Abstract
Axl is a receptor tyrosine kinase implicated in cell survival following growth factor withdrawal and other stressors. The binding of Axl's ligand, growth arrest-specific protein 6 (Gas6), results in Axl autophosphorylation, recruitment of signaling molecules, and activation of downstream survival pathways. Pull-down assays and immunoprecipitations using wildtype and mutant Axl transfected cells determined that Axl directly binds growth factor receptor-bound protein 2 (Grb2) at pYVN and the p85 subunit of phosphatidylinositol-3 kinase (PI3 kinase) at two pYXXM sites (pY779 and pY821). Also, p85 can indirectly bind to Axl via an interaction between p85's second proline-rich region and the N-terminal SH3 domain of Grb2. Further, Grb2 and p85 can compete for binding at the pY821VNM site. Gas6-stimulation of Axl-transfected COS7 cells recruited activated PI3 kinase and phosphorylated Akt. An interaction between Axl, p85 and Grb2 was confirmed in brain homogenates, enriched populations of O4+ oligodendrocytes, and O4– flow-through prepared from day 10 mouse brain, indicating that cells with active Gas6/Axl signal through Grb2 and the PI3 kinase/Akt pathways.
Keywords: Akt signaling, Axl receptor tyrosine kinase, growth arrest-specific protein 6, growth factor receptor-bound protein 2, oligodendrocytes, p85 subunit of phosphatidylinositol-3 kinase
Axl, Tyro3, and Mer, comprise a family of cell adhesion molecule-related receptor tyrosine kinase. The receptors are expressed on cells of the immune, nervous, and reproductive systems where co-expression of one or more family member is often detected on the same cell type (Lai and Lemke 1991; O'Bryan et al. 1991; Graham et al. 1994; Lai et al. 1994; Lemke and Lu 2003). The focus of our studies was to examine growth arrest-specific protein 6 (Gas6)/Axl signaling in the nervous system. During rat brain development and myelination, in situ hybridization determined that Axl is highly expressed in oligodendrocytes and neurons (Prieto et al. 2000). Using an O4 antibody specific to a sulfatide on the surface of oligodendrocytes (Sommer and Schachner 1981; Hajihosseini et al. 1996) our laboratory determined that O4+ immunopanned oligodendrocytes express Axl by microarray analysis, RT-PCR, and western blot analysis (Shankar et al. 2003). Also, the Axl receptor is expressed in Schwann cells, and endothelial cells (Li et al. 1996; Avanzi et al. 1998).
In the nervous system, the ligand for Axl, Gas6, is secreted by neurons and endothelial cells (Stitt et al. 1995; Varnum et al. 1995; Avanzi et al. 1998; Allen et al. 1999). While many cell types, including oligodendrocytes, express all three family members, receptor activation in response to Gas6 is Axl > Tyro3 > Mer (Nagata et al. 1996). Gas6 is the sole ligand for Axl, binding to the two Ig domains of Axl (Ebner et al. 2000). The major Gas6-binding site is found on the Axl receptor, while a minor Gas6-binding site is conserved by Tyro3 and Mer (Goruppi et al. 1999). Gas6 is widely expressed in the CNS and PNS suggesting that the interaction between Gas6 and its receptors has physiologically relevant functions (Li et al. 1996; Prieto et al. 2000).
Our laboratory determined that Gas6/Axl activation protects oligodendrocytes from the effects of growth factor withdrawal. Gas6-stimulated oligodendrocyte cultures treated with tumor necrosis factor α (TNFα), or serum starved were protected from caspase 3 activation and cell death. The effect was blocked with Axl-Fc decoy, or in the presence of the phosphatidylinositol-3 kinase (PI3 kinase) inhibitors LY294002 and wortmannin. Also, the addition of recombinant human Gas6 (rhGas6) to the cultures significantly increased the levels of phosphorylated Akt on the oligodendrocyte cell membrane indicating that Gas6/Axl signals through the PI3 kinase/Akt survival pathway (Shankar et al. 2006).
Gas6 activation of Axl results in autophosphorylation of tyrosine residues 779 (pYALM; site 1), 821 (pYVNM; site 2), and 866 (pYVLC; site 3) generating consensus sites within the cytoplasmic domain of Axl to recruit signaling molecules through consensus Src homology 2 (SH2) domains (Kazlauskas 1994; Heldin 1995; Pawson 1995; Ling et al. 1996; Fridell et al. 1998; Heiring et al. 2004). Our ongoing studies focus on Axl's role in CNS cell survival. As part of our analysis, we sought to identify binding partners for Axl in the CNS. We explored the binding of downstream signaling molecules to two of the consensus phosphotyrosine (pY) motifs following Axl autophosphorylation, at amino acids 779–782 and 821–824. We examined whether the SH2 domains of the p85 regulatory subunit of PI3 kinase and the adaptor protein growth factor receptor-bound protein 2 (Grb2) bind Axl, and whether these interactions occur in the brain. PI3 kinase consists of a p85 regulatory subunit (p85) and a p110 catalytic subunit (p110) that, when activated, signal to the Akt survival pathway (Fry 1994). The two subunits, p85 and p110, are present in mammalian tissue in a 1 : 1 ratio, with no free subunits that could negatively regulate PI3 kinase activity (Geering et al. 2007). Activation of PI3 kinase allows for the conversion of the lipid signaling molecule PIP2 to PI(3,4,5)P3, which then recruits Akt to the plasma membrane, where it is phosphorylated (threonine 308) and activated by phosphoinositide-dependent kinase 1 (Cantley 2002). Grb2 can signal to the Akt pathway and the MAPK extracellular regulatory kinase (ERK) 1/2 proliferative pathway; for review see (Tari and Lopez-Berestein 2001). In this study, we examined whether Grb2 and p85 can directly bind Axl following Axl autophosphorylation. In addition, we examined whether p85 can bind the SH3 domain of Grb2, indirectly allowing for the recruitment of p85 to Axl.
Experimental procedures
Cell culture, transient transfections, and protein extractions
COS7 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen) at 37°C in a 5% humidified CO2 atmosphere. For transient transfections, COS7 cells were plated at a density of 2 × 106 cells/60-mm dish the day before transfection. Four micrograms of the respective cDNAs were mixed with Lipofectamine 2000 (Invitrogen) in serum-free medium. After 4 h, 2 mL of DMEM in the absence of serum (serum starvation) or 10% serum was added for a total volume of 4 mL. Twenty-four hours later, cells were incubated with 200 ng/mL (2.6 nM) rhGas6 (AMGEN, Thousand Oaks, CA, USA) for 30 min at 37°C, and protein homogenates were prepared (see below).
Mutagenesis
To determine the binding sites on Axl for p85 and Grb2, site-directed mutagenesis of Axl cDNA at several amino acids surrounding the autophosphorylated tyrosines were performed. These mutations were within two of Axl's YXXM binding motifs, at residues 779 (YALM) and 821 (YVNM). The methionines were mutated to isoleucines (YALM → YALI and YVNM → YVNI). This substitution was chosen because of the similarities in their structures; both amino acids are hydrophobic and neutral at physiological pH. Also, the SH2 domain of p85 has no binding affinity for a pY motif containing an isoleucine at position Y + 3 (Songyang et al. 1993). Another generated and cloned mutant Axl cDNA consisted of a mutation of asparagine to glutamine (N → Q) in the YVNM consensus sequence; both amino acids are neutral and hydrophilic at physiological pH. Mutant forms of human Axl were made by PCR cloning as described in the QuickChange site-directed mutagenesis kit instruction manual (Stratagene, La Jolla, CA, USA). Double and triple mutations were performed sequentially. Constructs were sequenced to verify the mutations and to confirm the correct open reading frame. Western blot analysis was performed using an Axl polyclonal antibody (pAb; AMGEN) to confirm full-length Axl expression.
Fusion protein nomenclature
Human p85α constructs consisted of: p85(1–433): includes amino acids 1 through 433 of p85, containing the SH3 domain, 2 proline-rich domains, the bcr homology domain and the N-terminal SH2 domain; p85(1–333) (Hill et al. 2001): includes amino acids 1 through 333 of p85, and there lacks the N-terminal SH2 domain; p85Δpro1: same as p85(1–333) except for a deletion of the N-terminal proline-rich region (referred to as the first proline-rich region); p85Δpro2: same as p85(1–333) except for a deletion of the C-terminal proline-rich region (referred to as the second proline-rich region); p85Δpro1 + 2: same as p85(1–333) except for a deletion of both the N- and C-terminal proline-rich regions. Grb2 constructs consisted of: Grb2-FL consisting of full-length Grb2; Grb2-SH3N: contains only the N-terminal SH3 domain of Grb2; Grb2-SH3C: contains only the C-terminal SH3 domain of Grb2. All fusion proteins are glutathione S-transferase (GST)-tagged.
GST-mediated binding assays, western blot analysis and antibodies
Following transient transfection COS7 cells were lysed on ice with immunoprecipitation (IP) buffer (140 mM NaCl, 1 mM Tris, pH 7.4) containing 0.5% Triton X-100 and protease inhibitors [2 lg/ mL leupeptin; 2 mM ethylene glycol-bis(b-aminoethylether)-N,N,N′,N′-tetraacetic acid; 4 μg/mL pepstatin; 5 mM sodium pyrophosphate; 30 mM β-glycerophosphate; 30 mM sodium fluo-ride; 100 mM sodium orthovanadate; 100 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride]. The homogenates were cleared by centrifugation at 4°C at 300 g, and the supernatants were incubated with GST fusion proteins bound to glutathione-sepharose beads (GE Healthcare, Piscataway, NJ, USA) overnight at 4°Con a nutator. Subsequently, samples were washed five times in IP buffer, boiled for 5 min in 2X protein dye, spun at 7000 g, and the supernatants were separated in a 10% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE). Following electrophoresis, the proteins were transferred to nitrocellulose (Towbin et al. 1979). The blots were incubated with 5% non-fat dry milk and 5% goat serum in 1X Tris-buffered saline for 1 h at 22°C (Zamora-Leon et al. 2005). After blocking, the membranes were incubated with the respective primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies (Laemmli 1970; Albala et al. 1995). Primary monoclonal antibody (mAb) and pAb included: Axl pAb (1 : 1000), DT-12, a mAb generated to GST protein, provided by Dr Peter Davies, Albert Einstein College of Medicine (IgG1, 1 : 100), Grb2 mAb (IgG1, 1 : 5000; Transduction Laboratories, Lexington, KY, USA), HA mAb (IgG2A, 1 : 1000), myc mAb (IgG2A, 1 : 20 000; Cell Signaling, Beverly, MA, USA), p85 pAb (1 : 500), p-Akt Thr308 pAb (IgG, 1 : 1000; Cell Signaling), β-actin mAb (IgG2A, 1 : 5000; Sigma, St Louis, MO, USA). Secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) included: goat α-rabbit IgG (1 : 10 000), and goat α-mouse IgG1 (1 : 10 000), and IgG2A (1 : 10 000). The p85 pAb and the HA mAb were generated in the laboratory of Dr Jonathan Backer. The p85 pAb was generated to a fusion protein of p85 containing the N-terminal SH2 domain. The antibody recognizes p85 as well as GST. Visualization of all secondary antibodies was by enhanced chemiluminescence (GE Healthcare) except where noted in the figure legend.
To test the direct protein–protein interaction of Grb2 and p85, Grb2-GST fusion proteins were incubated with thrombin cut p85(1–433)-GST fusion proteins in vitro. First, the p85(1–433) construct was incubated with 1unit thrombin/20 μg p85(1–433) overnight at 22°C and the cleavage reaction was stopped by adding 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride to 1 mM. Four micrograms of either Grb2-GST or GST only were incubated with 30 ng of either cut p85(1–433) or uncut p85(1–433) for 1 h at 4°C on a nutator, followed by the addition of 12 lL glutathionesepharose beads (GE Healthcare) for 30 min. Subsequently, samples were washed five times in IP buffer and prepared as described for the GST-mediated binding assay above. The p85 pAb that recognizes the first SH2 domain of p85 was used to detect p85(1– 433). All other primary and secondary antibodies used are described above.
O4+ and O4– immunopanning and magnetic associated cell sorting
A standard protocol to purify oligodendrocytes by immunopanning with the O4 mAb was used (Gard and Pfeiffer 1989). O4 recognizes a sulfatide on the oligodendrocyte cell surface and does not cross-react with other cell types (Sommer and Schachner 1981; Hajihosseini et al. 1996). Following O4 binding, cells were incubated with an IgM isotype-specific secondary antibody linked to magnetic beads, allowing for viable oligodendrocytes to be purified by column binding, and subsequent elution (Supermacs; Miltenyi Biotech; Auburn, CA, USA) (Bansal et al. 1989; Gard and Pfeiffer 1990; Shankar et al. 2003). Enriched populations of O4+ oligodendrocytes, and O4– cells, were prepared from mouse brain at post-natal day 10. Following the removal of the meninges, 8–10 brains were pooled in calcium/magnesium free Hank's balanced salt solution (HBSS; Invitrogen, Grand Island, NY, USA). The tissue was minced and incubated with pre-warmed 0.025% trypsin/ 0.1 mM EDTA solution (Sigma) at 37°C for 20 min. Trypsinization was terminated by the (1 : 1) addition of 25 lg/mL soybean meal trypsin inhibitor (Sigma) for 2 min at 22°C, followed by the addition of 80 μg/mL Dnase I in HBSS for 5 min at 37°C. The tissue was centrifuged at 150 g for 5 min at 22°C, suspended in HBSS containing 80 μg/mL Dnase I, and 3.9% MgSO4, mechanically triturated by pipetting up and down 15 times, centrifuged at 150 g for 5 min at 22°C, and resuspended in cell-sorting buffer (1X phosphate-buffered saline, 2 mM EDTA, 0.5% bovine serum albumin). The cell suspension was filtered two times through a 40 μm nylon cell strainer to eliminate debris and cells larger than 40 μm (BD Biosciences, Bedford, MA, USA). The cells that passed through the strainer were counted, and incubated with sodium azide-free O4 mAb for 5 min on ice (Gard et al. 1988; Gard and Pfeiffer 1989; Pfeiffer et al. 1993). The cells were centrifuged at 300 g for 10 min, resuspended in cell sorting buffer at 80 μL/107 cells, and incubated on ice for 15 min with rat anti-mouse IgM-conjugated magnetic microbeads (20 μL/107 cells; Miltenyi Biotech). The cells were washed with 10 mL of cell-sorting buffer, centrifuged at 300 g for 10 min, resuspended in 0.5 mL of cell-sorting buffer, and separated on a ferromagnetic column by positive selection. This column sorts live cells according to cell surface markers (LS column; Miltenyi Biotech). The initial column flow through contained O4– cells, and this population was retained and assayed in parallel with the O4+ oligodendrocytes that were released from the column, once the column was removed from the magnet. Flow analysis, of one experiment, using an Alexa 488 antibody to detect O4 antibody determined that the O4+ population was 82% pure. It was determined by western blot analysis using antibodies for CD11b and glial fibrillary acidic protein that the O4– flow through population contained astrocytes and microglia (data not shown). The cells of each flow through were counted and resuspended at 3 × 106 cells/mL in HBSS containing 67 mM HEPES/6.7 mM sodium vanadate for 30 min at 37°C. After 30 min, 200 ng/mL rhGas6 was added to suspended cells and incubated for an additional 30 min at 37°C. The cells were centrifuged at 300 g for 5 min and protein homogenates were prepared as previously described.
Immunoprecipitations
Protein homogenates prepared from COS7 cells were lysed with IP buffer. For analysis of the total homogenates 20 μg from each sample were removed (unless otherwise stated in the figure legend), prior to addition of IP antibodies. Homogenates were pre-cleared overnight with an IgG1 irrelevant antibody, DT-12 and protein G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA) on a nutator at 4°C. Following the pre-clear, homogenates were spun at 300 g for 1 min in a microcentrifuge at 4°C, removed from protein G agarose beads, split into two equal parts, and each part was incubated with either the Axl mAb (5.76 μg; AMGEN) or DT-12 (5 μg) for 1 h. Protein G agarose beads were then added, and the samples were incubated overnight at 4°C on a nutator. Samples were washed five times in IP buffer. After each wash the samples were centrifuged at 300 g for 1 min in a microcentrifuge at 4°C. Following the washes and centrifugation, samples were boiled for 5 min in 2X β-mercaptoethanol-containing protein load dye, spun at 7000 g for 2 min, and the supernatants were separated in 10% SDS–PAGE and transferred to nitrocellulose.
PI3 kinase activity assay
COS7 cells were plated overnight at 2 × 106 in T75 flasks (for nonconfluency), transfected with Axl cDNA (8 μg) and serum starved overnight. DMEM media without serum was replaced with fresh DMEM media without serum, plus (+Gas6 and IgG control) or minus (No Gas6) 200 ng/mL (2.6 nM) rhGas6 for 1 min. Each condition was done in duplicate (IgG control) or in triplicate (+ or No rhGas6 with Axl mAb). Homogenates were prepared as described above, 20 μg were removed to test transfection efficiency, and homogenates were pre-cleared with DT-12 for 1 h, as described above. Following centrifugation, the homogenates were removed from the protein g agarose beads, and rhGas6 treated and untreated samples were incubated overnight on a nutator at 4°C with either the Axl mAb (5.76 μg; R&D Systems, Minneapolis, MN, USA) or DT-12 (IgG control). Following the overnight incubation, protein G agarose beads were added and rocked for 3 h at 4°C. The beads were washed three times with phosphate-buffered saline/1% Nonidet P-40 (Sigma), three times with 100 mM Tris pH 7.5, 500 mM LiCl2, two times with 10 mM Tris pH 7.5, 100 mM NaCl, 1 mM EDTA, and then resuspended in 60 μL of the final wash buffer, and the lipid kinase activity was assayed using phosphatidylinositol as a substrate as previously described (Layton et al. 1998; Yu et al. 1998). The complexes were incubated briefly in the presence of 10 mM MgCl2, 20 μg sonicated PI, and 100 lMATP containing 10 μCi [32P]ATP (New England Nuclear, Boston, MA, USA) for 10 min with agitation at 22°C. The reaction was stopped with 20 μL 8 N HCl, and the lipids were extracted with 160 μL chloroform : methanol (1 : 1). A 50 μL aliquot of the lower phase was spotted on baked (1 h at 100°C) Whatman indicator-free aluminum-backed silica plates, which were then developed in chloroform : methanol : water : ammonium hydroxide (60 : 47 : 11.3 : 2), dried, and analyzed using Phosphorimager cassettes (Molecular Dynamics, Sunnyvale, CA, USA).
Far Western
COS7 cells were transfected with wildtype (WT); I,I; or I,QI Axl, or empty vector cDNA, and cells were serum starved overnight. Cells were lysed and the protein homogenates were incubated with either the Axl mAb, or the DT-12 mAb, conjugated to protein G agarose beads. IP's, washes, gel electrophoresis and transfer to nitrocellulose were performed as described above with one difference; each of the immunoprecipitated (IP’ed) products were divided in half and duplicate blots were analyzed. One blot was pre-incubated with 5% non-fat dry milk, 5% goat serum, and 2% bovine serum albumin in 1X Tris-buffered saline (far western buffer) for 1 h, followed by incubation with 20 μg/mL GST-p85(1–433) at 22°C for 2 h. The blot was then washed with far western buffer, and incubated with a GST mAb (1 : 100), followed by incubation with horseradish peroxidase-conjugated goat-anti-mouse IgG1 secondary antibody; visualization was by enhanced chemiluminescence. The second blot was incubated with the Axl pAb to confirm efficient IP of Axl.
Results
Upon treatment with rhGas6, Axl undergoes autophosphorylation at tyrosines 779, 821, and 866. Surrounding these tyrosines are putative consensus SH2-binding motifs for the p85 regulatory subunit of PI3 kinase at 779 (pYALM) and 821 (pYVNM), a consensus Grb2-binding motif (pYVN) imbedded within the motif beginning at amino acid 821, and a third putative SH2 binding motif beginning at amino acid 866 (pYVLC). To determine the binding of active Axl to downstream signaling molecules, Axl cDNA was transfected into COS7 cells, treated with rhGas6 and pull-down assays were performed on total protein homogenates. This was accomplished by incubating the total protein homogenates with GST-fusion proteins expressing the SH2 domains of the p85 regulatory subunit of PI3 kinase, Abl, Abelson-related tyrosine kinase (ARG), phospholipase C gamma (PLCγ), p190RhoGAP and Grb2, coupled to glutathione agarose beads. Complexes between Axl and these select SH2 domains were examined by immunoblotting with an Axl pAb. Figure 1a shows that Axl associates with the SH2 domains of p85, ARG, and Grb2. Conversely, the SH2 domains of Abl, PLCγ, and p190RhoGAP do not bind with Axl. Additional pull-downs failed to show an interaction between activated Axl and Src, Lyn, or Fyn (data not shown).
Fig. 1.
p85 and Grb2 pull-down and co-IP with Axl. (a) Pull-downs were performed from Axl transfected COS7 cell homogenates using GST-SH2 domain fusion proteins. Confirmation of Axl in the pull-down was performed by western blot analysis using an Axl pAb (1 : 1000). Total Homog refers to the 140 kDa Axl protein in Axl transfected protein homogenate. Visualization was by enhanced chemiluminescence. (b) Axl and ARG co-transfected COS7 cell homogenates were IP’ed with DT-12, an irrelevant IgG1 mAb (negative control), an ARG pAb, or an Axl mAb. Western blot analysis was performed using an ARG pAb (1 : 1000). (c) Axl and p85 co-transfected COS7 cell homogenates were IP’ed with either the Axl mAb or the irrelevant IgG1 mAb as described in (b). Western blot analysis confirms the presence of Axl (c-i; Axl pAb, 1 : 1000), p85 (c-ii; p85 pAb, 1 : 400), and Grb2 (c-iii; Grb2 mAb, 1 : 5000). (d) Pull-downs were performed on GST-SH2 domain fusion proteins and protein homogenates from WT or YVQM mutant Axl transfected COS7 cells. Confirmation of Axl in the pull-down was performed by western blot analysis using an Axl pAb (1 : 1000). The lower panel shows the 26 kDa GST-protein and the GST-expressed fusion protein using a GST mAb (DT-12, 1 : 100). (e) Axl and p85 co-transfected cells were serum starved overnight, washed and administered DMEM plus or minus 200 ng/mL rhGas6 for 30 min. IP's were performed as detailed above. Blots were incubated with either an Axl pAb or a Grb2 mAb. The amount of IP’ed p85 and Grb2 was normalized to the total amount of the respective protein expressed in 20 μg of total protein (lighter exposure used for Grb2 normalization not shown) relative to β-actin and the amount of IP’ed Axl (top panel). (f) PI3 kinase activity increased following rhGas6 stimulation of Axl transfected COS7 cells. T75 flasks of subconfluent COS7 cells were transfected with Axl, serum starved, and stimulated for 1 min plus and minus rhGas6. Total homogenates were IP’ed with an Axl mAb (+Gas6, No Gas6), or DT-12 (IgG Control), and PI3 kinase activity assays were performed. Western blot analysis using an Axl pAb and b-actin mAb on 20 lg of total homogenates shows equal transfection efficiency (f-i), and using an Axl pAb shows the ability of the Axl mAb to IP Axl (f-ii). f-iii shows a two-fold increase in activity in +Gas6 samples relative to the unstimulated samples. Values are means ± SEM combined from three independent experiments (n = 3 for No Gas6 and +Gas6; n = 2 for IgG control for each experiment). Asterisk represents significance (p = 0.023) between No Gas6 and +Gas6.
To confirm the interactions with full-length molecules, Axl was co-transfected into COS7 cells with full-length ARG, or p85, and co-IPs on the protein homogenates were performed with an Axl mAb. Although the SH2 domain of ARG binds to Axl in pull-down assays, Fig. 1b shows that full-length ARG did not co-IP with Axl in rhGas6 stimulated COS7 cells co-transfected with Axl and ARG. Figure 1c shows that Axl co-IPs with p85 (Fig. 1c-ii) and endogenous Grb2 (Fig. 1c-iii). Neither p85 nor Grb2 were IP’ed when a negative control IP was performed using an irrelevant isotype-specific IgG1 antibody. Note that Axl is efficiently IP’ed by the Axl mAb (Fig. 1c-i). In our studies, the Axl band often appears as a doublet because, in vivo, Axl is glycosylated (Heiring et al. 2004).
The SH2 domain of Grb2 can bind to proteins containing a consensus pYXN motif, therefore, to determine whether Grb2 binds directly to Axl only at pYVN, COS7 cells were transfected with either WT Axl, or Axl with a mutation at the YVNM motif (YVQM). Fig. 1d shows the asparagine of pYVN is critical for Grb2 binding; Grb2 binds to WT but not pYVQM Axl. SH2-ARG binds to both WT and YVQM mutant Axl, and PLCγ binds to neither. This confirms that the SH2 domain of Grb2 directly binds to Axl via the consensus pYVN motif.
It is well established that Gas6 stimulates Axl and results in autophosphorylation of putative SH2 binding domains. To confirm that Axl forms complexes with SH2-containing proteins following Gas6 stimulation, Axl and p85 co-transfected COS7 cells were stimulated with rhGas6 and the amount of p85 and Grb2 that co-IP’ed with Axl was compared with unstimulated co-transfected COS7 cells. Figure 1e shows that more p85 co-IP’ed with Axl following a 30 min stimulation with rhGas6, than without rhGas6. Although p85 is over-expressed in these cells, we still detected twofold more Grb2 that co-IP’ed with Axl following stimulation with rhGas6 versus no stimulation, demonstrating that Gas6 enhances the ability of Axl to bind SH2-containing proteins. Therefore, we routinely treated cells and tissue with rhGas6 to maximally activate Axl and further enhance the interactions between activated Axl and its binding partners.
As rhGas6 administration activates Axl and leads to binding of SH2 domain containing proteins we examined whether the p85 being recruited to Axl was active, by performing PI3 kinase activity assays. COS7 cells were plated at low confluency, to prevent intercellular autophosphorylation of Axl that can occur when cells are highly confluent, transfected with Axl cDNA (8 μg), and serum starved overnight. Then cells were either stimulated with rhGas6 for 1 min (+Gas6) or left unstimulated (No Gas6). Total homogenates were prepared and incubated with an Axl mAb, or, in duplicate, a +rhGas6 sample was incubated with an irrelevant isotype control antibody (DT-12). Figure 1f-i shows that when 20 μg of total homogenate was loaded in each lane the Axl transfection efficiency was similar in all replicates (β-actin is the lane load control). Figure 1f-ii shows that Axl was efficiently IP’ed with the Axl mAb compared with an irrelevant isotype control antibody (DT-12; control IP). When we compared the relative PI3 kinase activity of the Axl transfected rhGas6 stimulated COS7 cells with the unstimulated Axl transfected COS7 cells significantly more active PI3 kinase IP’ed with Axl from rhGas6 stimulated Axl transfected COS7 cells (Fig. 1f-iii). These data in Fig. 1 demonstrate that rhGas6 stimulation leads to activation and autophosphorylation of Axl, and subsequently, recruitment of active PI3 kinase, and Grb2.
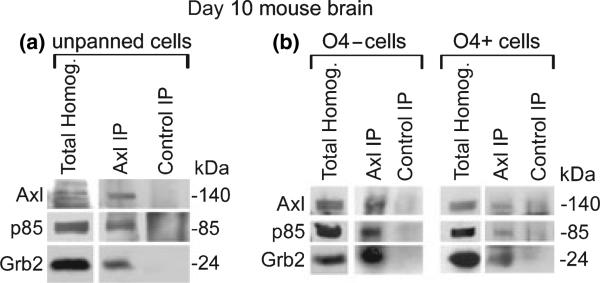
Based on these experiments, we examined the expression and interaction of Axl and its binding partners in the CNS. In our previous studies we detected Axl expression on human oligodendrocytes in fetal spinal cord tissue by immunofluorescent microscopy, and showed Axl expression in post-natal day 10 mouse brain homogenates by immunoblot analysis (Shankar et al. 2003). Therefore, we selected day 10 mouse brain to determine whether Axl co-IP's with p85 and Grb2 in total brain homogenates, and O4+ (oligodendrocytes) and O4– cell subpopulations. Figure 2a shows that in total brain homogenate, Axl, p85, and Grb2 co-IP’ed with an Axl mAb, but not with DT-12 (control IP).
Fig. 2.
p85 and Grb2 co-IP with Axl from O4+ and O4– protein extracts prepared from post-natal day 10 mouse brain. Cells from total brain, O4– flow through, and O4+ immunopanning were stimulated in suspension with rhGas6 for 30 min at 37°C. Homogenates from post-natal day 10 mouse brain (a) total brain; (b) O4+ and O4– Homogenates, were incubated with either an Axl or DT-12 (irrelevant IgG1 negative control antibody) mAb. Total homogenates and IP’ed proteins were separated by SDS/PAGE and transferred to nitrocellulose. Western blot analysis of total homogenates (40 μg) with the Axl pAb (1 : 1000) confirms the presence of Axl in total brain, O4+, and O4– populations. The blot was cut and immunoblot analysis was performed using Axl pAb (1 : 1000), p85 pAb (1 : 500), and Grb2 mAb (1 : 5000). A faint background band only in the O4+ DT-12 control lanes was consistently observed and most likely is a result of an interaction between residual O4 antibody (IgM) used during immunopanning and the DT-12 antibody (IgG1) used in the control IP. IP buffer with O4 and IgG1 antibody, but no protein shows a similar faint background band, and pre-clearing with Protein-l agarose beads did not eliminate the faint background (data not shown). Visualization was by enhanced chemiluminescence, except for Grb2 which was visualized by Super-signal west femto (Pierce, Rockford, IL, USA).
Once it was determined that the Axl/p85/Grb2 interactions occur in brain homogenates, O4+ oligodendrocytes were panned from day 10 mouse brain, and the O4+ and O4– populations were examined. Using these two separate CNS populations, we sought to determine whether Axl interacts with the same binding partners in these subpopulations as in the total homogenates from mouse brain and COS7 cells. As shown in Fig. 2b, O4+ and O4– protein homogenates express Axl; and p85 and Grb2 co-IP’ed with Axl when IP’ed with an Axl mAb, but not with the control antibody, DT-12. These data show that the Axl receptor interacts with p85 and Grb2 in multiple CNS populations, including oligodendrocytes (O4+).
To determine the specific site(s) on Axl that interact with p85 and Grb2, we generated a series of Axl mutants that eliminated one or both of the putative p85-binding motifs.
Figure 3a shows a schematic of the various Axl mutants in which the consensus YXXM motif was mutated to YXXI at one or both sites (YALI; YVNI), and the consensus Grb2-binding motif YVN was mutated to YVQ (YVQM). Axl constructs were transfected into COS7 cells and the p85 and Grb2 interactions with Axl were examined by both pull-downs and IPs. Figure 3b demonstrates that all Axl mutants with the exception of I,QI are pulled down with SH2-p85. To our surprise, p85 was able to bind and pull-down the I,I Axl mutant even though methionine at the Y + 3 position is known to be preferred for p85 binding at pYXXM. To confirm the pull-down assays, the Axl constructs and full-length HA-tagged p85 were co-transfected into COS7 cells. As demonstrated in Fig. 3c, when a single pYXXM-binding site was mutated on Axl, either YALI or YVNI, the mutant Axl still interacted with p85, presumably at the unaltered pYXXM site. Consistent with the pull-down data in Fig. 3b, p85 co-IP’ed with the Axl double mutant I,I; but did not co-IP with the Axl triple mutant construct I,QI, which is unable to bind Grb2, suggesting that p85 is able to indirectly bind to Axl via Grb2. Based on the results from Fig. 3b and c, we examined whether p85 directly bound Axl in the absence of the Grb2 binding site (pYXN). To accomplish this, HA-tagged full-length p85, and WT or Axl mutants were co-transfected into COS7 cells, the homogenates were IP’ed with the Axl mAb, and blots containing the IP’ed proteins were incubated with an HA mAb. As shown in Fig. 3d, p85 IP’ed with WT; I,QM; M,QM; and M,QI Axl; whereas, endogenous Grb2 only bound to WT Axl. Thus, p85 can bind both directly to Axl as well as indirectly via Grb2. To further substantiate that when the two methionines are mutated p85 cannot bind directly to Axl, we performed a far western in which purified p85(1–433)-GST fusion protein was incubated with a blot containing IP’ed WT Axl; I,I; and I,QI mutant Axl, and empty vector. As shown in Fig. 3e (upper panel) p85 bound only to WT Axl. The lower panel of Fig. 3e shows the transfection and IP efficiency of Axl.
Fig. 3.
Transfection of WT and Axl mutant constructs into COS7 cells, followed by pull-downs and IPs, demonstrate that p85 binding to Axl is both a direct and indirect interaction. (a) Schematic showing the three tyrosine residues (779, 821, and 866) on Axl that are autophosphorylated following Gas6 binding to the extracellular IgG domains. Seven mutant Axl constructs were generated and sequenced. The single Axl mutants (YVNI; YALI; and M,QM); double Axl mutants (I,I; I, QM; and M,QI); and the triple Axl mutant (I,QI) are shown. For details see Experimental procedures. (b) WT and Axl mutant constructs were transfected into COS7 cells and protein homogenates were incubated with SH2-p85-GST fusion protein, or GST-only protein (negative control). Western blot analysis confirmed the presence of Axl in the WT Axl Total homogenate. DT-12, an IgG1 antibody to GST (1 : 100), identifies GST proteins and the GST fusion proteins. The M,QI and I,QI Axl mutant pull-downs were done on a different blot than the rest of the Axl mutants, and therefore, have a separate WT positive control. (c) Protein homogenates from serum-starved COS7 cells co-transfected with Axl constructs and HA-tagged full-length p85 were IP’ed with the Axl mAb, or DT-12. Western blot analysis was performed with an HA mAb (top; 1 : 1000). The lower panel was incubated with an Axl pAb (1 : 1000) and an HA mAb confirming the expression of Axl and HA-p85 in the total protein homogenates (30 μg); β-actin (1 : 5000) was used as a loading control. (d) Serum starved COS7 cells were co-transfected with HA-tagged full-length p85, and WT or mutant Axl constructs. Protein homogenates were IP’ed with an Axl mAb or DT-12. The blot was cut and western blot analysis was performed with an Axl pAb (1 : 1000), HA mAb (1 : 1000), and a Grb2 mAb (1 : 5000). The Axl pAb confirms the presence of Axl in the total homogenates (5 μg) and in the IP. Because of the low amount of protein loaded in the total homogenate lanes the exposure time for the total homogenate lanes with the HA mAb was 90 s whereas the exposure time was 5 s for the IP lanes (Axl and control). All other antibodies had the same exposure time for their respective total homogenates and IP lanes. (e) Serum starved COS7 cells were transfected with WT or mutant Axl constructs, or empty vector controls. Protein homogenates were IP’ed with an Axl mAb or DT-12. Blots were incubated with GST-p85(1-433) fusion protein and subsequently incubated with a GST mAb (1 : 100), to detect p85-GST bound to Axl at 140 kDa. Visualization was by enhanced chemiluminescence.
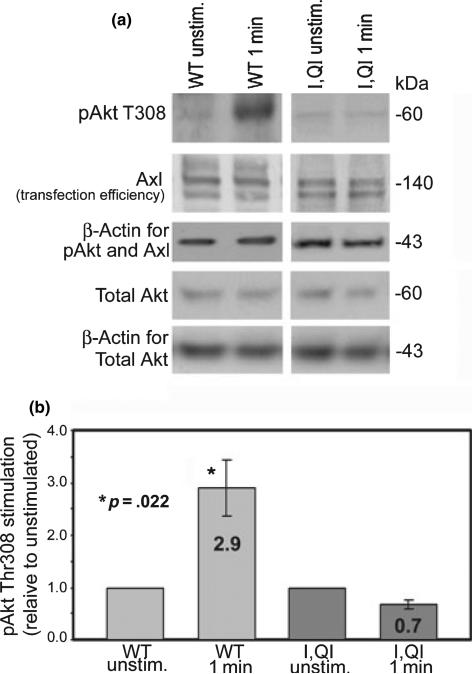
To determine whether the binding of endogenous p85/ p110 to Axl leads to activation of Akt, COS7 cells were transfected with WT or I,QI mutant Axl and either left unstimulated, or stimulated for 1 min with rhGas6. When normalized to β-actin as the loading control, COS7 cells transfected with WT Axl, and rhGas6 stimulated had a significant 2.9-fold increase in p-Akt at threonine 308 relative to unstimulated WT Axl transfected COS7 cells. In addition, COS7 cells transfected with I,QI mutant Axl showed no significant increase between unstimulated and rhGas6 stimulated COS7 cells (Fig. 4). This demonstrates that active Axl binds active PI3 kinase which relays signals resulting in activation of Akt by phosphorylation of Akt at threonine 308.
Fig. 4.
A 1-min rhGas6 stimulation of WT Axl transfected COS7 cells results in a 2.9-fold increase in Akt phosphorylation at Thr308 relative to unstimulated WT Axl expressing cells; there was no increase in rhGas6 stimulated I,QI mutant Axl transfected COS7 cells relative to its unstimulated counterpart. COS7 cells were transfected with WT or I,QI mutant Axl, serum starved overnight, left unstimulated or stimulated for 1 min with rhGas6. After 1 min, the media was removed, replaced by 1X Tris-buffered saline, and protein homogenates were prepared immediately. (a) Representative experiment of western blots; p-Akt T308 (1 : 1000), Axl (1 : 1000), total Akt (1 : 1000), and β-actin (1 : 5000, load control). (b) Values are means ± SEM combined from three experiments. Asterisk represents significant increase (p = 0.022) of pAkt Thr308 levels in 1 min rhGas6 stimulated WT Axl transfected COS7 cells versus unstimulated WT Axl transfected COS7 cells. There is no significant increase between stimulated or unstimulated I,QI mutant Axl transfected COS7 cells. Significance is based on three experiments.
To further examine the binding of Grb2 to Axl in the presence of p85, COS7 cells were co-transfected with p85 plus WT or mutant Axl constructs, and IP’ed with Axl. As shown in Fig. 5 (+p85 panel), Grb2 co-IP’ed with WT Axl, single Axl mutant YVNI, and the double Axl mutant (I,I). Grb2 did not co-IP with Axl when pY site 1 (pYALM) was mutated to YALI, or with the triple mutant, I,QI. This suggests that in the presence of excess p85, Grb2 can be blocked from binding to pY site 2 (pYVNM). COS7 cells expressing endogenous Grb2 and p85 transfected with WT or mutant Axl constructs, (Fig. 5, –p85 panel), IP’ed with the Axl mAb, and immunoblotted with a Grb2 mAb demonstrated that Grb2 bound WT Axl and all Axl mutant proteins, except the triple mutant, I,QI. This demonstrates that when p85 is bound to Axl at pY site 2 (pYVNM) p85 does not interact with Grb2 and Grb2 does not bind at an alternative site, however, as shown in Fig. 3b and c, when the SH2 domain of Grb2 is bound to Axl, Grb2 can interact and recruit p85.
Fig. 5.
Transfection of WT, or mutant Axl constructs, plus and minus full-length p85, show a competition between p85 and Grb2 for the pYVNM site on Axl. WT and Axl mutant constructs were co-transfected into serum starved COS7 cells with HA-tagged p85 (+p85) or empty vector (–p85) cDNA constructs. Protein homogenates were IP’ed with an Axl mAb. Western blot analysis was performed using the Grb2 mAb (1 : 5000). In the total homogenates, the presence of Axl was determined using an Axl pAb (1 : 1000).
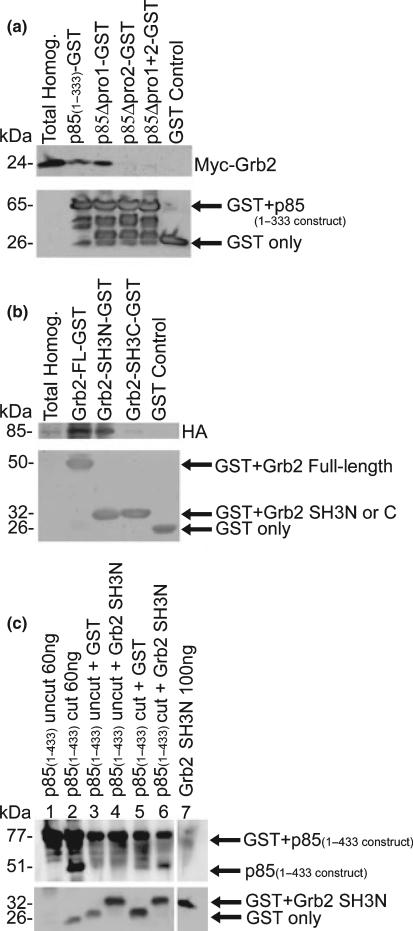
To define the sites of interaction between Grb2 and p85, full-length myc-tagged Grb2 was transfected into COS7 cells, and pull-downs were performed on protein homogenates using p85(1–333)-GST fusion proteins or one of several p85(1–333)-GST mutants lacking one or both of the proline-rich regions. The p85(1–333)-GST fusion protein spans amino acids 1–333 of p85 containing both the first and second proline-rich regions. The p85(1–333)-GST mutant constructs contain deletions (Δ) of the proline-rich region 1 (p85Δpro1-GST), the proline-rich region 2 (p85Δpro2-GST), or both proline-rich regions (p85-Δpro1 + 2-GST). Figure 6a shows that WT, and p85Δpro1-GST are equally efficient at binding and pulling down Grb2. GST-only, p85Δpro2-GST, and p85Δpro1 + 2-GST fusion proteins did not bind and pull-down Grb2. These data demonstrate that the second proline-rich region of p85 is required for binding full-length Grb2. To examine the p85-binding site on Grb2, N-terminal-SH3-Grb2-GST (Grb2-SH3N-GST), C-terminal-SH3-Grb2-GST (Grb2-SH3C-GST), full-length Grb2-GST, and GST only expressed fusion proteins were used in pull-down assays with full-length HA-tagged-p85 expressed protein. As demonstrated in Fig. 6b, full-length Grb2-GST efficiently pulls down p85. The Grb2 SH3N domain pulled down p85, but the efficiency is reduced relative to full-length Grb2. The Grb2-SH3C domain minimally pulled down p85, indicating that the predominant interaction between p85 occurs via the SH3N domain of Grb2. To determine if a direct interaction between the proline-rich region of p85 and the SH3N domain of Grb2, in the absence of other proteins, pull-downs were performed using Grb2-SH3N-GST fusion protein and p85(1–433)-GST fusion protein. The GST portion of the p85(1–433)-GST fusion protein was cleaved from p85 using thrombin. Fig. 6c, lanes 1 and 2, shows that when cleaved by thrombin, p85(1–433) migrates at 51 kDa, and a GST only band is detected (lane 2). Pull-down assays were performed by incubating either thrombin cut or uncut p85(1–433) (30 ng) with either Grb2-SH3N-GST or GST only (4 μg). Pull-down products were compared by incubating the blot with a p85 pAb. As expected, when uncut p85(1–433) was used, no p85 protein product was detected at 51 kDa (lanes 3 and 4). By contrast, there is a 51 kDa band after cutting with thrombin, and considerably more cut p85(1–433) product was pulled down with Grb2-SH3N-GST (lane 6) than with GST only (lane 5). This figure shows that there is direct binding between the proline-rich region of p85 and the SH3N domain of Grb2.
Fig. 6.
Pull-downs demonstrate that p85 directly binds to the SH3 domain of Grb2 via its second proline-rich region. (a) Full-length myc-tagged Grb2 was transfected into COS7 cells and pull-down assays were performed on protein homogenates with p85(1–333)-GST fusion proteins spanning both proline-rich regions [amino acids 1–333; GST-fusion protein containing p85Δpro1, p85Δpro2-GST, and p85Δpro1 + 2-GST; or GST only protein (negative control)]. Western blot analysis performed with a myc mAb (1 : 20 000) confirmed the presence of Grb2 in the total homogenates (b) COS7 cells were transfected with HA-tagged p85, and pull-down assays were performed on the protein homogenates using full-length or the N- or C-terminal SH3 domain of Grb2 fused to GST, or GST only protein (negative control). Western blot analysis performed with an HA mAb (1 : 1000) confirmed the presence of p85. (c) Pull-down assays were performed using either Grb2-SH3N-GST or GST only incubated with either thrombin cut p85(1–433), or uncut p85(1–433) GST. The p85(1–433) constructs span both proline-rich regions and the first SH2 domain of p85. The presence of p85 on the blot was detected using a pAb generated to a p85-GST fusion protein. The lower panels (a–c) show GST with or without the expressed fusion protein, using a GST mAb (DT-12, 1 : 100).
In summary, our data show that upon Gas6 activation and autophosphorylation, Axl can recruit p85 directly and indirectly. In addition, Axl directly recruits Grb2, and in the absence of the Grb2-consensus motif pYVN, Grb2 can not indirectly bind Axl.
Discussion
Our previous studies determined that the addition of rhGas6 to human oligodendrocytes during stress conditions, such as growth factor withdrawal or TNFα treatment, activates the Axl receptor, resulting in downstream signaling through the PI3 kinase/Akt pathway to maintain oligodendrocyte survival (Shankar et al. 2003). In this study, we demonstrate in vitro and in vivo that Gas6 stimulation of the Axl receptor recruits p85 and Grb2 to Axl via their respective SH2 domains, and alternatively, p85 can indirectly associate with Axl by binding to Grb2. Further, Gas6 stimulation of Axl results in recruitment of active PI3 kinase which leads to downstream activation of Akt at threonine 308.
Prior studies using epidermal growth factor (EGF) stimulation of an EGFR/Axl chimeric receptor determined that EGF–EGFR/Axl chimera binding activates the PI3 kinase pathway (Fridell et al. 1996). Also, an expression library screened with an EGFR/Axl cytoplasmic chimeric construct determined that the SH2 domains of p85, Grb2 and PLCγ can associate with the Axl chimeric receptor (Braunger et al. 1997). Unlike these earlier studies, our pull-down and IP studies were performed with Gas6 stimulated full-length Axl. Pull-down experiments confirm that p85 and Grb2 bind to Axl, however, we did not observe the reported interaction between Axl and c-Src, lck, or PLCγ (Braunger et al. 1997). In addition, we did not observe an interaction between Axl and Fyn, Abl, or p190RhoGAP. Although we demonstrate that the SH2 domain of the effector molecule ARG binds to Gas6-stimulated Axl, IP of full-length proteins demonstrate that Axl only interacts with p85 and Grb2. We confirm the interactions between Axl, p85 and Grb2 in vivo using homogenates from day 10 total mouse brain, and from brain subpopulations: O4+ immunopanned oligodendrocytes and O4– flow through cells.
In addition to showing that p85 and Grb2 bind to Axl's pYXXM and pYXN motifs, respectively (Braunger et al. 1997; Oligino et al. 1997), we demonstrate that when both consensus p85 motifs on Axl are mutated, p85 can indirectly bind Axl, via Grb2. Grb2 is an adaptor molecule that consists of one SH2 domain flanked by SH3 domains. Previous competitive peptide experiments showed that Grb2 can bind to either proline-rich region of p85, and that p85 can bind to either SH3 domain of Grb2 (Wang et al. 1995). We determined that GST fusion proteins of p85 (amino acids 1–333) and p85 containing a deletion of the first proline-rich region bind Grb2 equivalently. However, deletion of the second proline-rich region of p85 significantly diminishes binding to Grb2, demonstrating that the second proline-rich region of p85 is required for binding to Grb2. Furthermore, the SH3N domain of Grb2 is most effective at binding p85, although, an additive effect between the two Grb2 SH3 domains cannot be ruled out. Far western analysis demonstrated that the SH2 domain of p85 is unable to bind to the I,I Axl mutant in the absence of Grb2. These data show that the methionines of the YXXM motifs on Axl are critical for the direct binding of p85 and that Grb2 is required for p85 to indirectly bind to Axl, leading to downstream signaling.
Axl's conserved YXXM motif(s) are necessary for Gas6-activated Axl to signal to Akt. When WT Axl transfected COS7 cells are stimulated with rhGas6, a significant 2.9-fold increase in phosphorylated Akt at threonine 308 is observed. No significant increase in phosphorylated Akt is seen when I,QI mutant Axl is transfected into COS7 cells, indicating that the binding of p85 directly and/or indirectly via Grb2 to Axl is critical for Axl to signal to Akt.
Although p85 can indirectly bind to Axl via Grb2, direct binding of p85 to Axl at pY site 2 (pYVNM) excludes Grb2. We show that endogenous Grb2 is able to co-IP with the Axl mutant YALI by interacting with Axl at pY site 2 (pYVN) (Fig. 5, –p85 panel). However, over-expression of p85, by co-transfecting p85 with the YALI Axl mutant, inhibits the Grb2 and Axl interaction (Fig. 5, +p85 panel). Although it appears that p85 can compete with Grb2 for binding to pY site 2 (YVNM) another explanation for the loss of Grb2 binding is that the interaction and activation of the PI3 kinase cascade recruits additional molecules that bind PI3 kinase and sterically block Grb2 binding. Alternatively, in vivo, the binding of p85 and activation of PI3 kinase is known to result in conformational changes in the secondary structure of p85 that likely masks the proline-rich regions of p85, making them inaccessible to Grb2 binding (Shoelson et al. 1993).
While Gas6/Axl/PI3 kinase/Akt is a survival pathway, the binding of Grb2 to Axl may play a role in cell proliferation. The addition of Gas6 to NIH 3T3 cells in serum-containing media induced proliferation, whereas, Gas6 addition to serum-starved cells resulted in protection from apoptosis, indicating that during stress, Gas6 stimulation signals only to the survival pathway (Crosier and Crosier 1997). We speculate that during cellular stress, resulting in Gas6 activation of Axl, PI3 kinase can bind either YXXM site, and inhibit the binding of Grb2 to Axl at the YVN site. These interactions can activate the survival pathway, and inhibit the Grb2-mediated proliferative pathway. When the SH2 domain of Grb2 is bound to Axl, Grb2 has two free SH3 domains to interact with other signaling molecules. However, some intercellular interactions, such as the binding of Grb2 and son of sevenless, require that both Grb2 SH3 domains are available (Wang et al. 1995). Therefore, a p85 interaction with the SH3N domain of Grb2 would block the putative Grb2–son of sevenless mediated ERK proliferative pathway. Studies have shown that Axl activation following growth factor withdrawal, or serum starvation, does not activate the ERK/MAPK pathway (Fridell et al. 1996; Shankar et al. 2003). We demonstrate that 1 min following rhGas6 stimulation, serum starved Axl transfected COS7 cells show an increase in phosphorylation of Akt (threonine 308). In the absence of proliferation, PI3 kinase can also function in actin-based motility and aid in cell adhesion (Sotsios et al. 1999; Santy and Casanova 2001; Alahari et al. 2002; Nicholson and Anderson 2002; Valentijn and Gilmore 2004).
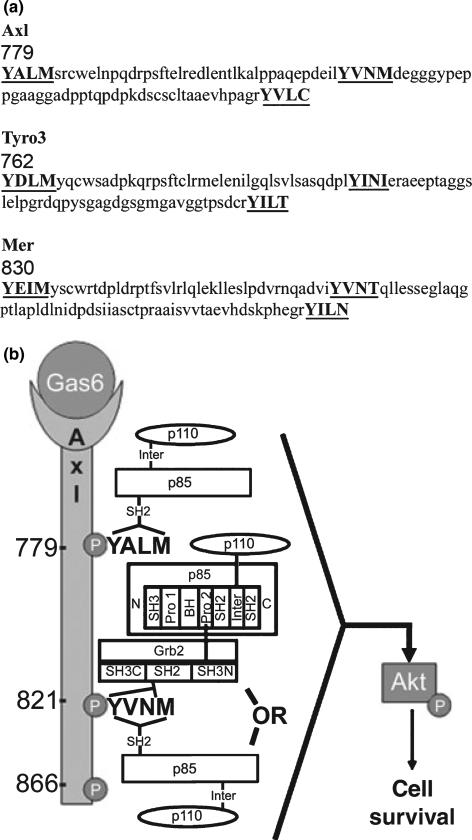
It is worth noting that while the Axl, Tyro3, and Mer receptors share highly conserved positioning and sequence homology for each of the autophosphorylated pY motifs located within the carboxy terminus, Tyro3, and Mer have one consensus YXXM motif, and Axl has two (Fig. 7a). Examination of the relative amount of p85 associated with Axl following the pull-down and the IP experiments does not support simultaneous p85 binding at both pYXXM motifs as the relative amount of p85 associated with Axl in the single mutants appears similar to the WT. However, under conditions of extreme stress when cell survival is at stake, it is feasible that p85 could bind to both sites.
Fig. 7.
Conserved amino acid alignment of the human Axl, Tyro3, and Mer autophosphorylation sites and schematic representation of the proposed Gas6/Axl downstream cell survival signaling pathway. (a) The number represents the position of the amino acid within the respective protein. Note that the spacing between the first YXXM motif and the second YXN motif is conserved amongst all three receptors. The accession number for human Axl is BC050914; human Rse (Tyro3) is U05682, and human c-Mer is U08023. (b) Gas6 stimulation of Axl results in autophosphorylation at Y779ALM, Y821VNM, and Y866VLC. Direct recruitment of p85 can occur via its SH2 domains binding to one or both of the pY779ALM, pY821VNM motifs, whereas direct recruitment of Grb2, via its SH2 domain, can only bind to the pY821VN motif. Indirect association of p85 and Axl can occur through Grb2 via the second proline-rich region of p85 binding to one of the SH3 domains of Grb2. The direct or indirect recruitment of p85/p110 PI3 kinase can signal downstream to activate Akt resulting in enhanced cell survival.
The significance of the third pY consensus motif is unknown. Although this motif was reported to be a binding site for PLCγ (Braunger et al. 1997), we were unable to pull-down Axl with the SH2 domain of PLCc. The possibility exists that the third motif is the binding site for C1 domain-containing phosphatase and TENsin homolog (C1-TEN). The over-expression resulted in reduced cell proliferation, migration and cell survival, therefore, C1-TEN is considered a negative regulator of the Akt pathway and it may serve to balance the signaling pathway induced by Gas6/Axl/PI3K/ Akt (Hafizi et al. 2005).
Based on our present study and previous data from our laboratory a proposed signaling model is shown in Fig. 7b. In a stressful situation Gas6 activation of Axl recruits PI3 kinase directly to Axl at the pYALM or pYVNM motifs, leading to cell survival. Alternatively, the second proline-rich region of p85 can indirectly bind to Axl, predominantly by the N-terminal SH3 domain of Grb2, and may also signal through the Akt pathway. As shown in Fig. 4, Akt and the cell survival pathway is activated by Gas6 stimulation of Axl in as little as 1 min. Akt activation is eliminated if the YXXM motifs are not available to p85 and/or Grb2, showing that these binding sites are critical for this pathway. Although our data shows that the triple mutant I,QI abolishes both the binding of p85 and Grb2 to Axl, we cannot rule out a scenario in which pY866 cooperates with pY779 or pY821 for the recruitment of these two proteins.
We previously showed that Gas6 can protect against TNFα-induced apoptosis in mouse and human oligodendrocyte cultures. Blocking the Akt pathway by administration of the PI3 kinase inhibitors wortmannin or LY294002 eliminated this protective effect (Shankar et al. 2003, 2006). We have determined that both an O4+ oligodendrocyte population and mixed O4– population, from mouse brain, can recruit p85 and Grb2 to the Axl receptor. Further investigation is needed to determine the downstream survival signaling pathway in the nervous system. Recent studies have determined that Gas6/ Axl signaling activates the basic loop helix transcription factor repressors TWIST which bind to the E box in the TNF promoter and inhibits nuclear factor kappa B-dependent transcription (Sharif et al. 2006). This pathway may be one of the ways Axl protects against cell injury.
Acknowledgements
We thank Dr Bjorn Dahlback, University of Lund; Malmo, Sweden, for the full-length Axl construct in pcDNA3. We thank our colleagues at Albert Einstein College of Medicine, Dr Celia Brosnan and Dr Laura Santambrogio for advice. We thank Kathleen Norman O’Guin for her technical support. We acknowledge the receipt of Axl mAb, Axl pAb and rhGas6 from Dr Brian Varnum at AMGEN, Thousand Oaks, CA. We acknowledge the receipt of the Grb2 construct from the laboratory of Dr Bruce Terman at Albert Einstein College of Medicine. This study was funded by the National Multiple Sclerosis Research Grant RG3020 (BS-Z), NIH Grant GM55692 and a grant from the Janey Fund (JMB), and the Training Program in Cellular and Molecular Biology and Genetics grant, National Institutes of Health, NIGMS GM07491.
Abbreviations used
- ARG
Abelson-related tyrosine kinase
- DMEM
Dulbecco's modified Eagle's medium
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- Gas6
growth arrest-specific protein 6
- Grb2
growth factor receptor-bound protein 2
- GST
glutathione S-transferase
- HBSS
Hank's balanced salt solution
- IP
immunoprecipitation
- IP'ed
immunoprecipitated
- mAb
monoclonal antibody
- pAb
polyclonal antibody
- PI3
phosphatidylinositol-3
- PLCγ
phospholipase C gamma
- pY
phosphotyrosine
- rhGas6
recombinant human Gas6
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SH2
Src homology 2
- TNFα
tumor necrosis factor α
- WT
wildtype
References
- Alahari SK, Reddig PJ, Juliano RL. Biological aspects of signal transduction by cell adhesion receptors. Int. Rev. Cytol. 2002;220:145–184. doi: 10.1016/s0074-7696(02)20005-4. [DOI] [PubMed] [Google Scholar]
- Albala JS, Kress Y, Liu WK, Weidenheim K, Yen SH, Shafit-Zagardo B. Human microtubule-associated protein-2c localizes to dendrites and axons in fetal spinal motor neurons. J. Neurochem. 1995;64:2480–2490. doi: 10.1046/j.1471-4159.1995.64062480.x. [DOI] [PubMed] [Google Scholar]
- Allen MP, Zeng C, Schneider K, Xiong X, Meintzer MK, Bellosta P, Basilico C, Varnum B, Heidenreich KA, Wierman ME. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol. Endocrinol. 1999;13:191–201. doi: 10.1210/mend.13.2.0230. [DOI] [PubMed] [Google Scholar]
- Avanzi GC, Gallicchio M, Bottarel F, et al. GAS6 inhibits granulocyte adhesion to endothelial cells. Blood. 1998;91:2334–2340. [PubMed] [Google Scholar]
- Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J. Neurosci. Res. 1989;24:548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, Bartram CR, Janssen JW. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene. 1997;14:2619–2631. doi: 10.1038/sj.onc.1201123. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Crosier KE, Crosier PS. New insights into the control of cell growth; the role of the AxI family. Pathology. 1997;29:131–135. doi: 10.1080/00313029700169744. [DOI] [PubMed] [Google Scholar]
- Ebner S, Dunbar M, McKinnon RD. Distinct roles for PI3K in proliferation and survival of oligodendrocyte progenitor cells. J. Neurosci. Res. 2000;62:336–345. doi: 10.1002/1097-4547(20001101)62:3<336::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Fridell YW, Jin Y, Quilliam LA, Burchert A, McCloskey P, Spizz G, Varnum B, Der C, Liu ET. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol. Cell. Biol. 1996;16:135–145. doi: 10.1128/mcb.16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell YW, Villa J, Jr, Attar EC, Liu ET. GAS6 induces Axl-mediated chemotaxis of vascular smooth muscle cells. J. Biol. Chem. 1998;273:7123–7126. doi: 10.1074/jbc.273.12.7123. [DOI] [PubMed] [Google Scholar]
- Fry MJ. Structure, regulation and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta. 1994;1226:237–268. doi: 10.1016/0925-4439(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989;106:119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Two proliferative stages of the oligodendrocyte lineage (A2B5 + O4– and O4+ GalC–) under different mitogenic control. Neuron. 1990;5:615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Gard AL, Warrington AE, Pfeiffer SE. Direct microculture enzyme-linked immunosorbent assay for studying neural cells: oligodendrocytes. J. Neurosci. Res. 1988;20:46–53. doi: 10.1002/jnr.490200108. [DOI] [PubMed] [Google Scholar]
- Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc. Natl. Acad. Sci. USA. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goruppi S, Ruaro E, Varnum B, Schneider C. Gas6-mediated survival in NIH3T3 cells activates stress signalling cascade and is independent of Ras. Oncogene. 1999;18:4224–4236. doi: 10.1038/sj.onc.1202788. [DOI] [PubMed] [Google Scholar]
- Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 1994;5:647–657. [PubMed] [Google Scholar]
- Hafizi S, Ibraimi F, Dahlback B. C1-TEN is a negative regulator of the Akt/PKB signal transduction pathway and inhibits cell survival, proliferation, and migration. FASEB J. 2005;19:971–973. doi: 10.1096/fj.04-2532fje. [DOI] [PubMed] [Google Scholar]
- Hajihosseini M, Tham TN, Dubois-Dalcq M. Origin of oligodendrocytes within the human spinal cord. J. Neurosci. 1996;16:7981–7994. doi: 10.1523/JNEUROSCI.16-24-07981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiring C, Dahlback B, Muller YA. Ligand recognition and homophilic interactions in Tyro3: structural insights into the Axl/Tyro3 receptor tyrosine kinase family. J. Biol. Chem. 2004;279:6952–6958. doi: 10.1074/jbc.M311750200. [DOI] [PubMed] [Google Scholar]
- Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Hill KM, Huang Y, Yip SC, Yu J, Segall JE, Backer JM. N-terminal domains of the class in phosphoinositide 3-kinase regulatory subunit play a role in cytoskeletal but not mitogenic signaling. J. Biol. Chem. 2001;276:16374–16378. doi: 10.1074/jbc.M006985200. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A. Receptor tyrosine kinases and their targets. Curr. Opin. Genet. Dev. 1994;4:5–14. doi: 10.1016/0959-437x(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- Lai C, Gore M, Lemke G. Structure, expression, and activity of Tyro 3, a neural adhesion-related receptor tyrosine kinase. Oncogene. 1994;9:2567–2578. [PubMed] [Google Scholar]
- Layton MJ, Harpur AG, Panayotou G, Bastiaens PIH, Waterfield MD. Binding of a diphosphotyrosine-containing peptide that mimics activated platelet-derived growth factor receptor beta induces oligomerization of phosphatidylinositol 3-kinase. J. Biol. Chem. 1998;273:33379–33385. doi: 10.1074/jbc.273.50.33379. [DOI] [PubMed] [Google Scholar]
- Lemke G, Lu Q. Macrophage regulation by Tyro 3 family receptors. Curr. Opin. Immunol. 2003;15:31–36. doi: 10.1016/s0952-7915(02)00016-x. [DOI] [PubMed] [Google Scholar]
- Li R, Chen J, Hammonds G, Phillips H, Armanini M, Wood P, Bunge R, Godowski PJ, Sliwkowski MX, Mather JP. Identification of Gas6 as a growth factor for human Schwann cells. J. Neurosci. 1996;16:2012–2019. doi: 10.1523/JNEUROSCI.16-06-02012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Templeton D, Kung HJ. Identification of the major autophosphorylation sites of Nyk/Mer, an NCAM-related receptor tyrosine kinase. J. Biol. Chem. 1996;271:18355–18362. doi: 10.1074/jbc.271.31.18355. [DOI] [PubMed] [Google Scholar]
- Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, 3rd, Le Beau MM, Earp HS, Liu ET. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell. Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oligino L, Lung FD, Sastry L, et al. Nonphosphorylated peptide ligands for the Grb2 Src homology 2 domain. J. Biol. Chem. 1997;272:29046–29052. doi: 10.1074/jbc.272.46.29046. [DOI] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Prieto AL, Weber JL, Lai C. Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J. Comp. Neurol. 2000;425:295–314. [PubMed] [Google Scholar]
- Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SL, O'Guin K, Cammer M, McMorris FA, Stitt TN, Basch RS, Varnum B, Shafit-Zagardo B. The growth arrest-specific gene product Gas6 promotes the survival of human oligodendrocytes via a phosphatidylinositol 3-kinase-dependent pathway. J. Neurosci. 2003;23:4208–4218. doi: 10.1523/JNEUROSCI.23-10-04208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SL, O'Guin K, Kim M, Varnum B, Lemke G, Brosnan CF, Shafit-Zagardo B. Gas6/Axl signaling activates the phosphatidylinositol 3-kinase/Akt1 survival pathway to protect oligodendrocytes from tumor necrosis factor alpha-induced apoptosis. J. Neurosci. 2006;26:5638–5648. doi: 10.1523/JNEUROSCI.5063-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif MN, Sosic D, Rothlin CV, Kelly E, Lemke G, Olson EN, Ivashkiv LB. Twist mediates suppression of inflammation by type I IFNs and Axl. J. Exp. Med. 2006;203:1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Sivaraja M, Williams KP, Hu P, Schlessinger J, Weiss MA. Specific phosphopeptide binding regulates a conformational change in the PI 3-kinase SH2 domain associated with enzyme activation. EMBO J. 1993;12:795–802. doi: 10.1002/j.1460-2075.1993.tb05714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev. Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Sotsios Y, Whittaker GC, Westwick J, Ward SG. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J. Immunol. 1999;163:5954–5963. [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Tari AM, Lopez-Berestein G. GRB2: a pivotal protein in signal transduction. Semin. Oncol. 2001;28:142–147. doi: 10.1016/s0093-7754(01)90291-x. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn AJ, Gilmore AP. Translocation of full-length Bid to mitochondria during anoikis. J. Biol. Chem. 2004;279:32848–32857. doi: 10.1074/jbc.M313375200. [DOI] [PubMed] [Google Scholar]
- Varnum BC, Young C, Elliott G, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–626. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- Wang J, Auger KR, Jarvis L, Shi Y, Roberts TM. Direct association of Grb2 with the p85 subunit of phosphatidylinositol 3-kinase. J. Biol. Chem. 1995;270:12774–12780. doi: 10.1074/jbc.270.21.12774. [DOI] [PubMed] [Google Scholar]
- Yu J, Wjasow C, Backer JM. Regulation of the p85/p110alpha phosphatidylinositol 3′-kinase. Distinct roles for the n-terminal and c-terminal SH2 domains. J. Biol. Chem. 1998;273:30199–30203. doi: 10.1074/jbc.273.46.30199. [DOI] [PubMed] [Google Scholar]
- Zamora-Leon SP, Bresnick A, Backer JM, Shafit-Zagardo B. Fyn phosphorylates human MAP-2c on tyrosine 67. J. Biol. Chem. 2005;280:1962–1970. doi: 10.1074/jbc.M411380200. [DOI] [PubMed] [Google Scholar]