI] [PubMed] [Google Scholar]
- 2.Bieglmayer C, Kaczirek K, Prager G, et al. Parathyroid hormone monitoring during total parathyroidectomy for renal hyperparathyroidism: pilot study of the impact of renal function and assay specificity. Clin Chem. 2006;52:1112. doi: 10.1373/clinchem.2005.065490. [DOI] [PubMed] [Google Scholar]
- 3.Block G, Martin K, de Francisco A, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Mack E, Starling J. Radioguided parathyroidectomy is equally effective for both adenomatous and hyperplastic glands. Ann Surg. 2003;238:332. doi: 10.1097/01.sla.0000086546.68794.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou F, Lee C, Chen H, et al. Persistent and recurrent hyperparathyroidism after total parathyroidectomy with autotransplantation. Ann Surg. 2002;235:99. doi: 10.1097/00000658-200201000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou F, Lee C, Chen J, et al. Intraoperative parathyroid hormone measurement in patients with secondary hyperparathyroidism. Arch Surg. 2002;137:341. doi: 10.1001/archsurg.137.3.341. [DOI] [PubMed] [Google Scholar]
- 7.Coates T, Kirkland G, Dymock R, et al. Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis. 1998;32:384. doi: 10.1053/ajkd.1998.v32.pm9740153. [DOI] [PubMed] [Google Scholar]
- 8.Cohen E, Moulder J. Parathyroidectomy in chronic renal failure: has medical care reduced the need for surgery? Nephron. 2001;89:271. doi: 10.1159/000046084. [DOI] [PubMed] [Google Scholar]
- 9.Cohen M, Dilley W, Wells SJ, et al. Long-term functionality of cryopreserved parathyroid autografts: a 13-year prospective analysis. Surgery. 2005;138:1033. doi: 10.1016/j.surg.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Gagné E, Ureña P, Leite-Silva S, et al. Short- and long-term efficacy of total parathyroidectomy with immediate autografting compared with subtotal parathyroidectomy in hemodialysis patients. J Am Soc Nephrol. 1992;3:1008. doi: 10.1681/ASN.V341008. [DOI] [PubMed] [Google Scholar]
- 11.Girotto J, Harmon J, Ratner L, et al. Parathyroidectomy promotes wound healing and prolongs survival in patients with calciphylaxis from secondary hyperparathyroidism. Surgery. 2001;130:645. doi: 10.1067/msy.2001.117101. [DOI] [PubMed] [Google Scholar]
- 12.Haciyanli M, Lal G, Morita E, et al. Accuracy of preoperative localization studies and intraoperative parathyroid hormone assay in patients with primary hyperparathyroidism and double adenoma. J Am Coll Surg. 2003;197:739. doi: 10.1016/S1072-7515(03)00676-8. [DOI] [PubMed] [Google Scholar]
- 13.Haustein S, Mack E, Starling J, et al. The role of intraoperative parathyroid hormone testing in patients with tertiary hyperparathyroidism after renal transplantation. Surgery. 2005;138:1066. doi: 10.1016/j.surg.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Jorna F, Jager P, Lemstra C, et al. Utility of an intraoperative gamma probe in the surgical management of secondary or tertiary hyperparathyroidism. Am J Surg. 2008;196:13. doi: 10.1016/j.amjsurg.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 15.Kaczirek K, Prager G, Riss P, et al. Novel parathyroid hormone (1–84) assay as basis for parathyroid hormone monitoring in renal hyperparathyroidism. Arch Surg. 2006;141:129. doi: 10.1001/archsurg.141.2.129. [DOI] [PubMed] [Google Scholar]
- 16.Kaczirek K, Riss P, Wunderer G, et al. Quick PTH assay cannot predict incomplete parathyroidectomy in patients with renal hyperparathyroidism. Surgery. 2005;137:431. doi: 10.1016/j.surg.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Kebebew E, Duh Q, Clark O. Tertiary hyperparathyroidism: histologic patterns of disease and results of parathyroidectomy. Arch Surg. 2004;139:974. doi: 10.1001/archsurg.139.9.974. [DOI] [PubMed] [Google Scholar]
- 18.Kerby J, Rue L, Blair H, et al. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg. 1998;227:878. doi: 10.1097/00000658-199806000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilgo M, Pirsch J, Warner T, et al. Tertiary hyperparathyroidism after renal transplantation: surgical strategy. Surgery. 1998;124:677. doi: 10.1067/msy.1998.91483. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa W, Shimizu K, Akasu H, et al. Radioguided parathyroidectomy for renal hyperparathyroidism. Med Sci Monit. 2003;9:CS9. [PubMed] [Google Scholar]
- 21.Lorenz K, Ukkat J, Sekulla C, et al. Total parathyroidectomy without autotransplantation for renal hyperparathyroidism: experience with a qPTH-controlled protocol. World J Surg. 2006;30:743. doi: 10.1007/s00268-005-0379-0. [DOI] [PubMed] [Google Scholar]
- 22.Malberti F, Marcelli D, Conte F, et al. Parathyroidectomy in patients on renal replacement therapy: an epidemiologic study. J Am Soc Nephrol. 2001;12:1242. doi: 10.1681/ASN.V1261242. [DOI] [PubMed] [Google Scholar]
- 23.Martin K, González E, Gellens M, et al. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9:1427. doi: 10.1681/ASN.V981427. [DOI] [PubMed] [Google Scholar]
- 24.Memmos D, Williams G, Eastwood J, et al. The role of parathyroidectomy in the management of hyperparathyroidism in patients on maintenance haemodialysis and after renal transplantation. Nephron. 1982;30:143. doi: 10.1159/000182451. [DOI] [PubMed] [Google Scholar]
- 25.Milas M, Weber C. Near-total parathyroidectomy is beneficial for patients with secondary and tertiary hyperparathyroidism. Surgery. 2004;136:1252. doi: 10.1016/j.surg.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 26.Nichol P, Mack E, Bianco J, et al. Radioguided parathyroidectomy in patients with secondary and tertiary hyperparathyroidism. Surgery. 2003;134:713. doi: 10.1016/s0039-6060(03)00335-0. [DOI] [PubMed] [Google Scholar]
- 27.Nichol P, Starling J, Mack E, et al. Long-term follow-up of patients with tertiary hyperparathyroidism treated by resection of a single or double adenoma. Ann Surg. 2002;235:673. doi: 10.1097/00000658-200205000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ockert S, Willeke F, Richter A, et al. Total parathyroidectomy without autotransplantation as a standard procedure in the treatment of secondary hyperparathyroidism. Langenbecks Arch Surg. 2002;387:204. doi: 10.1007/s00423-002-0307-9. [DOI] [PubMed] [Google Scholar]
- 29.Oyama Y, Kazama J, Maruyama H, et al. Combined radioguided parathyroidectomy and intravenous vitamin D therapy for the treatment of uraemic hyperparathyroidism. Nephrol Dial Transplant. 2003;18 Suppl 3:iii76. doi: 10.1093/ndt/gfg1019. [DOI] [PubMed] [Google Scholar]
- 30.Punch J, Thompson N, Merion R. Subtotal parathyroidectomy in dialysis-dependent and post-renal transplant patients. A 25-year single-center experience. Arch Surg. 1995;130:538. doi: 10.1001/archsurg.1995.01430050088015. [DOI] [PubMed] [Google Scholar]
- 31.Rothmund M, Wagner P, Schark C. Subtotal parathyroidectomy versus total parathyroidectomy and autotransplantation in secondary hyperparathyroidism: a randomized trial. World J Surg. 15:745. doi: 10.1007/BF01665309. [DOI] [PubMed] [Google Scholar]
- 32.Schlosser K, Veit J, Witte S, et al. Comparison of total parathyroidectomy without autotransplantation and without thymectomy versus total parathyroidectomy with autotransplantation and with thymectomy for secondary hyperparathyroidism: TOPAR PILOT-Trial. Trials. 2007;8:22. doi: 10.1186/1745-6215-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sebag F, Hubbard J, Maweja S, et al. Negative preoperative localization studies are highly predictive of multiglandular disease in sporadic primary hyperparathyroidism. Surgery. 2003;134:1038. doi: 10.1016/j.surg.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Sippel R, Bianco J, Chen H. Radioguided parathyroidectomy for recurrent hyperparathyroidism caused by forearm graft hyperplasia. J Bone Miner Res. 2003;18:939. doi: 10.1359/jbmr.2003.18.5.939. [DOI] [PubMed] [Google Scholar]
- 35.Skinner K, Zuckerbraun L. Recurrent secondary hyperparathyroidism. An argument for total parathyroidectomy. Arch Surg. 1996;131:724. doi: 10.1001/archsurg.1996.01430190046012. [DOI] [PubMed] [Google Scholar]
- 36.Slatopolsky E, Burke S, Dillon M. RenaGel, a nonabsorbed calcium- and aluminum-free phosphate binder, lowers serum phosphorus and parathyroid hormone. The RenaGel Study Group. Kidney Int. 1999;55:299. doi: 10.1046/j.1523-1755.1999.00240.x. [DOI] [PubMed] [Google Scholar]
- 37.Triponez F, Clark O, Vanrenthergem Y, et al. Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg. 2008;248:18. doi: 10.1097/SLA.0b013e3181728a2d. [DOI] [PubMed] [Google Scholar]
- 38.Triponez F, Dosseh D, Hazzan M, et al. Accuracy of intra-operative PTH measurement during subtotal parathyroidectomy for tertiary hyperparathyroidism after renal transplantation. Langenbecks Arch Surg. 2006;391:561. doi: 10.1007/s00423-006-0070-4. [DOI] [PubMed] [Google Scholar]
- 36.Triponez F, Kebebew E, Dosseh D, et al. Less-than-subtotal parathyroidectomy increases the risk of persistent/recurrent hyperparathyroidism after parathyroidectomy in tertiary hyperparathyroidism after renal transplantation. Surgery. 2006;140:990. doi: 10.1016/j.surg.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 40.Weber K, Misra S, Lee J, et al. Intraoperative PTH monitoring in parathyroid hyperplasia requires stricter criteria for success. Surgery. 2004;136:1154. doi: 10.1016/j.surg.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 41.Zou Q, Wang H, Zhou J, et al. Total parathyroidectomy combined with partial autotransplantation for the treatment of secondary hyperparathyroidism. Chin Med J (Engl) 2007;120:1777. [PubMed] [Google Scholar]