Abstract
The functional neuroanatomy of tasks that recruit different forms of response selection and inhibition has to our knowledge, never been directly addressed in a single fMRI study using similar stimulus-response paradigms where differences between scanning time and sequence, stimuli, and experimenter instructions were minimized. Twelve right-handed participants were scanned on two standard cognitive control tasks, a stimulus-response incompatibility task, and a response inhibition task. A compound trial design allowed comparison of preparing to inhibit an upcoming automatic response to wholly inhibiting an automatic response. Furthermore, inhibiting an automatic response to perform an alternative task-relevant response was compared to wholly inhibiting an automatic response. No differences were found in prefrontal activity when preparing to inhibit an automatic response was compared to wholly inhibiting an automatic response, suggesting a mostly common network. The left inferior frontal gyrus was found to be commonly recruited during both tasks when controlled responses were required, likely due to its role in response selection. In contrast, the right inferior frontal gyrus was found to be more involved when task demands were stronger for response inhibition. Our results are largely consistent with models of cognitive control that postulate that separate psychological constructs, such as response selection and inhibition, are related processes largely served by a common prefrontal network. This prefrontal network is recruited to a greater or lesser extent depending on specific task demands.
Keywords: functional magnetic resonance imaging, executive functioning, prefrontal cortex, inferior frontal gyrus, go/nogo, response incompatibility, cognitive control
Introduction
Localizing distinct cognitive processes to their underlying neural correlates is a fundamental goal of cognitive neuroscience. However, an early review of studies by Duncan and Owen (2000) demonstrated the challenge of such of an endeavor. That review demonstrated a broad range of tasks with various demands on attention, working memory, response selection, inhibition, and other forms of executive control tended to generally activate a network composed of the bilateral dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, and anterior cingulate cortex. There are a number of potential explanations for these findings. One explanation is that the psychological constructs themselves may involve the same underlying mechanisms and hence similar brain regions underlie these various processes. One candidate construct is “cognitive control,” which incorporates aspects of all these processes (Cohen, Dunbar, & McClelland, 1990). This theory suggests that attention, working memory, response selection, and inhibition are different manifestations of the same domain-general mechanism of goal representation and maintenance for the purpose of providing top-down support (Miller & Cohen, 2001).
In contrast, one potential methodological explanation for the findings is that many of the results that show co-activation of this network come from experiments that are unable to distinguish between the cognitive processes required at different points in each trial (e.g., preparatory-, maintenance-, or response-related activity). The apparent activity is then a result of collapsing several discrete cognitive processes. The distinction between a mechanistic and a methodological explanation for this common activation pattern across tasks has important implications. Studies that have distinguished between preparatory- and response-related activity have advanced our understanding of the components of executive control. For example, different forms of control may be present at different parts of a trial, with proactive control occurring earlier and reactive control occurring later in a trial, thereby manifesting different cortical recruitment (Braver, Gray, & Burgess, 2007; MacDonald, Cohen, Stenger, & Carter, 2000). Similarly, Schon and colleagues (2009) investigated three different facets of working memory, encoding, maintenance, and retrieval, and demonstrated that bilateral dorsolateral prefrontal activity was present during maintenance, but not during encoding or retrieval. Such observations have led some researchers to hypothesize that different aspects of a trial are associated with different cognitive processes and neural correlates.
A third explanation is that functional specialization is matter of degree as opposed to rather being present or absent. It is likely that all these explanations played a role in the functional imaging findings reviewed by Duncan and Owen (2000). The goal of the current study was to investigate two forms of response selection and inhibition at different points in a trial, while to the extent possible equate the cognitive paradigms utilized for methodological and superficial differences.
Cognitive control is the ability to engage in goal-oriented behaviors, allowing the brain to solve difficult, novel, or complex tasks, such as correcting errors or overcoming automatic responses in favor of weaker, task-relevant responses (Miller & Cohen, 2001). Response selection is a form of cognitive control that involves selecting an appropriate goal-related response. Schumacher and colleagues (2003) investigated the common and unique neural correlates of response selection processes during spatial and non-spatial tasks. The authors found different networks for the two tasks. Spatial response selection was mediated by a right dorsal prefrontal, bilateral premotor, and superior parietal network, whereas the non-spatial response selection task was mediated by a left dorsal prefrontal, ventral parietal, and temporal brain network. In contrast, Jiang and Kanwisher (2003) found fronto-parietal activations in both spatial and non-spatial task modalities. Differences between the findings could have been due to differential power linked to different sample sizes, the specific parametric manipulations, or the specific types of stimuli used.
Another critical component of cognitive control is response inhibition, which involves overcoming an inappropriate response. Wager and colleagues (2005) compared three forms of response inhibition: stimulus-response incompatibility, which involved inhibiting an automatic response to select a task-relevant response; a Go/NoGo paradigm, which involved wholly inhibiting a response (i.e., omitting a response); and a flanker task, which involved perceptual inhibition of distracters. This study found the bilateral insula, anterior prefrontal cortex, anterior cingulate, right dorsolateral prefrontal cortex, bilateral caudate/putamen, and posterior and right anterior intraparietal sulcus to be commonly activated for all three tasks. Activations unique to the stimulus-response incompatibility task involved solely the right motor cortex. Activations unique to the Go/NoGo task involved the thalamus, right inferior parietal cortex, and right anterior prefrontal cortex. One limitation of this study was that block designs were used for the flanker and stimulus-response incompatibility tasks whereas an event-related design was used for the Go/NoGo task. Differences in task design may result in subtle differences in functional activations (Goghari & MacDonald, 2008). In addition, resulting activations from block designs tend to involve conglomeration of distinct cognitive processes (i.e. conflict-monitoring, maintenance, error-related activity etc.). Bunge, Dudukovic, and colleagues (2002) contrasted two forms of response inhibition using a flanker and Go/NoGo task and a single event-related design. Regions commonly activated for both tasks included the right inferior frontal gyrus, left inferior and middle frontal gyrus, bilateral superior and inferior parietal cortex, precentral gyrus, right caudate, putamen, and temporo-occipital cortex. This study used similar trial designs for the two tasks; however intermixed the Go/NoGo and flanker trials, which may have resulted in a different task set than if the two tasks were blocked separately. In addition, a single event-related design did not allow parsing of distinct cognitive processes over a course of a trial, such as preparatory- and response-related activity. More recently, researchers have conceptualized response inhibition more broadly. Mostofsky and Simmonds (2008) propose that response inhibition is one facet of response selection, such that inhibiting a response is an intentional response involving a lack of movement. In support of this theory, behavioral, neuroimaging, and lesion data suggest that pre-supplementary motor circuits are critical to both engaging in a motor response as well as selecting to withhold a response (reviewed in Mostofsky & Simmonds, 2008).
Previous research has illuminated a number of factors to be aware of when evaluating and comparing the neuroanatomical correlates of cognitive processes. A number of studies have demonstrated that complex Go/NoGo tasks with working memory demands have different neural correlates than simple Go/NoGo tasks with a lower working memory load (for meta-analysis see Simmonds, Pekar, & Mostofsky, 2008). We have previously demonstrated that even when the same task is presented with different experimental designs (slow event-related, rapid jitter event-related, or rapid partial trial design) differences in brain activations are present (Goghari & MacDonald, 2008). In addition, even inter-session variability can create subtly disparate results (Smith et al., 2005).
The current study evaluated the neural correlates of two cognitive control tasks involving different forms of response selection and inhibition. Importantly, we evaluated both preparatory- and response-related activity, and blocked the task set, which allowed for the first time an evaluation of distinct cognitive processes within a trial. We used a stimulus-response incompatibility task, the Stimulus Response Reversal Task (SRRT) and a variant of the Go/NoGo task. The SRRT consisted of a colored square (green or red) which determined the participant’s subsequent response to the upcoming word (Left or Right). The green square denoted participants should respond with a button press on the same side the word indicated. The red square denoted participants should inhibit their automatic response in favor of a weaker task-relevant response and respond with a button press on the opposite side the word indicated. The Go/NoGo variant involved either viewing the word Left or Right and then a colored circle (green or red). The green circle denoted participants should respond in the direction of the previous word, whereas the red circle denoted participants should wholly inhibit their response to the previous word (i.e., omit a response). We used a within-subject design, similar stimuli and instructions, and the same experimental design and analysis techniques to reduce spurious differences in activations between tasks. The goal of this design was to allow us compare to the greatest extent possible preparatory-activity related to subsequently inhibiting an automatic response to wholly inhibiting an automatic response. In addition, this design would also allow us to compare inhibiting an automatic response to select a task-relevant response to wholly inhibiting an automatic response.
Consistent with the previous literature and theories of cognitive control, we expected to find broad regions of the prefrontal cortex, including the dorsolateral and ventrolateral prefrontal cortex, associated with preparing to perform and performing a controlled response. Specifically, for cue-related cognitive control activity in the SRRT and for probe-related cognitive control activity in both tasks, we expected activations in the middle frontal (BA 9, 10, 46), inferior frontal (BA 44, 45, 47), anterior cingulate (BA 24, 32), and parietal cortices (BA 7, 40; Barber & Carter, 2005; Brown & Braver, 2005; Goghari & MacDonald, 2008; Weissman, Gopalakrishnan, Hazlett, & Woldorff, 2005). As differences in the above threshold activation maps may be present solely due to the arbitrary cut-off between threshold and sub-threshold activations, we directly contrasted, (1) cognitive control preparatory activity in the SRRT with cognitive control response-related activity in the Go/NoGo variant and (2) cognitive control response-related activity in the SRRT with that in the Go/NoGo variant.
Methods
Participants
Twelve right-handed participants (mean age(sd)=26.2(6.6)) were included in this study. Gender distribution of the sample was 58% male. Participants were recruited from the community, had normal or corrected vision, and no substantial head injuries or loss of consciousness. Informed consent was obtained prior to participation and this protocol was approved by the University of Minnesota Internal Review Board.
Cognitive Control Tasks
The SRRT and Go/NoGo variant used similar stimuli. The SRRT was used to measure cognitive control processes associated with preparing to inhibit and then inhibiting an automatic response tendency. As depicted in Figure 1, a central cue (green or red square) appeared for 500ms, followed by a variable delay, after which a central probe (word ‘Left’ or ‘Right’) appeared for 500ms. The green or automatic cue denoted that participants should respond in the direction of the word using a button press. The red or cognitive control cue denoted participants should overcome their automatic tendency and respond in the direction opposite to the word. The type of cue and direction of the probe were fully randomized and occurred 50% of the time.
Figure 1.
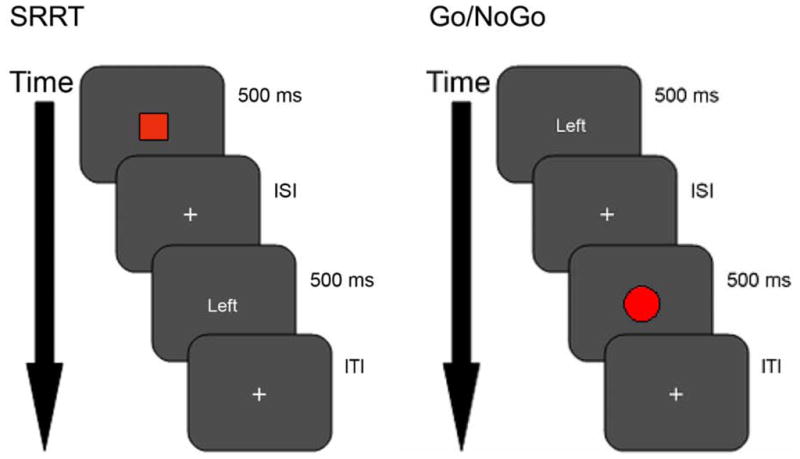
The Stimulus Response Reversal Task (SRRT). Trials began with a colored square indicating whether to respond on the same side (green or automatic condition) or on the opposite side (red or cognitive control condition) when the subsequent directional word (Left or Right) appeared. In the example trial illustrated, participants would respond with the right button.
Go/NoGo variant. Trials began with a directional word (Left or Right) followed by colored circle (green or automatic condition, red or cognitive control condition). A green circle indicated respond on the same side as the directional word. A red circle indicated the response should be withheld. In the example trial illustrated, participants would inhibit their response.
ISI (interstimulus interval) and ITI (intertrial interval) values ranged from 2000, 2250, 2750, 3250, 3750, 4000 ms.
A cued variant of the Go/NoGo task was used to measure cognitive control processes associated with wholly inhibiting an automatic tendency. As depicted in Figure 1, a central cue (word ‘Left’ or ‘Right’) appeared for 500ms, followed by a variable delay, after which a central probe (green or red circle) appeared for 500ms. The green or automatic probe denoted that participants should respond in the direction of the previous word using the button press. The red or cognitive control probe denoted participants should overcome their automatic tendency and inhibit their response. The direction of the cue was fully randomized and occurred 50% of the time and the green probe occurred 80% of the time, to create an automatic tendency to respond.
For both tasks the average trial length was 7 seconds and the interstimulus and intertrial intervals were jittered (2000, 2250, 2750, 3250, 3750, 4000 ms) to allow for better measurement of the hemodynamic response (Dale, 1999). The tasks were presented in two blocks totaling 16 minutes, 8 seconds and participants completed 112 trials per task. The task order was counterbalanced across participants. Participants practiced until competent on the tasks before scanning.
Neuroimaging Methods
Images were collected on 3T Siemens scanner using a standard CP head coil. The functional data were acquired using an EPI sequence (35 slices; TE=28; TR=2 sec; flip angle=90; slice thickness=3.5mm; base resolution=64; FOV=224). Standard T1-weighted anatomical data were collected in the same register, with the same center slice (240 slices; 1mm thickness). Motion during the functional images was calculated and corrected using Automated Image Registration (AIR). AIR was also used to align each participant’s structural sequence to a standard reference brain using a 12 parameter 3-D affine transformation. Those parameter estimates were then used to bring all participants’ functional T2*-weighted images into alignment with the standard brain. Registered functional data was then smoothed using a 7mm FWHM smoothing kernel to increase signal across individuals.
Statistical Analyses
Behavioral Data
Accuracy (%) and mean reaction times (ms) were calculated for in-scanner behavioral performance (see Table 1) using a repeated measures analysis of variance (ANOVA). Accuracy data was arcsine square-root transformed to reduce skew. A one sample t-test assessed whether the SRRT cognitive control mean response reaction time cost was greater than zero. Cognitive control cost was defined as the mean cognitive control trial response reaction time (ms) minus the mean automatic trial response reaction time (ms) for the SRRT. There was no equivalent of the cognitive control cost mean reaction time for the Go/NoGo variant. Inaccurate and inappropriate no-response trials were removed from behavioral reaction time analyses and fMRI analyses to ensure a better approximation of task-related behavior and brain functioning.
Table 1.
Behavioral data from the Stimulus Response Reversal Task (SRRT) and Go/NoGo variant.
| Measured Variables | SRRT | Go/NoGo |
|---|---|---|
| % accurate automatic trials | 94 (4) | 99 (2) |
| % accurate cognitive control trials | 92 (4) | 95 (8) |
| Automatic trial reaction time (ms) | 781 (214) | 478 (112) |
| Cognitive control trial reaction time (ms) | 895 (237) | - |
| Cognitive control cost (ms) | 114 (50) | - |
Abbreviations: All behavioral measures presented are mean (standard deviation) unless otherwise noted. Cognitive control cost = mean cognitive control trial response reaction time (ms) minus mean automatic trial response reaction time (ms) for the SRRT.
General Linear Model Analyses
Analyses were performed in two stages: first, statistical maps were calculated for each individual, and then the values of these maps were grouped for hypothesis testing. Four independent variables for the SRRT, cue activity, cue-activity differentiated by cue type, probe activity, and probe-activity differentiated by cue type and three independent variables for the Go/NoGo variant, cue activity, probe-activity, and probe-activity differentiated by probe type were used to account for variance in the MR signal from trial to trial on an event-related basis. Error and inappropriate no response trials were removed from the analysis by additionally providing a censor file, containing 1 for trials to be included and 0 for trials to be disregarded in fitting the hemodynamic response function. Activity for cognitive control condition versus automatic condition was contrast coded to avoid colinearity with main effects of cue and probe. Predictors for each task were entered into a GLM implemented by AFNI (one separate GLM per task). Each individual’s beta maps were extracted for subsequent analyses. T-tests were conducted on the betas for each regressor to determine where there were positive activations on average. A cluster-based correction was implemented. Functional activations were considered over threshold if 8 contiguous voxels were activated at p<0.005 (df=11) (Forman et al., 1995). However, this threshold is not as conservative as cluster-based corrections based on resampling methods. For the SRRT, the relationship between the cognitive control accuracy and cognitive control cost reaction time measure (cognitive control – automatic trial response reaction times) and prefrontal beta values for the cognitive control and automatic condition contrast for both the preparatory- and response-related activity was assessed using Pearson correlations. For the Go/NoGo, the relationship between the cognitive control accuracy and prefrontal beta values for the cognitive control and automatic condition contrast for the response-related activity was assessed using Pearson correlations.
A conservative intersection analysis using only above threshold activations was conducted to determine common activations to both tasks. To account for both above threshold and sub-threshold activations, a paired t-test was conducted to determine where functional activity differed significantly between regressors (Cognitive Control > Automatic) for the two tasks (p<0.005, df=11, contiguity threshold=8). Betas were extracted from the regions that showed a significant difference to determine the nature of the findings. Two contrasts were conducted to evaluate activation patterns: (1) cognitive control vs. automatic condition for the SRRT and (2) cognitive control vs. automatic condition for the Go/NoGo variant. Two additional contrasts were conducted to fully probe the differences between the cognitive control processes in those regions: (3) SRRT vs. Go/NoGo variant for the automatic condition and (4) SRRT vs. Go/NoGo variant for the cognitive control condition.
Results
Behavioral Data
A 2 (task: SRRT, Go/NoGo variant) × 2 (condition: automatic, cognitive control) repeated measures ANOVA on accuracy revealed a main effect of task (F(1,11)=13.79, p=0.003; partial eta-square=0.56), with the Go/NoGo variant being less difficult than the SRRT (97% vs. 93%; see Table 1). There was no main effect of condition (F(1,11)=1.53, p=0.24; partial eta-square=0.12) or most importantly task by condition interaction (F(1,11)=0.01, p=0.92; partial eta-square=0.001). A subsequently conducted one way t-test demonstrated a marginal main effect of condition (t(11)=1.24, p=0.12). There was a trend towards significance for the automatic condition to be more accurate than the cognitive control condition for the SRRT (t(11)=1.6, p=0.07) but not the Go/NoGo variant (t(11)=0.69, p=0.25).
As expected, a repeated measures ANOVA on reaction times for automatic trial responses (F(1,11)=56.7, p<0.001, partial eta-square=0.84) revealed an effect of task, with faster responses for the Go/NoGo variant where a response could be prepared, compared to the SRRT, where only a response tendency could be prepared (478 ms vs. 781 ms). Lastly, as predicted the cognitive control cost mean reaction time for the SRRT was significantly greater than zero (t(1,11)=7.88, p<0.001).
fMRI Data
SRRT
Brain activity is summarized in Table 2. For the cue-related period three functional activations were found in the cerebellar and occipital regions. For the probe-related period ten functional activations were found in the bilateral middle frontal, cingulate, bilateral basal ganglia, left precentral, right postcentral, right middle temporal, bilateral inferior parietal, and cerebellar regions.
Table 2.
Functional activations from Stimulus Response Reversal Task (SRRT).
| Region of Interest | BA | Coordinates |
Clus. | Max t | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
|
Cue-Related Activity |
||||||
| Cerebellum | - | 7 | −39 | −5 | 27 | 5.05 |
| Precuneus | 19 | 3 | −82 | 40 | 23 | 6.16 |
| Lingual | 18 | 2 | −87 | −8 | 243 | 7.28 |
|
Cue-Related Activity: Cognitive Control > Automatic |
||||||
| Cingulate/medial frontal | 24/32 6 |
−4 −4 |
6 7 |
41 54 |
103 | 5.55 |
| L precentral | 6/4 | −33 | −6 | 38 | 8 | 4.13 |
|
Probe-Related Activity |
||||||
| L middle frontal | 9/46 | −32 | 31 | 31 | 68 | 9.41 |
| R middle frontal | 9/46 | 37 | 28 | 29 | 67 | 5.87 |
| Cingulate/Bilateral basal ganglia/insula | 24/32 - - |
0 27 −27 |
10 −4 −3 |
31 8 8 |
897 | 8.74 |
| L precentral | 6 | −50 | −2 | 35 | 12 | 4.23 |
| R postcentral | 2 | 52 | −18 | 25 | 9 | 5.13 |
| R inferior parietal | 40 | 58 | −40 | 26 | 35 | 5.36 |
| R middle temporal | 21 | 45 | −47 | 1 | 26 | 5.29 |
| L inferior parietal | 40 | −41 | −51 | 47 | 281 | 5.87 |
| R inferior parietal | 40 | 31 | −53 | 39 | 40 | 4.50 |
| R cerebellum | - | 25 | −54 | −23 | 44 | 6.54 |
|
Probe-Related Activity: Cognitive > Automatic |
||||||
| R superior/medial frontal | 6/8 | 3 | 19 | 50 | 29 | 5.25 |
| L middle/inferior frontal | 9/44/45 | −51 | 19 | 21 | 20 | 6.84 |
| R sylvian fissure | - | 51 | 17 | 0 | 11 | 4.74 |
| L inferior frontal | 47 | −43 | 16 | 1 | 16 | 5.00 |
| R middle/inferior frontal | 9/44 | 46 | 9 | 32 | 16 | 4.04 |
| R middle temporal | 21 | 59 | −28 | −4 | 8 | 4.23 |
| L middle temporal | 21 | −56 | −39 | −2 | 24 | 7.08 |
| Cerebellum | - | −4 | −54 | −6 | 55 | 5.29 |
| L inferior parietal | 39/40 | −54 | −57 | 24 | 10 | 4.16 |
| L inferior parietal | 40 | −44 | −62 | 41 | 18 | 4.64 |
Note: Abbreviations: BA: Brodmann’s area; Clus.: Cluster size, number of contiguous voxels.
Analyses were thresholded at p<0.005, 11 degrees of freedom, with a contiguity threshold of 8.
For both cue- and probe-related processes associated with cognitive control, functional activations were expected in the middle frontal, inferior frontal, anterior cingulate, and parietal cortices. However, for the cue-related period associated with cognitive control only two functional activations were found in the cingulate/medial frontal and left occipital regions. For the probe-related period associated with cognitive control ten functional activations were found in the right superior frontal, right medial frontal, bilateral middle frontal, bilateral inferior frontal, bilateral temporal, left inferior parietal, and cerebellar regions (see Figure 2; Figure 4).
Figure 2.
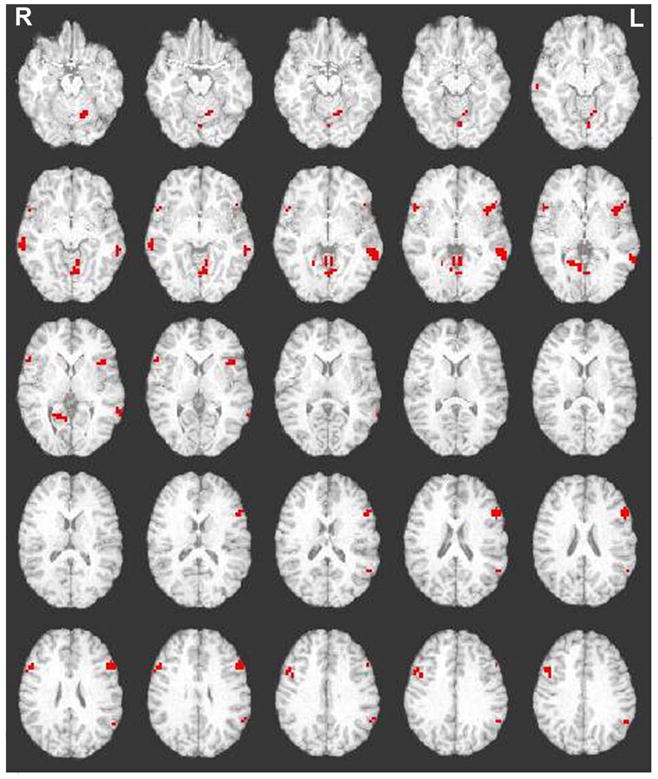
Activations for the Stimulus Response Reversal Task (SRRT) for cognitive control greater than automatic condition response-related activity. Abbreviations: R=Right; L=Left. Images were thresholded at p<0.005, 11 degrees of freedom, with a contiguity threshold of 8.
Figure 4.
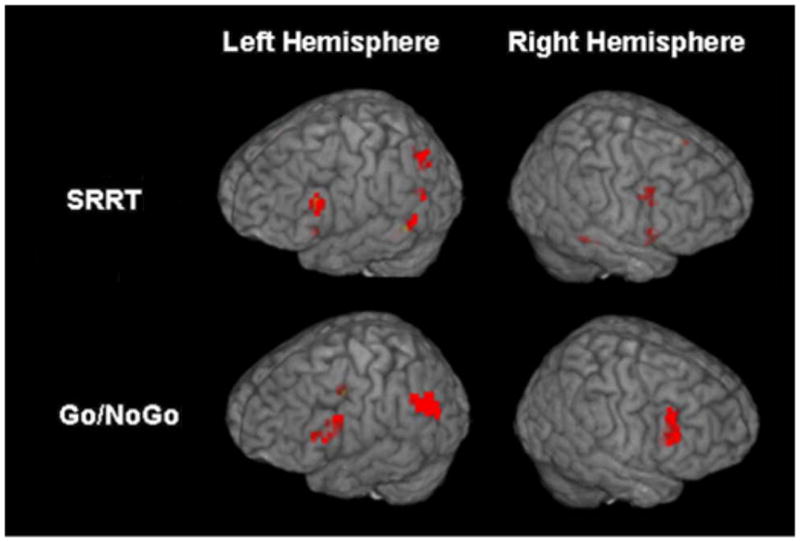
Activations for Stimulus Response Reversal Task (SRRT) and Go/NoGo variant for cognitive control greater than automatic condition response-related activity. Images were thresholded at p<0.005, 11 degrees of freedom, with a contiguity threshold of 8.
Individuals’ cognitive control vs. automatic condition contrast prefrontal functional beta values for the preparatory- and response-related period were correlated with participants’ cognitive control cost mean response reaction times. Increased betas significantly predicted increased cognitive control cost reaction times only for response-related activity for the right inferior/middle frontal (r=0.58, p=0.05) and the superior/medial frontal (r=0.64, p=0.03) functional activations. There were no significant associations for cognitive control condition accuracy with either preparatory- or response-related activity.
Go/NoGo Variant
Brain activity is summarized in Table 3. For the cue-related period functional activations were found in the left medial frontal and right fusiform/occipital regions. For the probe-related period eight functional activations were found in the bilateral inferior frontal, bilateral middle frontal, left precentral, bilateral postcentral, right cerebellar, and bilateral occipital regions.
Table 3.
Functional activations from the Go/NoGo variant.
| Region of Interest | BA | Coordinates |
Clus. | Max t | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
|
Cue-Related Activity |
||||||
| Left medial frontal | 6 | −4 | −3 | 52 | 26 | 4.80 |
| Right fusiform/inferior occipital | 18/19 | 38 | −80 | −16 | 9 | 4.37 |
|
Probe-Related Activity |
||||||
| Left frontal inferior/precentral | 44/6 | −43 | 18 | 8 | 27 | 4.61 |
| Right inferior frontal | 47 | 46 | 21 | −1 | 11 | 4.77 |
| Right middle frontal | 9 | 31 | 38 | 34 | 11 | 4.22 |
| Left middle frontal | 9/46 | −37 | 39 | 29 | 91 | 5.77 |
| Right postcentral | 40/43 | 54 | −13 | 14 | 19 | 4.88 |
| Left postcentral | 40 | −55 | −21 | 16 | 11 | 4.65 |
| Right cerebellum | - | 27 | −65 | −20 | 9 | 4.44 |
| Bilateral cuneus/occipital middle | 17/18 | 0 | −85 | 8 | 1108 | 12.12 |
|
Probe-Related Activity: Cognitive Control > Automatic |
||||||
| Left inferior frontal | 45 | −49 | 14 | 21 | 40 | 5.09 |
| Right inferior frontal | 47 | 36 | 24 | 0 | 18 | 4.63 |
| Right inferior/middle frontal | 44/46/9 | 49 | 26 | 27 | 49 | 5.32 |
| Left frontal middle/precentral | 9/6 | −37 | 0 | 41 | 10 | 5.03 |
| Right middle temporal | 21 | 51 | −16 | −10 | 10 | 4.96 |
| Left middle temporal | 21/37 | −45 | −50 | 8 | 10 | 5.52 |
| Left superior temporal | 39 | −59 | −57 | 30 | 26 | 4.85 |
| Left fusiform/lingual | 18/19 | −30 | −58 | −7 | 42 | 7.48 |
Note: Abbreviations: BA: Brodmann’s area; Clus.: Cluster size, number of contiguous voxels.
Analyses were thresholded at p<0.005, 11 degrees of freedom, with a contiguity threshold of 8.
For probe-related cognitive control processes functional activations were expected in the middle frontal, inferior frontal, anterior cingulate, and parietal cortices. For the probe-related period associated with cognitive control eight functional activations were found in the bilateral inferior frontal, bilateral middle frontal, left precentral, bilateral middle temporal, left superior temporal, and left fusiform/occipital regions (see Figure 3; Figure 4). There was no equivalent of the cognitive control cost mean reaction times to correlate with beta values for the Go/NoGo variant. There were no significant associations for cognitive control condition accuracy with response-related activity.
Figure 3.
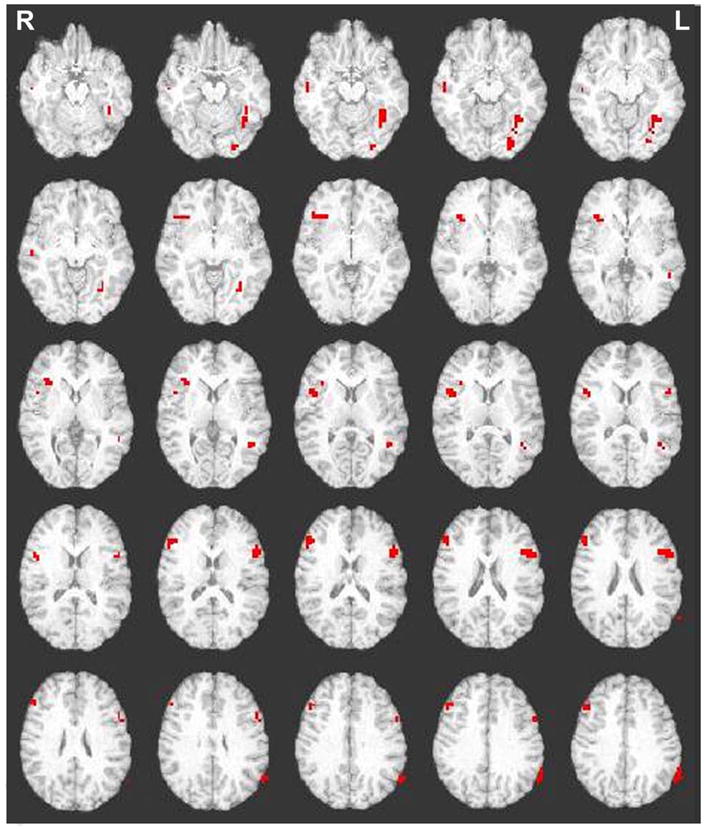
Activations for the Go/NoGo variant for cognitive control greater than automatic condition response-related activity. Abbreviations: R=Right; L=Left. Images were thresholded at p<0.005, 11 degrees of freedom, with a contiguity threshold of 8.
Preparing to Inhibit an Automatic Response in the SRRT Compared to Wholly Inhibiting an Automatic Response in the Go/NoGo Variant
We compared cue-related cognitive control activity for the SRRT to probe-related cognitive control activity for the Go/NoGo variant. This contrast compared the red vs. green cue of the SRRT to the red vs. green probe of the Go/NoGo variant, thereby attempting to equate superficial components of the stimuli the participants were presented with. Consistent with theories of cognitive control, we predicted a common prefrontal network mediating the different forms of cognitive control.
A conservative intersection analysis of the above threshold maps revealed no common functional activations. A direct contrast of the two tasks demonstrated functional activations that were significantly different (see Table 4). However, as predicted no prefrontal activations were found to be significantly different between the two contrasted processes for the two tasks.
Table 4.
Functional activations demonstrating a significant difference between SRRT cognitive control greater than automatic condition preparatory-related activity compared to Go/NoGo variant cognitive control greater than automatic condition response-related activity.
| Region of Interest | BA | Coordinates | Clus. | Max t | Condition | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Right middle temporal | 21 | 43 | −26 | −8 | 13 | 5.57 | NoGoCog>NoGoAuto |
| Left middle temporal | 21 | −54 | −27 | −2 | 13 | 4.05 | NoGoCog>NoGoAuto NoGoCog>SRRTCog |
| Right parietal | 40 | 36 | −34 | 44 | 14 | 4.68 | NoGoAuto>NoGoCog NoGoAuto>SRRTAuto |
| Left parietal | 40 | −43 | −35 | 51 | 55 | 5.54 | NoGoAuto>NoGoCog NoGoAuto>SRRTAuto |
| Left posterior cingulate | 29 | −2 | −43 | 7 | 58 | 6.95 | NoGoAuto>NoGoCog SRRTCog>NoGoCog |
| Left middle temporal | 21/37 | −49 | −47 | −1 | 18 | 5.59 | NoGoCog>NoGoAuto SRRTAuto>SRRTCog NoGoCog>SRRTCog |
| Left middle/superior temporal | 39/40 | −59 | −53 | 30 | 34 | 4.84 | NoGoCog>NoGo Auto SRRTAuto>SRRTCog |
Note: Abbreviations: BA: Brodmann’s area; Clus.: Cluster size, number of contiguous voxels; NoGoCog:Go/NoGo variant cognitive control condition; NoGoAuto:Go/NoGo variant automatic condition; SRRTCog:SRRT cognitive control condition; SRRTAuto:SRRT automatic condition.
The contrast column lists the paired t-tests that were significantly different for the following four tests conducted: cognitive control vs. automatic condition for Go/NoGo variant; cognitive control vs. automatic condition for SRRT; automatic condition for SRRT vs. Go/NoGo; cognitive control condition for SRRT vs. Go/NoGo. Analyses were thresholded at p<0.005, 11 degrees of freedom, with a contiguity threshold of 8.
A posterior cingulate functional activation was found to have greater activity when completing automatic responses compared to cognitive control responses during the Go/NoGo variant (t(1,11)=4.3, p=0.001). In addition, there was a difference between the cognitive control conditions, such that there was greater activity for cognitive control preparation during the SRRT compared to cognitive control response during the Go/NoGo variant (t(1,11)=−3.52, p=0.005).
Four temporal functional activations were found to be differentially activated during preparatory- versus response-related activity for the two tasks. A right middle temporal functional activation was found to have more activity when completing cognitive control responses than automatic responses for the Go/NoGo variant (t(1,11)=3.6, p=0.004). Two left temporal functional activations were found to have greater activity when completing cognitive control responses compared to automatic responses for the Go/NoGo variant (t(1,11)=4.07, p=0.002; t(1,11)=4.9, p=0.001). In contrast, for the SRRT, there was greater activity when preparing automatic responses compared to preparing cognitive control responses (t(1,11)=−2.05, p=0.07; t(1,11)=−2.59, p=0.03). In addition, cognitive control responses during the Go/NoGo variant was associated with greater activation than cognitive control preparation during the SRRT (t(1,11)=4.02, p=0.002; t(1,11)=3.08, p=0.01). A third left middle and superior temporal functional activation demonstrated a similar relationship with greater activity when completing cognitive control responses compared to automatic responses for the Go/NoGo variant (t(1,11)=4.03, p=0.002). In contrast, for the SRRT, preparing automatic responses resulted in greater activity compared to preparing cognitive control responses (t(1,11)=−2.8, p=0.02).
Lastly, bilateral inferior parietal functional activations were found to be differentially activated between the two tasks. Greater activation was found when completing automatic responses compared to completing cognitive control responses during the Go/NoGo variant (t(1,11)=−2.89, p=0.02; t(1,11)=−4.2, p=0.001). In addition, greater activity was found when completing automatic responses during the Go/NoGo variant compared to preparing automatic responses for the SRRT (t(1,11)=2.7, p=0.02; t(1,11)=3.2, p=0.008).
Inhibiting an Automatic Response to Select a Task-Relevant Response in the SRRT Compared to Wholly Inhibiting an Automatic Response in the Go/NoGo Variant
We compared probe-related cognitive control activity in the SRRT to that in the Go/NoGo variant. Consistent with theories of cognitive control, we predicted a largely common prefrontal network mediating the different forms of controlled responses.
Intersection analysis of the SRRT and Go/NoGo variant above threshold activation maps revealed a common activation in the left inferior frontal region (see Figure 5). Contrasting the two tasks revealed significantly greater activation in the right inferior frontal region when completing cognitive control responses compared to automatic responses for the Go/NoGo variant (t(1,11)=t=4.47, p=0.001) (see Table 5). The SRRT, which required inhibiting an automatic response to perform an alternative task-relevant response, did not demonstrate such an effect.
Figure 5.
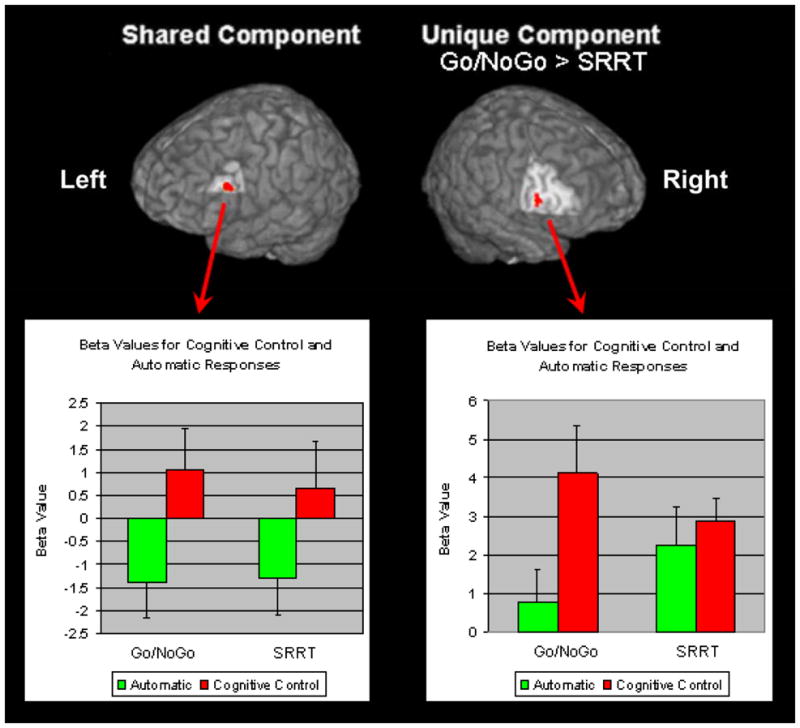
Shared and unique activations for the Stimulus Response Reversal Task (SRRT) and Go/NoGo variant for cognitive control greater than automatic condition response-related activity. Images were thresholded at p<0.005, 11 degrees of freedom, with a contiguity threshold of 8. Shared component: Left inferior frontal (BA 44/45; x=−49, y=17, z=21; cluster size=8 voxels). Unique component: Right inferior frontal (BA 44; x=46, y=11, z=10; cluster size=8 voxels, Max t=4.05).
Table 5.
Functional activations demonstrating a significant difference between SRRT and Go/NoGo variant for cognitive control greater than automatic condition response-related activity.
| Region of Interest | BA | Coordinates | Clus. | Max t | Condition | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Right inferior frontal | 44 | 46 | 11 | 10 | 8 | 4.05 | NoGoCog>NoGoAuto |
| Right midbrain | - | 13 | −26 | −13 | 16 | 5.69 | NoGoAuto>NoGoCog |
| Left cerebellum/lingual | 18 | −4 −1 |
−55 −66 |
−7 2 |
220 |
6.30 | NoGoAuto>NoGoCog SRRTCog>SRRTAuto NoGoAuto>SRRTAuto |
Note: Abbreviations: BA: Brodmann’s area; Clus.: Cluster size, number of contiguous voxels; NoGoCog:Go/NoGo variant cognitive control condition; NoGoAuto:Go/NoGo variant automatic condition; SRRTCog:SRRT cognitive control condition; SRRTAuto:SRRT automatic condition.
The contrast column lists the paired t-tests that were significantly different for the following four tests conducted: cognitive control vs. automatic condition for Go/NoGo variant; cognitive control vs. automatic condition for SRRT; automatic condition for SRRT vs. Go/NoGo; cognitive control condition for SRRT vs. Go/NoGo. Analyses were thresholded at p<0.005, 11 degrees of freedom, with a contiguity threshold of 8.
A midbrain functional activation was found to be more active when completing automatic responses compared to cognitive control responses during the Go/NoGo variant (t(1,11)=−3.1, p=0.01). Lastly, a cerebellar and lingual functional activation demonstrated greater activity when completing automatic responses compared to cognitive control responses for the Go/NoGo variant (t(1,11)=−3.42, p=0.006). In contrast, during the SRRT, greater activity was found for cognitive control responses compared to automatic responses (t(1,11)=3.61, p=0.004). Lastly, greater activity was found when completing automatic responses for the Go/NoGo variant compared to completing automatic responses for the SRRT (t(1,11)=5.11, p<0.001).
Task-Switching Processes in the SRRT
The SRRT involved task-switching between compatible and incompatible stimulus-response mappings, which was not present in the Go/NoGo variant. To investigate whether task-switching activity was being misattributed as cognitive control activity, we conducted additional behavioral and neuroimaging analyses.
Behavioral Analyses
A repeated measures ANOVA evaluated the behavioral effects of switch trials compared to repeat trials and a switch cost was calculated (mean response reaction time (ms) for switch trials minus mean response reaction time (ms) for repeat trials). The ANOVA revealed a significant effect of condition with switch trials having longer response reaction times than repeat trials (switch cost=33ms; F(1, 11)=6.67, p=0.03, partial eta-squared=0.38). As switching from a compatible mapping to incompatible mapping can be different from switching from an incompatible mapping to a compatible mapping, specific types of switch and repeat trials were analyzed. A 2 (previous trial: cognitive control, automatic) × 2 (current trial: cognitive control, automatic) repeated measured ANOVA demonstrated a significant interaction between the previous trial condition with current trial condition (F(1, 11)=7.81, p=0.02, partial eta-squared=0.42). This interaction was driven by a significant effect of the previous condition on the current automatic condition (t(1, 11)=4.16, p=0.002), but not the cognitive control condition (t(1, 11)=−1.31, p=0.22). That is, when an automatic trial was repeated there was a substantial decrease in response times compared to if there was a switch from a previous cognitive control trial to an automatic trial (specific switch cost=93ms). This raised the possibility that some of the brain regions identified in the direct contrast of cognitive control to automatic trials on the SRRT may in fact reflect activity associated with task-switching.
Neuroimaging Analyses
The fMRI task-switching analyses were conducted in a parallel manner to all other neuroimaging data. Behavioral analyses guided neuroimaging analyses. Paired t-tests evaluated cue- and probe-related activity for all switch trials compared to all repeat trials and the specific effect of a previous trial condition on the current automatic trial condition. For switch trials compared to repeat trials, greater cue-activity was found during repeat trials in the right parahippocampal gyrus (x=26, y=−1, z=−12, cluster size=8). For switch trials compared to repeat trials, greater probe-related activity was found during switch trials in the anterior cingulate gyrus (BA 24, x=1, y=2, z=33, cluster size=22). For specific task-switching effects of switching from a cognitive control trial to an automatic trial compared to repeating an automatic trial, greater probe-related activity was found during switch trials in the left cerebellum (x=−14, y=−48, z=−33, cluster size=8). These analyses suggest that task-switching mechanisms were not prominently influencing the cognitive control functional activations for the SRRT.
Discussion
This study compared the functional neuroanatomy of two related cognitive control processes, namely response selection and inhibition. For the first time, different aspects of trials corresponding to different cognitive processes (preparation and response) were distinguished. To reduce the influence of spurious factors, a within-subject design, similar stimuli and instructions, same experimental design, and same analysis techniques were implemented. The first goal of this study was to investigate how preparing to inhibit an automatic tendency differed from wholly inhibiting an automatic tendency. Therefore, we compared cue-related activity from the SRRT to response-related activity from the Go/NoGo. We did not find any preparatory prefrontal activation above statistical threshold as expected during the SRRT. This diminished our chance of finding commonly activated regions between the two tasks. However, the overlap technique is conservative as it only takes into account above threshold activations. Therefore we also conducted analyses that directly contrasted the two tasks to include activations both above and below statistical threshold. We did not find any prefrontal regions that were differentially activated for preparing to inhibit a response compared to wholly inhibiting a response when directly contrasted. This suggests a largely common prefrontal cognitive control network for these executive processes (Miller & Cohen, 2001).
We did find other brain regions to be differentially involved between the cognitive control processes of preparing to inhibit a response compared to wholly inhibiting a response. Bilateral middle temporal functional activations were more associated with wholly inhibiting a response compared to completing a response for the Go/NoGo variant. This relationship was not present for the SRRT; rather for two of the temporal regions, there was significantly greater activity for preparing an automatic response tendency than a cognitive control response tendency. The middle temporal gyrus has been found to be activated in a meta-analysis of complex Go/NoGo tasks involving working memory (Simmonds, Pekar, & Mostofsky, 2008). In addition, Badre and colleagues (2005) found that that middle temporal cortex was sensitive to the strength of stimulus-response associations rather than competition. The middle temporal cortical activity was also found to be coupled with anterior part of the inferior frontal gyrus. Our results taken together with Badre and colleagues (2005) suggest that the middle temporal gyrus is sensitive to stimulus-response mapping that are more general than just semantic stimulus-response mappings. One potential reason there was a reduced role for the middle temporal region during the SRRT may be because the stimulus-response mappings were weaker than that of the Go/NoGo variant. During the SRRT, participants were preparing their upcoming response (i.e., the first stimulus provides information on how to perform the upcoming response, which has not been determined yet) rather than actually performing the stimulus-response mapping as in Go/NoGo variant.
In addition, we found the posterior cingulate was more active for completion of an automatic response for the Go/NoGo variant, which may reflect processes related to completion of a motoric response (Vogt, Finch, & Olson, 1992). Surprisingly, we also found increased bilateral parietal activations for probe-related activity when completing a response was compared to inhibiting a response for the Go/NoGo variant. This relationship was not present for the SRRT. This unpredicted finding may reflect activations necessary for activating possible response selections on the basis of stimulus-response associations (Brass, Ullsperger, Knoesche, von Cramon, & Phillips, 2005; Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002) and may be more prominent for actual completion of a more simply mapped response. Parietal regions (BA 40) have also been found to be important for the short-term storage of verbal material (Jonides et al., 1998). Hence, parietal regions may have activated more during the response phase of the Go/NoGo variant, where completion of the response required retrieval of the previous directional word. Lastly, the left parietal cortex (BA 40) has also been associated with motor attention relating to hand movements (Rushworth, Krams, & Passingham, 2001), which is necessary for the actual completion of a response.
The second goal of this study was to compare the neural correlates of inhibiting an automatic response to select a task-relevant response to wholly inhibiting an automatic response. One of the main findings of this study was that the left inferior frontal gyrus was found to be similarly online for both response-related activity in the SRRT, as well as, in the Go/NoGo variant. In addition, to the significant difference between conditions, negative beta weights were found during the automatic condition for both tasks. Negative beta weights in a contrast-coded, multi-predictor general linear model can be interpreted as decreases in the fMRI signal associated with the particular condition relative to the other conditions included in that analysis. In this specific analysis, the general linear models for the tasks included regressors for general cue-related activity irrespective of condition, general probe-related activity irrespective of condition, probe-related activity differentiated by condition, and cue-related activity differentiated by condition in the SRRT analyses only. Thus, the negative beta weights suggest that there was less activity in the left inferior frontal gyrus associated with automatic responses compared with all other forms of activity, including response-related activity in general.
In support of these findings, the left inferior frontal gyrus has been found to be activated during response selection of task-relevant information during conflict in a number of studies. Moss and colleagues (2005) varied selection demands while controlling for retrieval demands and found evidence for increased activation in the left inferior frontal gyrus with increased selection demands. Similarly, Badre and colleagues (2005) demonstrated that the left mid-inferior frontal region (~BA 45), which is similar to the region we found, may be useful for general selection processes. In addition, the Badre and colleagues found that the role of the left mid-inferior frontal gyrus was distinct from the role of the left anterior inferior frontal gyrus, which was more involved in controlled retrieval processes. Furthermore, Zhang and colleagues (2004) demonstrated that the left inferior frontal gyrus activation increased as the need for selection among competing representations increased during the preparatory period. This association was independent of semantic retrieval. In the present study, during both tasks, participants had to select between two competing responses. In the SRRT, participants need to select between completing two alternative responses, whereas, in the Go/NoGo variant participants need to select between completing a response or omitting a response. These findings suggest that the inferior frontal gyrus is involved in broad forms of controlled selection at the response and motoric level.
In addition to finding common activations related to response selection in the left inferior frontal gyrus, we found the right inferior frontal gyrus to be significantly more activated when wholly inhibiting a response during the Go/NoGo variant compared to inhibiting an automatic response to complete a task-relevant response during the SRRT. A review of response inhibition studies provided evidence that the right inferior frontal gyrus has a specific role in response inhibition (Aron, Robbins, & Poldrack, 2004). Aron and colleagues (2003) demonstrated that greater damage to right inferior frontal gyrus was particularly related to slowed inhibition on a stop/signal task. Damage to the left inferior frontal gyrus and other frontal regions was not associated with stop/signal task performance. In addition, performance on the Go trials was not related to damage to the right inferior frontal gyrus. Similarly, Rubia and colleagues (2003) found successful inhibition of a stop/signal was associated with the right inferior prefrontal cortex, whereas unsuccessful inhibition signals were not. Furthermore, this association was not found for the right middle frontal or superior frontal gyri. Additional evidence is provided by a study which used repetitive transcranial magnetic stimulation and compared a stop/signal task (more response inhibition) to a flanker task (more response competition) and found the right inferior frontal gyrus to be especially crucial for inhibiting a response tendency especially in the face of increased response competition (Chambers et al., 2007).
It is important to note though in this study we found the right inferior frontal gyrus to be activated above threshold during response-related activity for both tasks and this is consistent with both tasks involving variations of response inhibition (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Konishi et al., 1999). However, when directly contrasted the Go/NoGo variant had greater right inferior frontal activation and this region was not found to overlap with the above threshold SRRT right inferior frontal activation. These results suggest that there may be a component of the right inferior frontal gyrus that is more sensitive to wholly inhibiting a motoric response. Alternatively this region may be more sensitive to the degree of response inhibition required which was greater for the Go/NoGo variant.
In this study, we attempted to control for spurious manipulations that could influence the cognitive processes and subsequently neural activity. Both tasks were developed to have high accuracy rates as the goal of this study was to investigate successful inhibition. Although we attempted to balance our tasks for difficulty, the SRRT was more difficult than the Go/NoGo variant as demonstrated by the accuracy and reaction time measures. Both tasks were constructed to have a working memory component and rely on repetition of the first stimulus to keep the response code active. However in SRRT, there was the added component of needing to remember the appropriate stimulus-response mapping; therefore the reliance on the phonological loop could have differed between the two tasks. Lastly, the SRRT had more cognitive control trials (50% of the trials) than the Go/NoGo variant (20% of the trials) and increased cognitive control trials can lead to greater prefrontal cortical activation (de Zubicaray, Andrew, Zelaya, Williams, & Dumanoir, 2000). Nonetheless, the main differences found in this study were associated with the Go/NoGo variant demonstrating more activity associated with cognitive control compared to the SRRT when directly contrasted. This suggests cognitive processes as opposed to task difficulty account for our current findings. Also, the SRRT additionally involved task-switching between the two alternative task responses. Supplemental analyses, however, demonstrated that switching between compatible and incompatible stimulus-response mappings were not prominently driving the primary results.
In stimulus-response paradigms it is difficult to know precisely what and when participants are preparing their response. This can make interpreting and comparing activations of specific processes in a trial difficult. To create a response-readiness state in participants and make it more likely participants would prepare a response tendency, we used a jitter technique. By varying the interval, participants were unable to predict when the probe would appear biasing the use of a proactive strategy, where the response code is kept active. We have previously demonstrated that varying the intervals in cue-probe paradigms likely leads to greater response readiness, evidenced by quicker response reaction times when the probe appears (Goghari & MacDonald, 2008).
Lastly, we found no above threshold prefrontal activations for the SRRT during preparation. Although, this could suggest that this task did not invoke the prefrontal cortex to a sufficient degree, supplementary evidence suggests that this is not the case. We have previously demonstrated in the same participants that the SRRT invoked the prefrontal cortex during preparation. When this task was presented using a partial-trial technique with the same amount of trials, robust prefrontal particles above statistical threshold were found. These same prefrontal activations were found not to be activated to a greater degree than the present data collected with the jitter technique (Goghari & MacDonald, 2008). Therefore, the lack of prefrontal cortex activation in this study is likely due to our choice of statistical threshold. In addition, rather than comparing above statistical threshold maps, we used direct contrasts of beta values that allowed us to test both above and sub-threshold regions for differences.
Our findings suggest a largely common cognitive control network, with subtle differences depending on specific tasks demands. Consistent with these findings, Fan and colleagues (2003) used three cognitive control tasks and found common prefrontal recruitment, with differences between the tasks in peak activation and spatial extent of those activations. These neuroimaging findings are consistent with accumulated behavioral findings arguing for similar underlying neural correlates or a central cognitive mechanism for different cognitive processes (e.g., Verbruggen, Liefooghe, Notebaert, & Vandierendonck, 2005; Verbruggen, Liefooghe, & Vandierendonck, 2006). The findings from this study are consistent with theories that suggest the prefrontal neural correlates of preparing to inhibit a response are largely similar to the neural correlates of wholly withholding a response (Miller & Cohen, 2001). In addition, we found that there was a strong association for the left inferior frontal gyrus to be involved in controlled response selection. Nevertheless, subtle differences in recruitment of regions were also found depending on the nature of the specific cognitive processes recruited. The right inferior frontal gyrus was more sensitive to response inhibition and especially involved in wholly inhibiting a response. Differences between this study and other studies may be due to experimental design, analyses, and degree to which specific cognitive processes are involved in the cognitive paradigms. Furthermore, there were many discrepancies between the two tasks when functional activations above statistical threshold were considered. These differences were not found when below threshold activations were included and the two tasks were directly contrasted This may be an important distinction for researchers to keep in mind as they view above threshold activation maps and attempt to reconcile seemingly disparate cognitive findings in the literature. In the future, investigators may wish to carefully consider and report how characteristics of their tasks, analysis pipeline, and imaging design may be affecting their results when attempting to reconcile the functional neuroanatomy of inter-connected cognitive processes (Poldrack et al., 2008).
Acknowledgments
Ms. Goghari was supported by a PGS Master’s and Doctoral Award from the Natural Sciences and Engineering Research Council of Canada and by the Graduate Research Partnership Program, University of Minnesota. Grant support was provided by: Grant-in-Aid of Research, Artistry and Scholarship University of Minnesota, BTRR P41 008079, and the MIND Institute. The authors gratefully acknowledge the assistance of Melissa Johnson, Kate Fissell, Rudrava Roy, and the staff at the Center for Magnetic Resonance Research. Data from this study were presented at the Human Brain Mapping Conference, June 11–15th, 2006 in Florence Italy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cerebral Cortex. 2005;15:899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. Journal of Cognitive Neuroscience. 2005;17:1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. New York, NY: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, et al. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. Journal of Neurophysiology. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray GI, Andrew C, Zelaya FO, Williams SC, Dumanoir C. Motor response suppression and the prepotent tendency to respond: a parametric fMRI study. Neuropsychologia. 2000;38:1280–1291. doi: 10.1016/s0028-3932(00)00033-6. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW., 3rd Effects of varying the experimental design of a cognitive control paradigm on behavioral and functional imaging outcome measures. Journal of Cognitive Neuroscience. 2008;20:20–35. doi: 10.1162/jocn.2008.20007. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanwisher N. Common neural substrates for response selection across modalities and mapping paradigms. Journal of Cognitive Neuroscience. 2003;15:1080–1094. doi: 10.1162/089892903322598067. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, et al. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, et al. Selecting among competing alternatives: selection and retrieval in the left inferior frontal gyrus. Cerebral Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE. Guidelines for reporting an fMRI study. Neuroimage. 2008;40:409–414. doi: 10.1016/j.neuroimage.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. Journal of Cognitive Neuroscience. 2001;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Schon K, Quiroz YT, Hasselmo ME, Stern CE. Greater working memory load results in greater medial temporal activity at retrieval. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D’Esposito M. Neural evidence for representation-specific response selection. Journal of Cognitive Neuroscience. 2003;15:1111–1121. doi: 10.1162/089892903322598085. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Ramnani N, Woolrich MW, Bannister PR, Jenkinson M, et al. Variability in fMRI: a re-examination of inter-session differences. Human Brain Mapping. 2005;24:248–257. doi: 10.1002/hbm.20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Notebaert W, Vandierendonck A. Effects of stimulus-stimulus compatibility and stimulus-response compatibility on response inhibition. Acta Psychologica (Amst) 2005;120:307–326. doi: 10.1016/j.actpsy.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Vandierendonck A. Selective stopping in task switching: The role of response selection and response execution. Experimental Psychology. 2006;53:48–57. doi: 10.1027/1618-3169.53.1.48. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Feng CM, Fox PT, Gao JH, Tan LH. Is left inferior frontal gyrus a general mechanism for selection? Neuroimage. 2004;23:596–603. doi: 10.1016/j.neuroimage.2004.06.006. [DOI] [PubMed] [Google Scholar]


