Abstract
The voltage sensor is a four-transmembrane helix bundle (S1–S4) that couples changes in membrane potential to conformational alterations in voltage-gated ion channels leading to pore opening and ion conductance. Although the structure of the voltage sensor in activated potassium channels is available, the conformation of the voltage sensor at rest is still obscure, limiting our understanding of the voltage-sensing mechanism. By employing a heterologously expressed Bacillus halodurans sodium channel (NaChBac), we defined constraints that affect the positioning and depolarization-induced outward motion of the S4 segment. We compared macroscopic currents mediated by NaChBac and mutants in which E43 on the S1 segment and the two outermost arginines (R1 and R2) on S4 were substituted. Neutralization of the negatively charged E43 (E43C) had a significant effect on channel gating. A double-mutant cycle analysis of E43 and R1 or R2 suggested changes in pairing during channel activation, implying that the interaction of E43 with R1 stabilizes the voltage sensor in its closed/available state, whereas interaction of E43 with R2 stabilizes the channel open/unavailable state. These constraints on S4 dynamics that define its stepwise movement upon channel activation and positioning at rest are novel, to the best of our knowledge, and compatible with the helical-screw and electrostatic models of S4 motion.
Introduction
Voltage-gated sodium channels (Navs) are large membrane proteins that sense and respond to changes in the membrane potential by opening and allowing for inward flux of sodium ions (activation) followed by fast inactivation. This ability underlies the generation of action potentials and their mediation in excitable cells and tissues (1). Whereas the eukaryotic Nav α-subunit is composed of four homologous domains (DI-DIV), the bacterial voltage-gated sodium channel from Bacillus halodurans (NaChBac) is a homotetramer of a 6TM segment (2). Like other members of the voltage-gated ion channel superfamily, NaChBac is composed of a voltage-sensor module (voltage-sensing domain (VSD)) formed by four membrane-spanning helices (S1–S4), and a pore-forming module consisting of two membrane-spanning helices (S5–S6) with the pore-lining loop in between, as was shown in the structure of the potassium channel KvAP (3). A basic feature of the S4 transmembrane segment in the VSD is the highly conserved pattern of up to eight basic residues, mostly arginines, at every third position that are involved in voltage sensing (4–7). Of these basic residues, the four outermost contribute the majority of the gating charge measured upon movement of the charges relative to the membrane electric field (8,9). Conserved acidic residues on S2 and S3 that interact electrostatically with basic residues on S4 have been shown to affect channel folding and gating properties (10,11) and stabilize, inter alia, via electrostatic interactions the transition states of the VSD (12). It has also been demonstrated that conservative mutations of noncharged residues on S4 affect channel gating due to small steric changes in the mutated residues (13). Although a high-resolution structure of the activated VSD is available (3,14,15), the voltage-dependent conformational changes it undergoes from the resting state (at hyperpolarized potentials) to the activated state are still unknown because the structure of the resting conformation has not yet been determined.
Theoretical analyses based on the crystal structure of homotetrameric Kvs predict a conserved extracellular acidic residue on S1 that stabilizes the positively charged S4 (15–17). Indeed, it has been shown that neutralization of the corresponding residue in the Shaker Kv results in stabilization of the channel closed state, likely by precluding an electrostatic interaction that stabilizes the channel conformation in the open state (18). However, the electrostatic constraints imposed by the negatively charged extracellular S1 residue on S4 dynamics have not been studied. Therefore, in this work we monitored energy perturbations resulting from single and double nonradical substitutions of the conserved extracellular acidic S1 residue (E43) and R1 (R113) and R2 (R116) on S4 of NaChBac. This approach enabled us to differentiate channel functional properties attributed to electrostatic interactions within the VSD, while avoiding the severe effects on the channel that may occur when modifying reagents or nonconservative point mutations are applied. On the basis of the helical-screw S4 trajectory (19), a thermodynamic double-mutant cycle analysis of our results reveals putative electrostatic constraints that define the conformational state of S4 at rest and upon transition to the activated state.
Materials and Methods
Site-directed mutagenesis, cell line culture, and transfection
The gene encoding NaChBac from Bacillus halodurans was amplified via polymerase chain reaction and subcloned into the vector pCDNA3.1 (Invitrogen, San Diego, CA). Mutants were generated using standard polymerase chain reaction techniques, and their sequence integrity was verified. Chinese hamster ovary (CHO) cells, grown in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, 10% fetal calf serum, and antibiotics, were maintained at 37°C in 5% CO2/air. Briefly, cells seeded on poly-L-lysine-coated glass coverslips (13 mm in diameter) in a 24-multiwell plate were cotransfected with pIRES-CD8, as a marker for expression, along with wild-type or mutant pCDNA3.1-NaChBac. For electrophysiology, the CHO cells were visualized 2 days after transfection using the anti-CD8 antibody-coated beads method (20). All transient transfections were performed with TransIT-LT1 (Mirus, Madison, WI) according to the manufacturer's instructions.
Patch-clamp electrophysiology
Recordings of macroscopic Na+ currents mediated by NaChBac-transfected CHO cells were performed using the whole-cell configuration of the patch-clamp technique (21). Signals were amplified using a HEKA EPC10 patch-clamp amplifier (HEKA Instruments, Lambrecht/Pfalz, Germany), sampled at 10 kHz and filtered at 2.9 kHz. Data were acquired using PatchMaster 2.4 software. The results were analyzed using Igor Pro v6.04 (WaveMetrics, Portland, OR). The patch pipettes were pulled from borosilicate glass (Warner Instrument Corp., Holliston, MA) with a resistance of 3–4 MΩ and were filled with 5 mM NaCl, 105 mM CsF, 10 mM EGTA-Cs, 10 mM HEPES. The pH was adjusted to 7.4 with CsOH. The bath solution contained 150 mM NaCl, 2 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES. The pH was adjusted to 7.4 with NaOH. Cell and electrode capacitance and 75–90% of the series resistance were compensated with internal voltage-clamp circuitry. Residual linear leak and capacitance were subtracted by using a P/4 or P/5 protocol. The currents were measured from a holding potential of −140 mV in response to various test potentials, followed by a 20 s interval at the holding potential to allow for recovery from inactivation. Because the channels activate, inactivate, and recover from inactivation more rapidly at 10°C above room temperature (22), all experiments were performed at 32°C using a heated bath platform (Warner Instrument Corp.) with a custom-made temperature controller.
Data analysis
Peak currents (I) were measured after a series of 300 ms depolarizing test pulses at 10 mV increment intervals, and were plotted versus the test potentials (I-V). The conductance of wild-type NaChBac and mutants (G) was calculated from steady-state peak currents obtained from the I-V plot using the equation G = I/(V − Vrev), where V is the test potential and Vrev is the reversal potential for the Na+ current calculated from the intercept of the linear fit of currents before and after reversal. G was normalized to the maximal conductance (G/Gmax). The data points of normalized G-V curves were fitted to the two-state Boltzmann function: G/Gmax = 1/{1 + Exp[zF(V0.5 − V)/RT]}, where G/Gmax is the normalized conductance relative to Gmax, V is the test potential, V0.5 is the voltage required to elicit 50% of Gmax, z is the effective valence, F is Faraday's constant, R is the gas constant, and T is the temperature in °K.
Steady-state availability (h∞) curves, which describe the fraction of channels that did not enter the inactivation process after a depolarization pulse, were constructed using a double-pulse protocol with a 2 s conditioning prepulse and 300 ms test pulse durations. The peak current amplitudes obtained from test pulses were normalized to their maximal values and plotted versus the corresponding conditioning pulse. The fitting was performed using the two-state Boltzmann function I/Imax = 1/{1 + Exp[zF(V0.5 − Vpp)/RT]}, where Vpp is the prepulse potential and V0.5 is the potential when the probability for channel availability is 50%. The V0.5- and z-values from the two-state Boltzmann fitting were used to estimate changes in Gibb's free energy (ΔG) for channel activation and availability assuming a two-state transition (from closed to open or from available to unavailable) at test potential Vt using the equation ΔG = 0.2389zF(V0.5 − Vt). To avoid errors arising from overextrapolation of ΔG, we used Vt-values as close as possible to the informative voltage range of channel activation (or availability) that elicits 10–90% of channel open probability (Po). Unless otherwise stated, Vt for ΔG calculations from steady-state activation and availability curves was −40 and −85 mV, respectively. Changes in ΔG (ΔΔG) for each mutation were estimated by ΔΔG = ΔGWT→Mut = ΔGMut − ΔGWT. The free energy that couples two sites in the protein was calculated by ΔΔGcoupling = (ΔGMut1 − ΔGWT) − (ΔGMut1+Mut2 − ΔGMut2).
Results
Effects of substitution E43C on the steady-state activation and availability of NaChBac
S1–S4 sequence alignment among representative members of the S4-based VSDs indicates that the acidic residue at the extracellular side of S1 (E43 in NaChBac) is highly conserved (Fig. 1). We neutralized this acidic residue by substitution with cysteine, as was previously demonstrated for other acidic residues on the VSD of NaChBac (23). We then tested the effect of this substitution on gating properties of NaChBac expressed in CHO cells by recording currents using the whole-cell, patch-clamp configuration. A series of increasing depolarizing pulses ranging from −140 mV to +60 mV, and depending on the mutant threshold of activation, were used to generate normalized steady-state activation curves (G-V) and steady-state inactivation curves (channel availability curves; Fig. 2 and Tables 1 and 2). A comparison of the normalized G-V curves obtained from the wild-type channel and the E43C channel mutant revealed a 47 mV shift in V0.5 toward the depolarizing potentials from V0.5(WT) = −55.6 mV ± 0.6 (n = 11) to V0.5(E43C) = −8.4 mV ± 2.4 (n = 12; Fig. 2, solid symbols). A 13 mV shift in the same direction was obtained for the availability curves from V0.5(WT) = −79.1 mV ± 1.3 (n = 7) to V0.5(E43C) = −66.4 mV ± 1.3 (n = 5; Fig. 2, open symbols). The shifts in G-V and the availability curves were reflected by changes in ΔG (ΔGWT→E43C) of 2.7 kcal/mol and 0.9 kcal/mol, respectively (see Fig. 4, A and B). Since higher potentials were required to activate the mutant channel compared to the unmodified channel, it is likely that E43 stabilizes the channel open state, whereas the E43C substitution stabilizes the channel in its closed state.
Figure 1.

Comparison of S1–S2 transmembrane segments in various VSDs. Sequence alignment of S4-based VSDs was performed using T-Coffee, and only the S1–S2 regions are presented. The sequences include NaChBac (NCBI Entrez Protein Database accession number BAB05220), rNav1.2 DI-DIV (NP_036779), Cav3.1 DI-DIV (O43497), Kv1.2 (NP_032443), Kv3.2 (P22462), Kv4.1 (BAA96454), KvAP (Q9YDF8), Hv1 (NP_001035196), and Ci-VSP (NP_001028998). The positions of conserved acidic residues are designated by black boxes. Intersegmental loops are represented by double slash signs. Residue numbers correspond to NaChBac.
Figure 2.
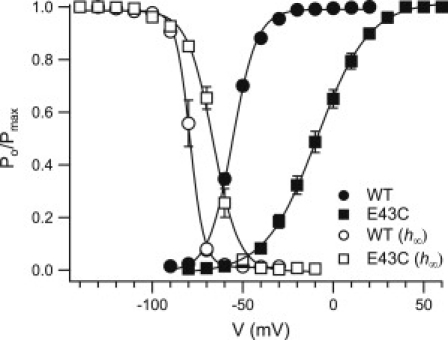
Gating properties of the E43C NaChBac mutant. The voltage dependence of activation (solid symbols) and channel availability (h∞; open symbols) for wild-type NaChBac and E43C mutant is shown. Conductance and availability-voltage relationships were determined as described in Materials and Methods. The mean value of the data points is expressed as the normalized channel open probability [Po/Pmax] ± SE. See Tables 1 and 2 for details.
Table 1.
Activation parameters for NaChBac and mutant channels derived from steady-state activation curves (G-V)
| Channel | V0.5 ± SE∗ (mV) | z ± SE∗ (e0) | ΔG ± SE∗ (kcal/mol)† | ΔG ± SE∗(kcal/mol)‡ | n |
|---|---|---|---|---|---|
| WT | −55.59 ± 0.61 | 3.94 ± 0.13 | −1.41 ± 0.08 | 1.29 ± 0.06 | 11 |
| E43C | −8.43 ± 2.40 | 1.88 ± 0.08 | 1.30 ± 0.10 | 2.55 ± 0.12 | 12 |
| R1K | −61.74 ± 1.50 | 2.54 ± 0.13 | −1.25 ± 0.16 | 0.44 ± 0.08 | 10 |
| R2K | −35.08 ± 1.57 | 1.83 ± 0.09 | 0.15 ± 0.06 | − | 12 |
| E43C-R1K | −13.58 ± 0.83 | 2.03 ± 0.03 | 1.23 ± 0.05 | 2.63 ± 0.06 | 9 |
| E43C-R2K | −45.16 ± 0.73 | 2.31 ± 0.22 | −0.28 ± 0.06 | − | 6 |
Mean ± standard error (SE).
At −40 mV.
At −70 mV.
Table 2.
Availability parameters for NaChBac and mutants derived from steady-state availability-V relationships
| Channel | V0.5 ± SE∗ (mV) | z ± SE∗ (e0) | ΔG ± SE∗ (kcal/mol)† | n |
|---|---|---|---|---|
| WT | −79.09 ± 1.32 | 6.46 ± 0.29 | 0.89 ± 0.25 | 7 |
| E43C | −66.44 ± 1.35 | 4.19 ± 0.19 | 1.78 ± 0.15 | 5 |
| R1K | −98.66 ± 1.27 | 5.28 ± 0.26 | −1.71 ± 0.19 | 8 |
| R2K | −76.66 ± 0.95 | 6.34 ± 0.13 | 1.23 ± 0.14 | 5 |
| E43C-R1K | −78.74 ± 1.11 | 2.34 ± 0.06 | 0.34 ± 0.06 | 7 |
| E43C-R2K | −86.45 ± 2.30 | 3.04 ± 0.20 | −0.13 ± 0.20 | 7 |
Mean ± SE.
At −85 mV.
Figure 4.
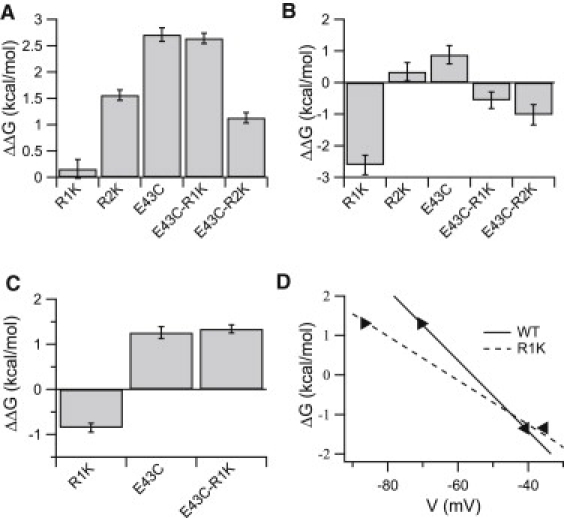
Calculated effects of the single and double mutations on activation and availability of NaChBac. (A and B) Bar graph depicting the effect of the indicated mutant on the ΔΔG value (ΔGWT→Mut = ΔGMut − ΔGWT) for (A) channel activation and (B) channel availability. (C) Bar graph describing the effect of E43C and R1K single and double mutations on ΔΔG for channel activation at −70 mV. Error bars represent the mean ± SE. (D) ΔG-voltage relationship for activation of the wild-type and R1K mutant channels. The left and right black triangles indicate voltages that elicited the open probability of 10% and 90% of the channels, respectively.
Evaluation of E43 interactions with R1 and R2 by double-mutant cycle analysis
E43 in NaChBac is analogous to E183 in Kv1.2, which belongs to an external negatively charged cluster on the VSD (15). In S4 of NaChBac, R1 and R2 (but not R3 or R4) are accessible for membrane-impermeant thiol reagents (24). Therefore, we examined the putative interactions of E43 with the two outermost arginines on S4 by their conservative substitution with another basic residue, lysine, to minimize perturbation in VSD function, as previously demonstrated by Chahine et al. (25). Energetic perturbations (ΔG) in channel activation and availability due to the mutations were calculated from normalized G-V and availability curves fitted with a two-state Boltzmann function (Fig. 3; curve parameters are shown in Tables 1 and 2). The ΔG perturbations (ΔGWT→Mut) of channel activation and availability for each single- and double-channel mutant were calculated. As is evident in Fig. 4, A–C, the calculated ΔΔG for each double mutant was not the sum of the ΔΔG values calculated for each mutation alone (nonadditive), indicating interdependence in each of the residue pairs. Since R1K substitution shifted the V0.5 of the G-V curve to more hyperpolarized potentials and simultaneously lowered its slope (z), the ΔG-V lines, derived from the normalized G-V curves of the wild-type and mutant R1K, intersect at ∼−45 mV where ΔΔG equals zero (Fig. 4 D). Accordingly, the significant left shift of the G-V curve of NaChBac upon R1K substitution is concealed if the reference potential, Vt, for the ΔG calculation is ∼≥45 mV (Figs. 3 A and 4 D). Therefore, for the analysis of steady-state activation involving R1, we calculated the ΔΔG values at both −40 mV and −70 mV. The changes in ΔG of the R1K mutant (ΔGWT→R1K) were −2.6 kcal/mol for channel availability (Fig. 4 B) and insignificant for the steady-state activation at −40 mV (Fig. 4 A). Nevertheless, at −70 mV, the ΔΔG value for the steady-state activation of R1K mutant was −0.85 kcal/mol (Fig. 4 C). These results indicate that the R1K mutation stabilized the open/unavailable state of the channel at relatively hyperpolarized potentials. Introduction of the R1K substitution into the background of mutant E43C resulted in a −1.4 kcal/mol perturbation in ΔG for channel availability (Fig. 4 B), without a significant effect on ΔΔG for activation (Fig. 4 A; ΔGE43C→E43C-R1K), implying that the stabilizing effect on the open state of the channel caused by the R1K substitution had diminished. Although the R1K effect on channel availability in the background of E43C was apparently significant at hyperpolarized potentials, the gradual availability curve obtained (Fig. 3 C and Fig. S2 in the Supporting Material) indicates a reduced contribution of R1K substitution to channel availability in the background of the E43C mutant.
Figure 3.
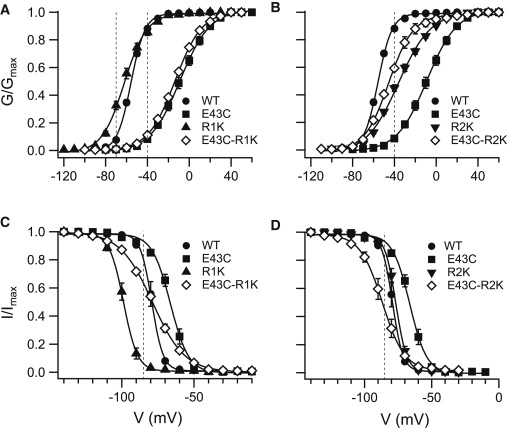
Gating properties of single and double NaChBac mutants E43C on S1, and R1K and R2K on S4. (A) Voltage dependence of activation for the single NaChBac mutants E43C, R1K and the double mutant E43C-R1K. (B) Voltage dependence of activation for the single NaChBac mutants E43C, R2K and the double mutant E43C-R2K. (C) Voltage dependence of channel availability for the single NaChBac mutants E43C, R1K and the double mutant E43C-R1K. (D) Voltage dependence of channel availability for the single NaChBac mutants E43C, R2K and the double mutant E43C-R2K. Conductance and availability-voltage relationships were determined as described in Materials and Methods. Vertical dashed lines indicate the reference potential (Vt) used in the ΔG calculations. The value of the data points is expressed as the mean ± SE. See Tables 1 and 2 for details.
In contrast to R1K, the ΔG perturbations in the R2K mutant were 1.6 kcal/mol for the steady-state activation (Fig. 4 A) and negligible (0.34 ± 0.29 kcal/mol) for the channel availability (Fig. 4 B). These results suggest that the R2K substitution stabilized the closed state of the channel. However, introduction of the R2K substitution into the background of mutant E43C resulted in a −1.6 kcal/mol perturbation in ΔG for activation (Fig. 4 A; ΔGE43C→E43C-R2K) and a −1.9 kcal/mol perturbation in ΔG for channel availability (Fig. 4 B). These results indicate that R2K in the background of E43C stabilized the open available state of the channel.
The coupling energy (ΔΔGcoupling) for the E43C/R1K pair was −1.2 kcal/mol, as calculated from the availability curves (Fig. 5, second bar from the right). This value is compatible (in magnitude and direction) with the coupling energy for channel activation at −70 mV with ΔΔGcoupling of −0.9 kcal/mol (Fig. 5, second bar from the left). The ΔΔGcoupling for the E43C/R2K pair was 3.15 kcal/mol and 2.2 kcal/mol, respectively, as per activation and channel availability (Fig. 5). Since <3 kcal/mol is required for wild-type channel activation in the linear range of the ΔG-V relation (10% < Po < 90%; Fig. 4 D, range between triangles), the coupling energies obtained here are physiologically significant. Overall, these results draw a dynamic picture of the interactions of E43 with either R1 or R2 during channel activation.
Figure 5.
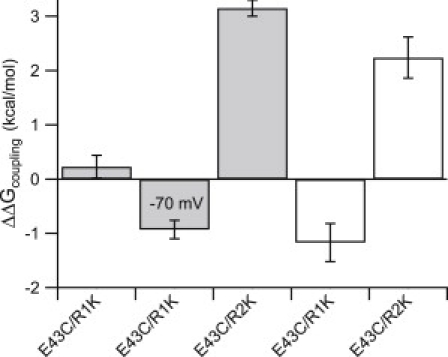
Double-mutant cycle analysis for NaChBac. The bar graph depicts the coupling energies (ΔΔGcoupling) of the indicated pairs calculated for channel activation (gray bars) and channel availability (white bars). Note that the second bar provides the ΔΔGcoupling for E43C/R1K at −70 mV (for more details, see Materials and Methods). Error bars represent the mean ± SE.
Discussion
The double-mutant cycle analysis suggests coupling (e.g., electrostatic) between E43 and R1 or R2. The 47 mV shift in NaChBac activation (V0.5) toward depolarizing potentials, when the charge of E43 was eliminated (substitution E43C), is in agreement with results previously obtained for neutralization of the corresponding residue in the Shaker Kv (18), and the right-shift upon substitution of the corresponding negatively-charged residue on DII of the rat brain sodium channel rNav1.2 (see Fig. 5 a in Cestèle et al. (26)). The coupling energies of E43C and R1K were evident from the insignificant contribution to the free-energy perturbations of the double mutant compared to the E43C mutant. The left shift of the activation and availability curves upon R1K substitution (destabilization of the closed/available state) together with the calculated coupling energy of E43C/R1K implies that the interaction of E43 with R1 in the wild-type channel is essential for stabilization of the closed/available state, as was apparent at membrane potentials below −45 mV. On the other hand, the right shift of the activation curve upon R2K substitution (stabilization of the closed state) along with the large E43C/R2K coupling energies implies that the interaction of E43 with R2 stabilizes the open/unavailable state during channel activation. Surprisingly, despite the stabilizing effect of either E43C or R2K substitutions on the closed/available state of the channel, the double mutation E43C/R2K stabilizes the open/unavailable state, as inferred from the perturbations in ΔG and the high coupling energies obtained (Figs. 4, A and B, and 5). This opposing effect may result from compensatory changes in energetic minima arising in each single channel mutant for the transition between states (Fig. 3, B and D).
The stabilization of the closed state of the Shaker Kv channel upon substitution of E247W (equivalent to E43 in NaChBac) was interpreted as a result of destabilization of the open state by precluding the formation of a salt bridge observed in the crystal structure of the open conformation (18). Our results obtained for the single and double mutations suggest that it is not the interaction with R1 but the elimination of the ability of E43 to interact with R2, a possible intermediate state during the activation process, that contributes to stabilization of the closed state of the channel. When R1 is substituted, E43 can readily interact with R2 (and probably with R3; see below) and stabilize the channel open/unavailable state, as shown in Fig. 3, A and C.
Overall, our double-mutant cycle analysis confirms the interactions between E43 on S1 and R1 and R2 on S4, and, on the basis of the structural and functional viewpoints of the helical screw (19) and electrostatic (12) models of S4 motion, enables us to dissect the conformational rearrangements of the VSD during channel activation as follows: According to the helical-screw model, S4 rotates by ∼60° about its axis and translates ∼4.5 Å along its axis for every moving step upon membrane depolarization, which brings every emerging arginine to the spatial position of the adjacent arginine three residues apart. In the electrostatic model, the S4 movement is funneled by a series of local energetic minima that correspond to electrostatic interactions of positive charges on S4 with negative charges on S2 and S3. Each helical-screw step corresponds to a shift of S4 between two energetic minima via thermal transitions. A recent study (17) suggested a number of open and closed putative conformations of the VSD in a structural model of NaChBac, two of which—Closed2 and Open1 (Fig. 6)—are most relevant to the mutations examined in our study. Therefore, in the initial stage of our modeling, we examined the transition of a single helical screw step between the Closed2 and Open1 conformations. Qualitative changes in equilibrium between these conformations that are defined by the electrostatic interactions of positively charged groups on S4 with negatively charged residues within the VSD upon mutagenesis are schematically illustrated in Fig. 7. According to this model, neutralization of the charge in E43 stabilizes the closed state of the channel (Fig. 7, A and B) by disabling its electrostatic interaction with R2. Along this line, the E43C substitution disables as well an electrostatic interaction with R1, and therefore subsequent R1K substitution has no net effect on the equilibrium in favor of the open or the closed states of the channel (Fig. 7 C). The R2K substitution in the background of E43C destabilizes the closed state because the probability of electrostatic interaction between D60 (on S2) and R2 decreases, whereas the interaction of D60 with R3 is promoted, thus stabilizing the open state of the channel (Fig. 7 D). Our results fit well with the qualitative projections of this model, which unequivocally explains changes in channel gating properties upon each combination of substitutions, including the apparently puzzling reduced energetic barrier for channel activation of the double mutant E43C-R2K compared to the corresponding single mutants.
Figure 6.
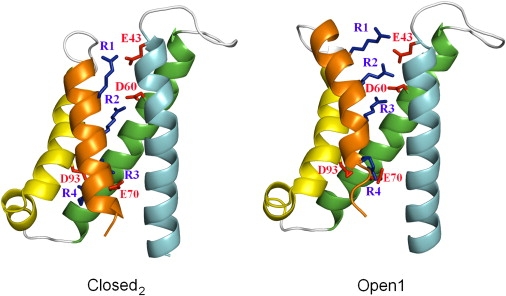
Ribbon representations of NaChBac VSD. S1, cyan; S2, green; S3, yellow; S4, orange. Negatively charged residues of S1–S3 are colored in red, R1–R4 on S4 are colored in blue. The Closed2 model of NaChBac (left) and Open1 model of NaChBac (right) are shown. The VSD model of NaChBac was provided by Shafrir et al. (17) and the structural representation was generated using PyMOL (DeLano Scientific).
Figure 7.
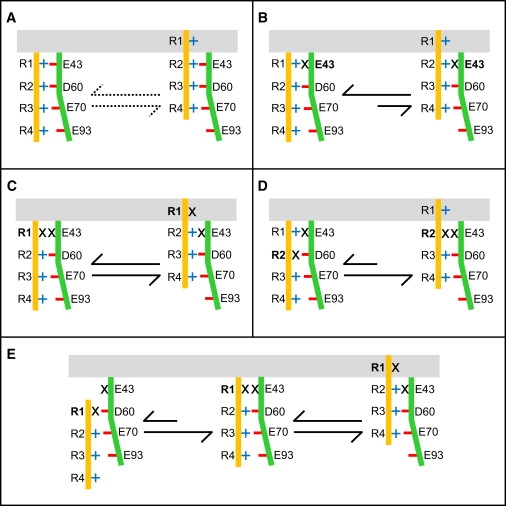
Transition among putative VSD conformations in NaChBac and mutants. The schematic model describes electrostatic interactions and movement of S4 (orange rod) relative to S1-S2-S3 (green). Interacting residues are according to Shafrir et al. (17). Blue plus signs represent arginines (R1–R4) on S4; red minus signs represent acidic residues E43 (S1), D60 (S2), E70 (S2), and E93 (S3); black X signs on S4 represent R1K or R2K substitutions, as well as the E43C substitution. The gray area designates the putative lipid/water interface. (A) Dotted arrows represent the baseline equilibrium in the wild-type. Black arrows represent equilibrium according to the electrostatic model between the Closed2 to Open1 conformation upon substitutions (in bold) of (B) E43C, (C) R1K in the background of E43C, and (D) R2K in the background of E43C. Note that all transitions comply with the experimental results. (E) Transition through Closed1-Closed2-Open1 conformations upon R1K substitution in the background of E43C stabilizes the open state according to the electrostatic model. The incongruity of the transition from Closed1 to Closed2 conformation with the results of channel activation negates the prevalence of the Closed1 conformation at rest.
Because more than two transition states have been suggested for the entire process of channel activation (17,19,27,28), we sought to determine whether additional states other than Closed2 and Open1 would comply with our model. One of these transition states, Open2, positions S4 one helical screw step outward relative to the Open1 conformation, such that R3 (R119 in NaChBac) opposes E43 (17). According to our model, the transition from Closed2 to Open2 in any of the mutants examined here would not affect the equilibrium trend described for the transition from the Closed2 to Open1 conformation. This may suggest that an open channel includes the Open2 conformation. Another transition state, Closed1, positions S4 one helical screw step inward relative to the Closed2 state, such that R1 opposes D60 (Fig. 7 E, left) (17). The predicted effect of E43C/R1K double mutation on the equilibrium among three channel states in the activation process, including the Closed1 conformation, is illustrated in Fig. 7 E. The R1K substitution in the background of E43C (Fig. 7 E, left) stabilizes the Closed2 conformation over Closed1 (Fig. 7 E, middle) because 1K (lysine that replaces R1) may weakly interact with D60, whereas the interaction of R2 with D60 is energetically favored. The transition that follows to the Open1 state (right) is then similar to that described in Fig. 7 C, with no change in the equilibrium of channel activation. Of note, the transition of the E43C-R1K double mutant from the Closed1 (Fig. 7 E, left) to Closed2 conformation (Fig. 7 E, middle) is analogous to the transition that the E43C-R2K double mutant undergoes from the Closed2 to Open1 conformation (Fig. 7 D). A Closed1-Closed2-Open1 transition upon R1K substitution in the background of E43C, as depicted in Fig. 7 E, would overall stabilize the open state of the channel. However, this transition, as shown on the left-hand side of Fig. 7 E, is incompatible with the lack of effect shown in our results, and is simulated to demonstrate that the trajectories of R1 and R2 regarding their interactions with E43 are likely dissimilar. This consideration and the opposing effects of the single R1K and R2K substitutions compared with their effects in the background of E43C suggest that the prevalent resting conformation of the VSD is unlikely to position S4 further inward than its position in the Closed2 conformation where R1 opposes E43. Yang et al. (29) reached a similar conclusion regarding the position of S4 at rest when the N-terminal residue on S4 of the Shaker K+ channel was substituted (L361R). Of note, our conclusion about the prevailing VSD conformation at rest disagrees with previous models suggested for the Shaker channel (15,30,31).
The transition from the Closed2 to Open2 conformation will relocate S4 ∼9 Å along its axis and rotate it by ∼120° if a rigid S4 α-helix is assumed (19). This S4 trajectory is in agreement with recent luminescence resonance energy transfer measurements of 10 ± 5 Å with a vertical component of 5 ± 2 Å (32).
Our suggested constraint for the resting state is also supported by previous ω-scan assays (33). Substitution of R1 on S4 in the Shaker Kv to histidine (34) or to a smaller uncharged residue renders the hydrophobic plug that separates the external and internal solutions in the VSD permeable to ions (the ω current) in the resting conformation (35). The ω conductance pathway was analyzed using a series of mutations added in the background of an ω conducting R1S Shaker channel mutant (33). The large perturbations in the ω current upon substitution of E247D on S1 (corresponding to E43 in NaChBac), E283D on S2 (corresponding to D60 in NaChBac), and R2Q on S4 suggest that they are in the pathway of the ω current. These data are consistent with R1 and R2 being opposed to E247 and E283, respectively, at rest (analogously to the Closed2 conformation) because they support an ω current pathway < 10 Å in length. In this respect, the previously suggested constraint obtained when R1 is in close proximity to E283 (30,31) or further inward (15) is inconsistent with a narrow (<10 Å) hydrophobic plug within the VSD (36–39).
In conclusion, the S1 extracellular negatively charged residue imposes constraints (presumably electrostatic) on the S4 position at rest and upon activation of NaChBac. Because our results are in agreement with previous findings for the Shaker potassium channel (33), the highly conserved charged residues and architecture of the VSD module suggest that these constraints may fit other S4-based VSDs. The definition of the S4 position at rest can be used as a baseline constraint for constructing models that can be examined experimentally and computationally to elucidate the detailed trajectory of S4 upon channel activation. It also provides a starting point for further calculations of the driving force that enables channel opening.
Supporting Material
Two figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(10)00551-5.
Supporting Material
Acknowledgments
The authors thank R. Guy for providing the coordinates of the NaChBac models shown in Fig. 6 and for his critical reading of the manuscript, and A. Peretz for valuable technical assistance and comments on the manuscript.
This research was supported by United States-Israel Binational Agricultural Research and Development grant IS-3928-06 and Israeli Science Foundation grant 107/08.
References
- 1.Hille B. Sinauer Associates; Sunderland, MA: 2001. Ion Channels of Excitable Membranes. [Google Scholar]
- 2.Ren D., Navarro B., Clapham D.E. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y., Lee A., MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 4.Liman E.R., Hess P., Koren G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature. 1991;353:752–756. doi: 10.1038/353752a0. [DOI] [PubMed] [Google Scholar]
- 5.Logothetis D.E., Movahedi S., Nadal-Ginard B. Incremental reductions of positive charge within the S4 region of a voltage-gated K+ channel result in corresponding decreases in gating charge. Neuron. 1992;8:531–540. doi: 10.1016/0896-6273(92)90281-h. [DOI] [PubMed] [Google Scholar]
- 6.Papazian D.M., Timpe L.C., Jan L.Y. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- 7.Stühmer W., Conti F., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S.K., MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- 9.Seoh S.A., Sigg D., Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- 10.Papazian D.M., Shao X.M., Wainstock D.H. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron. 1995;14:1293–1301. doi: 10.1016/0896-6273(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari-Woodruff S.K., Schulteis C.T., Papazian D.M. Electrostatic interactions between transmembrane segments mediate folding of Shaker K+ channel subunits. Biophys. J. 1997;72:1489–1500. doi: 10.1016/S0006-3495(97)78797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecar H., Larsson H.P., Grabe M. Electrostatic model of S4 motion in voltage-gated ion channels. Biophys. J. 2003;85:2854–2864. doi: 10.1016/S0006-3495(03)74708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith-Maxwell C.J., Ledwell J.L., Aldrich R.W. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J. Gen. Physiol. 1998;111:421–439. doi: 10.1085/jgp.111.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long S.B., Campbell E.B., Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 15.Long S.B., Tao X., MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 16.Nishizawa M., Nishizawa K. Molecular dynamics simulation of Kv channel voltage sensor helix in a lipid membrane with applied electric field. Biophys. J. 2008;95:1729–1744. doi: 10.1529/biophysj.108.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shafrir Y., Durell S.R., Guy H.R. Models of voltage-dependent conformational changes in NaChBac channels. Biophys. J. 2008;95:3663–3676. doi: 10.1529/biophysj.108.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S.-Y., Banerjee A., MacKinnon R. Two separate interfaces between the voltage sensor and pore are required for the function of voltage-dependent K(+) channels. PLoS Biol. 2009;7:e47. doi: 10.1371/journal.pbio.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy H.R., Seetharamulu P. Molecular model of the action potential sodium channel. Proc. Natl. Acad. Sci. USA. 1986;83:508–512. doi: 10.1073/pnas.83.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurman M.E., Boland L.M., Yellen G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- 21.Hamill O.P., Marty A., Sigworth F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 22.Correa A.M. Temperature modulation of NaChBac gating. Biophys. J. 2004;86:114a. (Abstract) [Google Scholar]
- 23.Blanchet J., Pilote S., Chahine M. Acidic residues on the voltage-sensor domain determine the activation of the NaChBac sodium channel. Biophys. J. 2007;92:3513–3523. doi: 10.1529/biophysj.106.090464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchet J., Chahine M. Accessibility of four arginine residues on the S4 segment of the Bacillus halodurans sodium channel. J. Membr. Biol. 2007;215:169–180. doi: 10.1007/s00232-007-9016-1. [DOI] [PubMed] [Google Scholar]
- 25.Chahine M., Pilote S., Sato C. Role of arginine residues on the S4 segment of the Bacillus halodurans Na+ channel in voltage-sensing. J. Membr. Biol. 2004;201:9–24. doi: 10.1007/s00232-004-0701-z. [DOI] [PubMed] [Google Scholar]
- 26.Cestèle S., Yarov-Yarovoy V., Catterall W.A. Structure and function of the voltage sensor of sodium channels probed by a β-scorpion toxin. J. Biol. Chem. 2006;281:21332–21344. doi: 10.1074/jbc.M603814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catterall W.A. Molecular properties of voltage-sensitive sodium channels. Annu. Rev. Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- 28.Durell S.R., Hao Y., Guy H.R. Structural models of the transmembrane region of voltage-gated and other K+ channels in open, closed, and inactivated conformations. J. Struct. Biol. 1998;121:263–284. doi: 10.1006/jsbi.1998.3962. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y.-C., Own C.-J., Kuo C.-C. A hydrophobic element secures S4 voltage sensor in position in resting Shaker K+ channels. J. Physiol. 2007;582:1059–1072. doi: 10.1113/jphysiol.2007.131490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathak M.M., Yarov-Yarovoy V., Isacoff E.Y. Closing in on the resting state of the Shaker K(+) channel. Neuron. 2007;56:124–140. doi: 10.1016/j.neuron.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Yarov-Yarovoy V., Baker D., Catterall W.A. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K(+) channels. Proc. Natl. Acad. Sci. USA. 2006;103:7292–7297. doi: 10.1073/pnas.0602350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posson D.J., Selvin P.R. Extent of voltage sensor movement during gating of Shaker K+ channels. Neuron. 2008;59:98–109. doi: 10.1016/j.neuron.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tombola F., Pathak M.M., Isacoff E.Y. The twisted ion-permeation pathway of a resting voltage-sensing domain. Nature. 2007;445:546–549. doi: 10.1038/nature05396. [DOI] [PubMed] [Google Scholar]
- 34.Starace D.M., Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- 35.Tombola F., Pathak M.M., Isacoff E.Y. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 36.Ahern C.A., Horn R. Focused electric field across the voltage sensor of potassium channels. Neuron. 2005;48:25–29. doi: 10.1016/j.neuron.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Asamoah O.K., Wuskell J.P., Bezanilla F. A fluorometric approach to local electric field measurements in a voltage-gated ion channel. Neuron. 2003;37:85–97. doi: 10.1016/s0896-6273(02)01126-1. [DOI] [PubMed] [Google Scholar]
- 38.Campos F.V., Chanda B., Bezanilla F. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc. Natl. Acad. Sci. USA. 2007;104:7904–7909. doi: 10.1073/pnas.0702638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islas L.D., Sigworth F.J. Electrostatics and the gating pore of Shaker potassium channels. J. Gen. Physiol. 2001;117:69–89. doi: 10.1085/jgp.117.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


