Abstract
There are no ideal ways to identify and isolate viable and purified Foxp3+ regulatory T cells so far. Here we developed a novel procedure for the isolation of highly purified Foxp3+ cells using flow cytometry. This method relies on an identification and sorting of the lymphoblast cell population identified on a scatter plot using flow cytometry. We confirmed that greater than 98% of the cells sorted using this technique expressed Foxp3 and displayed a potent suppressive activity. This method provides a valuable tool for the study of the T regulatory cell biology and their therapeutic manipulation.
Keywords: Foxp3, TGF-β, regulatory T cells
Introduction
Foxp3+CD4+ regulatory T (Treg) cells play a crucial role in maintaining the immune homeostasis against self-tissues (Shevach, 2002; Sakaguchi, 2004). These cells also prevent autoimmune and inflammatory diseases through suppressing potentially deleterious activities of T helper (Th) cells. Lack or dysfunction of Treg cells in mice and human is responsible for many autoimmune diseases (Miyara et al., 2005; Pop et al., 2005; Valencia et al., 2006). Foxp3, a fork head transcription factor, is expressed mainly in Treg cells and essential for the development and function of Tregs (Khattri et al., 2003). A mutation in Foxp3 is responsible for expression of both scurfy mouse and immune dysregulation, polyendocrineopathy, enteropathy and X-Linked syndrome (IPEX) in humans (Bennett et al., 2001; Fontenot et al., 2005). Tregs can be classified as either thymus-derived, naturally occurring (nTreg) or those that can be induced with antigen stimulation and cytokines such as TGF-β (iTreg). Both are essential for immune homeostasis and tolerance (Shevach, 2002; Sakaguchi, 2004).
Therapeutic manipulation of purified Foxp3+ cells provides a logical rationale for a promising alternative approach to treat many autoimmune diseases, yet isolating and enriching viable purified Foxp3-positive cells remains a major challenge. As Foxp3 is a transcript factor that is expressed in the nucleus, it is not possible to directly isolate viable Foxp3+ cells using anti-Foxp3 antibody staining and cell sorting. Other markers such as CD25 (IL-2 receptor alpha chain), CTLA-4 (cytotoxic T lymphocyte-associated antigen 4), GITR (glucocorticoid-induced tumor necrosis factor receptor family-related gene) and lymphocyte activation gene-3, provide possible molecular markers for the identification of Foxp3+ cells, however, these markers are not strictly Treg specific since all are upregulated upon T cell activation (Takahashi et al., 2000; McHugh et al., 2002; Huang et al., 2004). Downregulation of CD127, the α chain of the IL-7 receptor, has been described as useful in the discrimination of Tregs from conventional T helper cells (Tconv) (Seddiki et al., 2006), but unfortunately, CD127 expression is also downregulated upon T cell activation and the loss of CD127 may be a characteristic feature of both activated Tconv cells and follicular helper T cells (Tfh), which provide help for B cells. Moreover, using conventional molecular markers, the most enrichment of Tregs one can hope to achieve is ∼75%. Thus, other approaches that are able to specifically identify Foxp3-positive cells would be invaluable research tools to study the Treg cell biology and manipulate Treg cell therapy.
In this study, we have focussed on how to isolate purified Foxp3+ cells in both expanded natural (nTregs) and TGF-β induced Treg cells (iTregs) since both cell populations have the potential to be exploited for the clinical use.
Results
Lymphoblast population of nTregs in scatter plot identifies purified and viable Foxp3+ cells
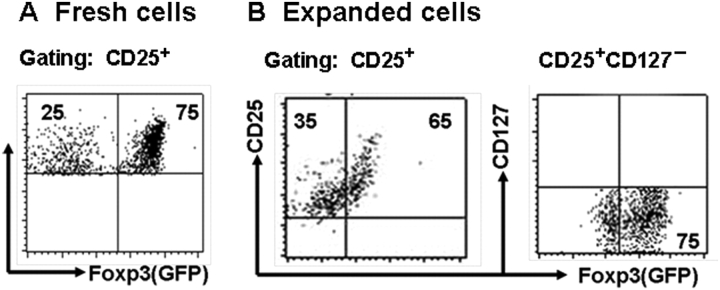
We confirmed that CD4+GFP+ cells (nTreg cells) sorted from spleen T cells in Foxp3gfp knock-in mice where green fluorescence protein (GFP) is inserted in the Foxp3 gene promoter exclusively expressed Foxp3. We revealed that CD25+ cells gated on the CD4+ cells only expressed about 75% of Foxp3 (GFP) (Figure 1A), gating on CD127− cells did not markedly improve the purity of Foxp3 expression on CD4+CD25+ cells (data not shown). When CD4+CD25+ cells sorted from spleen as above were expanded with anti-CD3 and CD28-coated beads and IL-2 for 4 days, these cells maintained CD25 expression and almost completely lost CD127 expression. Foxp3 expression on the CD25+ cell population was slightly decreased following cell expansion ex vivo. Gating on CD25+CD127− cell population did not significantly improve the purity of Treg cells since these cells populations were still contaminated about 25% Foxp3− cells (Figure 1B).
Figure 1.
Foxp3 expression of fresh or expanded nTregs. (A) Foxp3 expression on fresh nTregs in spleen on CD25brght gate; (B) spleen CD4+CD25+ cells in Foxp3gfp knock-in mice (C57BL/6 strain) were stimulated with anti-CD3/CD28-coated beads (one bead to five cells) in the presence of IL-2 (200 units/ml) for 4 days. Foxp3 expression on expanded nTregs on CD25bright and CD127−/dim gates. All data shown in (A) and (B) represent at least four separate experiments.
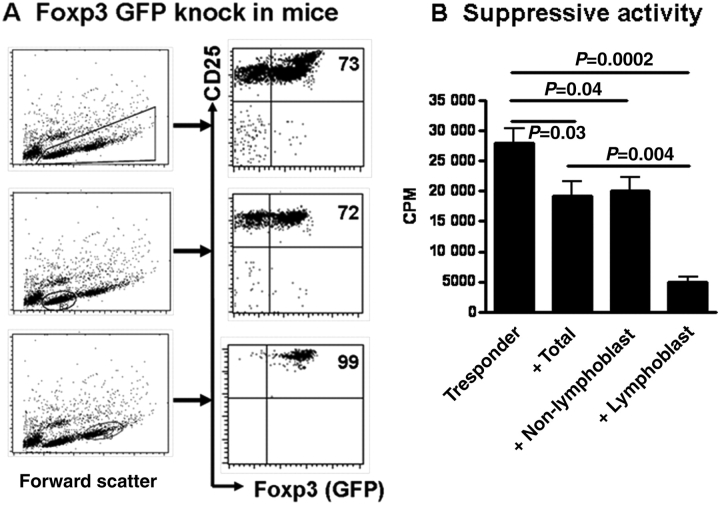
Of interest, when CD4+CD25+ cells isolated from spleen in Foxp3gfp knock-in mice were expanded with anti-CD3/CD28-coated beads and IL-2 for 4 days, two distinct populations were clearly displayed in the scatter plot in flow cytometry, one is the lymphocyte population, representing about 45% of total viable cells, that has been over-activated, called ‘lymphoblast cell population’ and another one is mostly resting cells and sometimes referred to as a ‘non-lymphoblast cell population’ (Figure 2A). Two cell populations were separately sorted and the expression of the Foxp3 and their suppressive activity have been analyzed and compared. As shown in Figure 2A, while total expanded lymphocyte population expressed similar levels of Foxp3 with fresh CD4+CD25+ cells (∼70%), more than 98% of lymphoblast cell population expressed Foxp3 and their CD25 and Foxp3 mean fluorescent intensity (MFI) were significantly higher than either total cells or non-lymphoblast cell populations. Conversely, the non-lymphoblast cell population was composed of ∼70% Foxp3+ and ∼30% Foxp3− cells. Using CD4+CD25+ cells sorted from C57BL/6 wild-type mice, we observed that after 4 days of anti-CD3/CD28 and IL-2 stimulation, scatter plot in flow cytometry also clearly displayed two distinct cell populations of lymphoblast and non-lymphoblast cells and similar levels of Foxp3 expression in the different cell populations (data not shown).
Figure 2.
Lymphoblast population of expanded CD4+CD25+ nTregs in the scatter plot identifies purified and viable Foxp3+ cells. (A) CD4+CD25+ cells in Foxp3gfp knock-in mice (C57BL/6 strain) were stimulated as Figure 1B. Foxp3 (GFP) expression on total expanded nTregs, non-lymphoblast or lymphoblast cell populations was determined by flow cytometry. (B) T cells were isolated from C57BL/6 mice and stimulated with anti-CD3 (0.25 µg/ml) in the presence of γ-irradiated (20 cGy) APCs (1:1 ratio) for 3 days. Total expanded nTregs, non-lymphoblast or lymphoblast cell populations sorted as Figure 2A were added to some culture wells (one conditioned cell to four T responder cells). 3H (1 µCi) was added to each well (96-well plates) in the final 16 h of cultures and the proliferation was determined by 3H incorporation using a liquid scintillation counter. All data shown in (A) and (B) represent at least four separate experiments.
We next determined their suppressive activities against T cell proliferation using a standard in vitro proliferation experiment. As shown in Figure 2B, while expanded nTreg cells harvested from total or sorted from non-lymphoblast cell populations significantly suppressed T cell proliferation, expanded nTreg cell sorted from lymphoblast population displayed more potent suppressive activity. In fact, Foxp3− (GFP−) cells sorted from expanded CD4+CD25+ cells had no suppressive ability. This is consistent with previous reports that suppressive activity of Treg cells is closely associated with the level of Foxp3 expressed (Zheng et al., 2007; Lu et al., 2010a, b).
Lymphoblast population of iTregs in scatter plot also identifies purified and viable Foxp3+ cells
We and others have reported that the combination of IL-2 and TGF-β is able to induce CD4+Foxp3+ iTregs that mostly share similar phenotype and functional characteristics with nTregs (Kohm et al., 2002; Zheng et al., 2002; Chen et al., 2003; Zheng et al., 2004). As only the CD25+ subset of iTregs displayed suppressive activity (Mottet et al., 2003) and as with nTregs, only ∼75% of the CD25+ cells expressed Foxp3, we next examined the purity of iTregs using similar methods as above. As shown in Figure 3A, naïve CD4+GFP− cells stimulated with anti-CD3/CD28 beads with IL-2 and TGF-β emerged two-cell populations after 3-day cultures. Compared with total lymphocyte and non-lymphoblast populations, the lymphoblastic iTregs expressed significantly higher levels of Foxp3, CD25, CD103, CD122, PD1, GITR and CTLA-4 and lower level of CD127 (Figure 3B), suggesting lymphoblast population holds the phenotypic characteristics of purified Treg cells. These data suggest that current procedure we established has a greater advantage on isolating purified and viable Foxp3+ cells compared with other methods used currently.
Figure 3.
Lymphoblast population of activated iTregs in the scatter plot also identifies purified and viable Foxp3+ cells. (A) Scatter plot of iTreg induction in the different time points. Naïve CD4+GFP− cells isolated from Foxp3gfp knock-in mice were stimulated with anti-CD3/CD28 beads (1:5) and IL-2 (40 U/ml) and TGF-β (2 ng/ml) for 1–4 days. (B) Treg cell relative phenotypes were analyzed and compared between total cell and two distinct cell populations. Data are representative of three independent experiments.
Purified Foxp3+ cells from lymphoblast cell population display more potent suppressive activity
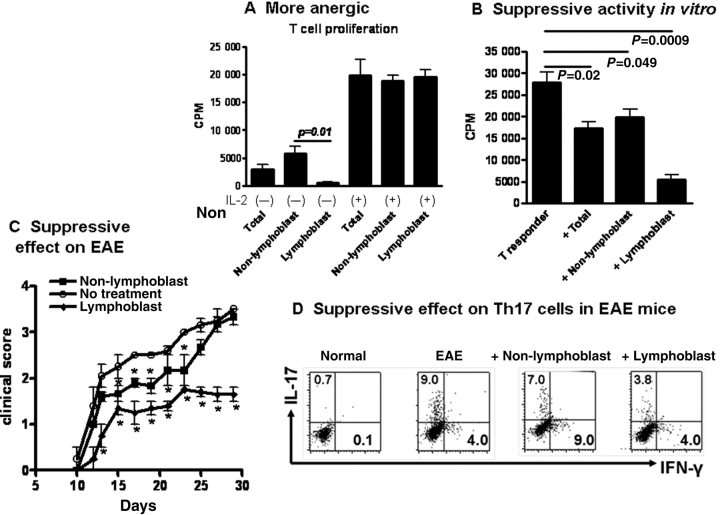
One of the important features for Treg cells is the anergic status. We have compared their anergic status of two populations of TGF-β-induced iTregs following cell sorting. Cells were induced and sorted similarly as in Figure 3 and total or two separate cell populations were stimulated with anti-CD3 in the presence of antigen-presenting cells (APC) for 3 days, cell proliferation was evaluated with 3H-thymidine as previously reported (Zheng et al., 2004). While total lymphocytes and non-lymphoblast cell population displayed partial proliferation, lymphoblast cell population did not proliferate at all (Figure 4A). Addition of exogenous IL-2 restored the proliferative capacity of lymphoblast cell population (Figure 4A), suggesting these cells are typical anergic cells.
Figure 4.
Purified Foxp3+ cells obtained from lymphoblast cell population display more potent suppressive activity in vitro and in vivo. (A) Lymphoblast, non-lymphoblast and total cell population were stimulated with soluble anti-CD3 (0.25 μg/ml) in the presence of irradiated APC (1:1) with or without IL-2 (50 U/ml) for 3 days. The proliferation was determined by 3H incorporation. (B) These cells were added to T responder cells (1:4 ratio as in Figure 2B) and stimulated with anti-CD3 and their proliferation was similarly conduced as in (A). (C) EAE was induced by immunizing mice with MOG35–55 peptide following pertussis toxin administration as detailed in the Materials and methods. 2 × 106 lymphoblast or non-lymphoblast cells were i.v administered to C57/BL6 mice at the time of disease induction. Clinical scores were monitored every other day. (D) On Day 26 some of these mice were sacrificed and draining lymph node cells were stimulated with PMA and ionomycin for 5 h and BFA for 4 h for intracellular cytokine staining on CD4+ cell gate.
We further developed the suppressive assays to determine the suppressive capacity of two-cell populations of TGF-β-induced iTregs. As shown in Figure 4B, addition of one non-lymphoblast cell to four T responder cells partially suppressed the proliferation of anti-CD3-stimulated T responder cell in vitro. However, addition of similar doses of lymphoblast cell population displayed more potent suppressive activity (Figure 4B). Moreover, injection of 2 million of non-lymphoblast cell population slightly suppressed the development of the experimental autoimmune encephalomyelitis (EAE) during the early stage of disease but lost the suppressive activity after 25 days following myelin oligodendrocyte glycoprotein (MOG) peptide immunization, conversely, adoptive transfer of similar doses of lymphoblast cell population markedly inhibited the EAE development during entire period we examined (Figure 4C) and downregulated IL-17 production by CD4+ cells in the draining lymph nodes (LN) in day 26 after MOG immunization (Figure 4D), suggesting that adoptive transfer of lymphoblast cell population results in more sufficient and persistent suppressive effect on autoimmune diseases.
Discussion
Adoptive transfer of CD4+CD25+ nTreg cells has been proved to alleviate many autoimmune diseases including EAE, colitis and diabetes and is therefore considered as a promising strategy for the treatment of autoimmune diseases (Kohm et al., 2002; Green et al., 2003; Mottet et al., 2003). Clinical use exploring this strategy requires efficient in vitro expansion of this rare cell population. Approaches developed thus far depend upon highly purified Treg cells prior to culture initiation, a process still hampered by the lack of Treg cell-specific surface markers.
Current methods using cell surface markers such as CD25hi or CD127low usually gain about 75% of purified CD4+Foxp3+ cells. When CD4+CD25+ or CD4+CD25+CD127− cells are expanded, CD4+Foxp3− non-Treg cells are also expanded. In autoimmune diseases and inflammatory condition, these cells may represent pathogenic T effector cells. We have observed that after 4-day ex vivo expansion, Foxp3 expression by CD4+CD25+ cells gradually decreased. In fact, natural Treg cells have lost their cell-type-specific characteristics after repetitive TCR stimulation (Hoffmann et al., 2009).
Several factors may explain the observed cell heterogeneity. It is likely that CD4+CD25+Foxp3− non-Treg cells contaminated in starting CD4+CD25+ cells have been preferentially expanded in culture over time. Additionally, Foxp3+ Treg cells have predominately experienced activation-induced apoptosis or lost Foxp3 phenotype and/or converted into T effector cells. We and others recently have reported that nTregs can be converted into Th17 cells when stimulated with IL-6 or into Th1 cells when they were strongly stimulated with anti-CD3 (Xu et al., 2007; Zheng et al., 2008; Lu et al., 2010a, b). nTregs can also be converted into fTh cells (Fantini et al., 2004) . This problem has greatly hampered the clinical use of Treg cells in the treatment of autoimmune diseases.
One of the ideal ways is to develop specific cell surface markers to exclusively recognize Foxp3 expression. Many researchers have identified the Treg-associated signatures, such as CTLA-4, GITR, CD103, CD127, CD73 and CD39 (Takahashi et al., 2000; Lehmann et al., 2002; McHugh et al., 2002; Huang et al., 2004; Liu et al., 2006; Deaglio et al., 2007), as represented by Foxp3 expression. Nonetheless, none of these molecules is specific Treg signature. Although Foxp3 itself is a best marker to identify purified Treg cells, the detection of Foxp3 expression using the current method requires fixation and permeabilization of the cells, it is, therefore, impossible to isolate viable Tregs for biological studies and ex vivo expansion as a prelude to therapeutic administration since Foxp3 is only expressed in the nucleus.
In this study we find a novel approach to isolate the purified Foxp3+ Tregs. Lymphoblast population of expanded CD4+CD25+ nTregs and TGF-β-induced Treg cells in scatter plot identifies purified and viable Foxp3+ cells. After 3-day expansion and induction, both nTregs and iTregs displayed two distinct cell populations in scatter plot of flow cytometry. Almost all lymphoblast population expressed Foxp3 and other Treg relative markers. These cells have typical anergy characteristic and strongly suppressed T cell proliferation in vitro. Compared to non-lymphoblast cell population, injection of lymphoblast cell population resulted in efficiently suppressive effects on the progression of EAE, a chronic inflammatory demyelinating disease of the central nervous system. Although previous study revealed that injection of CD4+CD25+ nTregs failed to control Th17 cell differentiation in autoimmune gastritis (Huter et al., 2008), our data demonstrate that injection of purified Foxp3+ cells can overcome this problem since we observed only purified but not non-purified Foxp3+ cells suppressed Th17 production in the EAE model. In fact, injection of non-purified Foxp3+ Treg cells resulted in somewhat increase of IFN-γ-producing cells. Although IFN-γ is able to suppress Th17 cell production, it is likely it also contributes to the pathogenesis of EAE.
Taken together, we have identified a novel approach using sorting of lymphoblast cell population on scatter plot of flow cytometry to isolate viable and purified Foxp3+ regulatory T cells, which is better than current methods to isolate Foxp3+ cells depending upon CD25 expression and/or CD127 downexpression. We demonstrate that this cell population is highly Foxp3 expressed and has potent suppressive activities in vitro and in vivo. This approach is especially valuable for the isolation of purified Foxp3+ Tregs and decrease of contaminated Tconv cells after expansion of nTregs ex vivo. It has been known that repeated expansion of nTregs ex vivo leads to downregulation of Foxp3 and also increases the expansion of Foxp3− Tconv cells. This method provides a valuable tool to study biology of regulatory T cells and their therapeutic manipulation in autoimmune diseases and other diseases. We are currently investigating if similar approach also can identify purified Treg cells in the human and patients with autoimmune diseases. If successful, the injection of purified and viable Foxp3+ cells to patients with autoimmune diseases not only ensures the treatment of disease but also decreases and avoids the risk of contaminated T effector cells.
Materials and methods
Mice
Specific pathogen-free, female C57/BL6 mice were purchased from Jackson Laboratory and Foxp3gfp knock-in mice were a gift from Dr. A. Y. Rudensky (University of Washington, Seattle, WA). All experiments using mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of University of Southern California and Tongji University.
Cell sorting, in vitro stimulation and suppressor assay
Spleen CD25+ or GFP+ cells gated on the CD4 from C57BL/6 or Foxp3gfp knock-in mice were obtained by cell sorting using a FACSAria (BD). These cells were further expanded with anti-CD3/CD28-coated beads (1:5, one bead to five cells) (Invitrogen), rmIL-2 (200 U/ml) (R&D) for 4 days. TGF-β-iTreg cells were induced from naïve CD4+GFP− cells. CD4+GFP− cells sorted as above in the gate of CD4+ and GFP− cells. These cells were further labeled with PE-conjugated anti-CD62L beads, and CD4+CD62L+ cells were obtained by MACS (CD4+CD62L+GFP− cells purity >98%). These cells were stimulated with anti-CD3/CD28 beads (1:5), rmIL-2 (50 U/ml) and TGF-β (2 ng/ml) for days as indicated. Lymphoblast and non-lymphoblast cell populations were sorted for the phenotypic and functional analysis. Foxp3, CD25, CD103, CD122, CD127, PD-1, GITR, CTLA-4 (purchased from eBioscience and BD Pharmingen) expression were analyzed by flow cytometry. To measure the in vitro suppressive assay, T cells isolated from C57BL/6 mice were stimulated with soluble anti-CD3 (0.25 μg/ml) in the presence of γ-irradiated APC (1:1) with or without lymphoblast or non-lymphoblast cell population in the 1:4 ratio (1 Treg subset to 4 T responder). 3H-thymidine (1 µCi/96 well) was added to cultures in the last 16 h and cell proliferation was measured by using a liquid scintillation counter.
Induction of EAE and adoptive transfer
EAE was induced by immunization with MOG35–55 emulsified in CFA (Difco Laboratories) at a dose of 100 µg per mouse, followed by the administration of pertussis toxin (150 ng per mouse, Sigma) on Days 0 and 2 as described (Stromnes and Goverman, 2006). 2 × 106 lymphoblast cell and non-lymphoblast cell population sorted form iTregs were adoptively transferred to mice at the time of disease induction. Clinical signs of EAE were assigned scores according to the following: 0, no symptoms; 1, loss of muscle tone in tail; 2, hind limp weakness; 3, hind limp paralysis of one (3.0) or both (3.5); 4, hind and fore limp paralysis; 5, loss of temperature control or moribund. Scores are shown as Mean daily clinical scores for all mice per group.
Cytokines staining and production
Mice were sacrificed 26 days after MOG35–55 peptide immunization with or without Treg subset injection. Draining LN were collected and single cell suspension were prepared from LN and stimulated with PMA (50 ng/ml) and ionomycin (100 ng/ml) for 5 h and brefeldin A (5 µg/ml) for 4 h. Cells were stained for surface CD4, fixed, permeabilized and then stained for intracellular IL-17 and IFN-γ.
Funding
This work was supported by grant NIH R01 [HL068597], ACR Research and Education Foundation's Within Our Reach, Arthritis Foundation, Webb Foundation, Outstanding Youth Scientist Investigator Award from National Natural Science Foundation of China [30728007], National Natural Science Foundation of China [30872557] and Program of Shanghai Subject Chief Scientist, China [08XD14033].
Acknowledgments
We thank all the members of Zheng's laboratory for helpful discussion.
Conflict of interest: none declared.
References
- Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. doi:10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N, Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. doi:10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S., Dwyer K.M., Gao W., Friedman D., Usheva A., Erat A., Chen J.F., Enjyoji K., Linden J., Oukka M., et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. doi:10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini M.C., Becker C., Monteleone G., Pallone F., Galle P.R., Neurath M.F. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. doi:10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Green A., Gorelik L., McGregor C.M., Tran E.H., Flavell R.A. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-β–TGF-β receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. doi:10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P., Boeld T.J., Eder R., Huehn J., Floess S., Wieczorek G., Olek S., Dietmaier W., Andreesen R., Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. doi:10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- Huang C.T., Workman C.J., Flies D., Pan X., Marson A.L., Zhou G., Hipkiss E.L., Ravi S., Kowalski J., Levitsky H.I. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. doi:10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Huter E.N., Stummvoll G.H., DiPaolo R.J., Glass D.D., Shevach E.M. Cutting edge: antigen-specific TGF-β-induced regulatory T cells suppress Th17-mediated autoimmune disease. J. Immunol. 2008;181:8209–8213. doi: 10.4049/jimmunol.181.12.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R., Cox T., Yasayko S.A., Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. doi:10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kohm A.P., Carpentier P.A., Anger H.A., Miller S.D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Huehn J., de la Rosa M., Maszyna F., Kretschmer U., Krenn V., Brunner M., Scheffold A., Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc. Natl. Acad. Sci. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. doi:10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Putnam A.L., Xu-Yu Z., Szot G.L., Lee M.R., Zhu S., Gottlieb P.A., Kapranov P., Gingeras T.R., Fazekas de St Groth B., et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. doi:10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Ma J., Wang X., Wang J., Zhang F., Xu B., Brand D.D., Horwitz D.A., Shi W., Zheng S.G. Synergistic effect of TGF-beta superfamily members on the induction of Foxp3+ Treg. Eur. J. Immunol. 2010a;40:142–152. doi: 10.1002/eji.200939618. doi:10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Wang J., Zhang F., Chai Y., Brand D., Wang X.H., Horwitz D.A., Shi W., Zheng S.G. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J. Immunol. 2010b;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh R.S., Whitters M.J., Piccirillo C.A., Young D.A., Shevach E.M., Collins M., Byrne M.C. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. doi:10.1016/S1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Miyara M., Amoura Z., Parizot C., Badoual C., Dorgham K., Trad S., Nochy D., Debré P., Piette J.C., Gorochov G. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J. Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- Mottet C., Uhlig H.H., Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- Pop S.M., Wong C.P., Culton D.A., Clarke S.H., Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J. Exp. Med. 2005;201:1333–1346. doi: 10.1084/jem.20042398. doi:10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. doi:10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A., Solomon M., Selby W., Alexander S.I., Nanan R., et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. doi:10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E.M. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Stromnes I.M., Goverman J.M. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. doi:10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. doi:10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia X., Stephens G., Goldbach-Mansky R., Wilson M., Shevach E.M., Lipsky P.E. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. doi:10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Kitani A., Fuss I., Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Gray J.D., Ohtsuka K., Yamagiwa S., Horwitz D.A. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J. Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J.H., Gray J.D., Soucier H., Horwitz D.A. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J., Wang P., Gray J.D., Horwitz D.A. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J., Horwitz D.A. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-{beta} are resistant to Th17 conversion by IL-6. J. Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]