Abstract
Increasing evidence suggests that intracellular H+ directly stimulates large-conductance Ca2+- and voltage-activated K+ (Slo1 BK) channels, thus providing a crucial link between membrane excitability and cell metabolism. Here we report that two histidine residues, His365 and His394, located in the intracellular RCK1 domain serve as the H+ sensors of the Slo1 BK channel. Activation of the channel by H+ requires electrostatic interactions between the histidine residues and a nearby negatively charged residue involved in the channel’s high-affinity Ca2+ sensitivity. Reciprocally, His365 and His394 also participate in the Ca2+-dependent activation of the channel, functioning as Ca2+ mimetics once protonated. Therefore, a common motif in the RCK1 domain mediates the stimulatory effects of both H+ and Ca2+, and provides a basis for the bidirectional coupling of cell metabolism and membrane electrical excitability.
Normal functions of excitable cells, such as neurons and muscle cells, critically depend on an intimate and finely tuned regulation of membrane excitability by the cellular metabolic state. The metabolism, in turn, is reciprocally influenced by homeostasis of intracellular ions, and dysregulation of the ion concentrations accompanies many forms of abnormal excitability such as excitoxicity following ischemia/hypoxia 1 and also in neurodegenerative diseases 2. Among the intracellular ion species, Ca2+ and H+ are particularly important, functioning in an intertwined manner; H+ can interfere with numerous Ca2+-dependent processes and alters the intracellular Ca2+ concentration ([Ca2+]i) 3–5 and, in turn, alterations in [Ca2+]i modulate the intracellular H+ homeostasis 6.
One of the key coupling mechanisms between the cellular metabolism and electrical excitability is the Slo1 (also known as KCNMA1) BK channel 7,8. The two distinguishing features of the channel, large conductance and synergic activation by membrane depolarization and intracellular Ca2+, allow the channel to exert a negative feedback influence on cellular excitability under many physiological conditions, especially in neurons and muscle cells 9. Consistent with this functional role, opening of BK channels often plays a cell-protective role against excitoxicity following hypoxia/ischemic insults 10,11.
As in other voltage-gated K+ channels, four pore-forming Slo1 subunits form a functional BK channel complex, frequently with up to four auxiliary β subunits in a tissue-specific manner 12. The transmembrane segments with its N-terminus facing the extracellular side contain the voltage sensor, and the large cytoplasmic domain is postulated to possess multiple divalent cation sensors within a putative cytoplasmic structured termed a “gating ring”, most probably comprised of four sets of dimers made of RCK1 (regulator of conductance for K+) and RCK2 domains 13,14. The octameric gating ring is considered to expand when Ca2+ binds to the putative high-affinity divalent cation sensor in the RCK1 domain and/or the Ca2+ bowl in the RCK2 domain near the distal C-terminus 15. This ligand binding then facilitates opening of the channel’s gate 15 perhaps by increasing the tension on the spring-like linker segment between the gate and the RCK domains 16. While many aspects of the Ca2+-dependent activation of the channel, such as the molecular and biophysical characteristics of the Ca2+ sensors, remain elusive, electrophysiological studies collectively suggest that Ca2+ facilitates activation of the voltage sensor and opening of the gate so that the channel opens more frequently at more negative voltages 15,17. This allosteric gating of the BK channel is modulated by a variety of intracellular signaling events, and the RCK1/RCK2 domains contain multiple sites critical for modulation of the BK channel such as that by oxidation 18, heme binding 19,20, and phosphorylation 21.
Intracellular H+ is also a potent modulator of BK channel function 22–24. While some conflicting reports exist whether native BK channels are stimulated or inhibited by a decrease in intracellular pH (pHi), recent evidence shows that, unlike most other K+ channels, Slo1 BK channels measured under defined conditions are robustly activated by a decrease in pHi near the physiological level 22,23. This stimulatory effect of low pHi may be pathophysiologically significant in early stages of hypoxia/ischemia during which pHi typically falls by 0.5 to 1 unit 1. Despite the potential physiological and pathophysiological relevance, the molecular mechanism of the Slo1 BK channel activation by low pHi has remained elusive. Our study presented here using human Slo1 BK channels reveals that each Slo1 subunit has a common molecular sensing domain for Ca2+ and H+ containing two histidine residues and a negatively-charged residue and that the electrostatic interactions encompassing these residues is altered by H+ or by Ca2+ to facilitate opening of the channel. Therefore, the multi-ligand sensor pockets in the Slo1 BK channel provide a direct molecular mechanistic link between the cellular excitability and metabolism.
RESULTS
Low intracellular pH activates native and recombinant BK channels
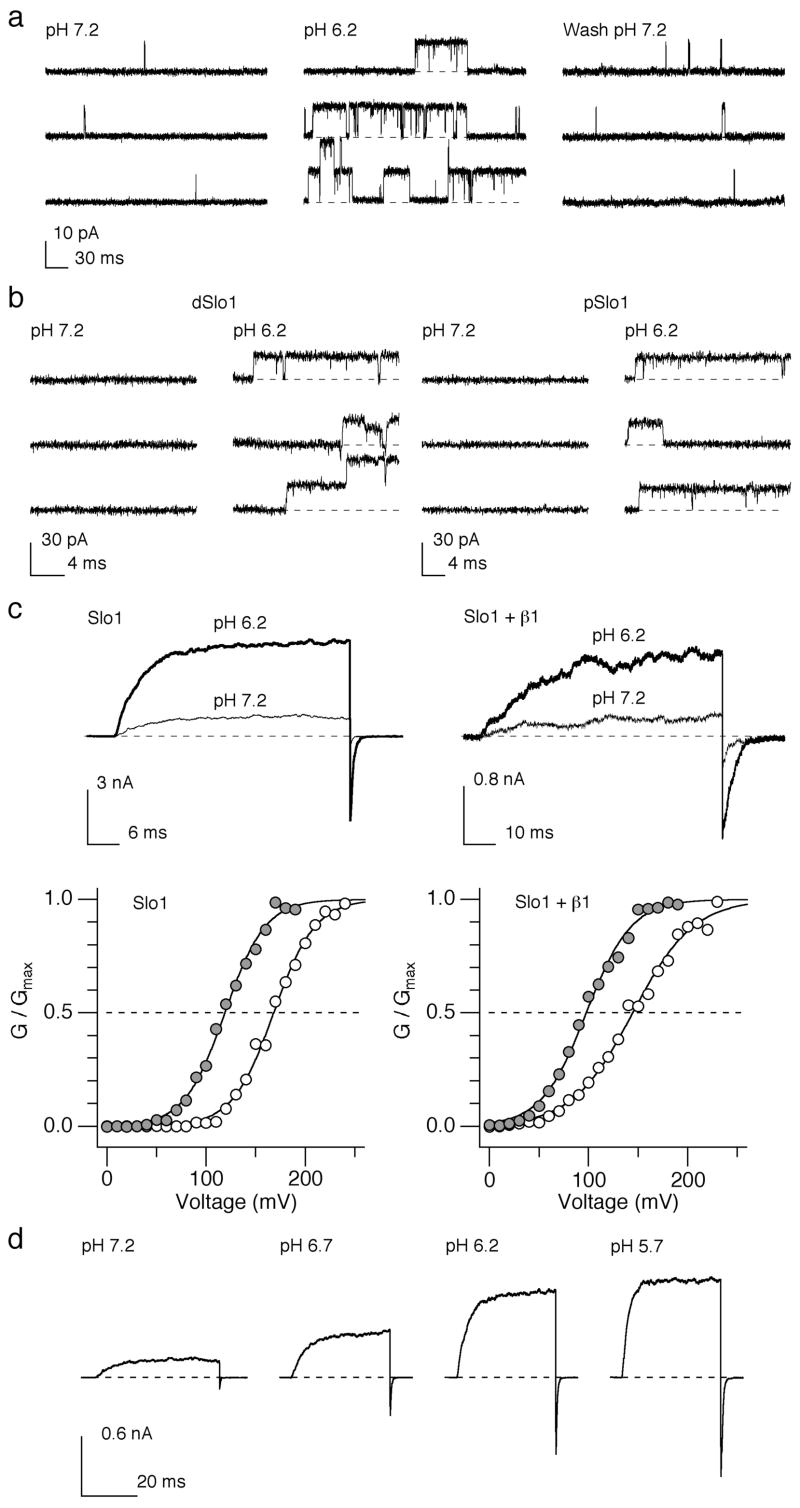
The activity of native Slo1 BK channels in rat aortic smooth muscle cells substantially increased when pHi was lowered from 7.2 to 6.2 (Fig. 1a). The channel open probability and mean open duration at –40 mV in the presence of 200 nM [Ca2+]i increased by 13.3 ± 2.8 and 3.2 ± 0.8 fold, respectively, without any effect on the single-channel current size. In contrast, a decrease in extracellular pH from 7.2 to 6.2 did not produce any significant effect (data not shown). The stimulatory effect of low pHi was observed not only for recombinant human Slo1 (hSlo1) BK channels 23 but also for Slo1 channels from the fruit fly Drosophila melanogaster (dSlo1) and the cockroach Periplaneta americana (pSlo1; Fig. 1b), suggesting that the underlying molecular machinery is conserved in Slo1 channels from different species and resides in the pore-forming Slo1 α subunit of the BK channel. To identify whether the β1 subunit, the predominant type of auxiliary β subunits in vascular smooth muscle cells, has any modulating effect, we compared the effects of a pHi decrease on heterologously-expressed hSlo1 and hSlo1+β1 channels. A decrease in pHi by 1 unit from 7.2 prominently increased the macroscopic hSlo1 and hSlo1+β1 K+ currents and shifted the conductance-voltage (G-V) curve to the negative voltage by about 50 mV in both types of channels (Fig. 1c). The simulation effect of H+ was concentration dependent with an apparent EC50 of ~0.3 ⎧M or pH 6.5 (Fig. 1d) 23. In addition to the leftward shift in G-V, lowering pHi noticeably accelerated activation kinetics and slowed deactivation kinetics of the channel (Supplementary Fig. 1) 23.
Figure 1.
Low pHi enhances the native and recombinant BK channel activity. (a) Representative single-channel current openings in response to pulses from –80 to –40 mV from a rat aortic smooth muscle cell at pHi=7.2, 6.2 and after wash at 7.2. (b) Representative openings elicited by pulses from 0 mV to 160 mV in membrane patches from HEK cells expressing Drosophila melanogaster Slo1 (dSlo1; left) and Periplaneta americana Slo1 (pSlo1; right). (c) Representative macroscopic current traces at pHi=7.2 and 6.2 through by heterologously-expressed hSlo1 and hSlo1+β1 channels. The currents were activated by depolarization from 0 mV to 100 mV (hSlo1) and 70 mV (hSlo1+β1) in the absence of Ca2+, respectively. Normalized G-V curves at pHi=7.2 (open circles) and 6.2 (filled circles) are shown below the current traces. The smooth curves represent Boltzmann fits. (d) Currents from hSlo1 channels elicited by pulses from 0 to 100 and then to –80 mV at the different pHi indicated in the absence of Ca2+.
Two histidine residues in the RCK1 domain mediate the H+ sensitivity
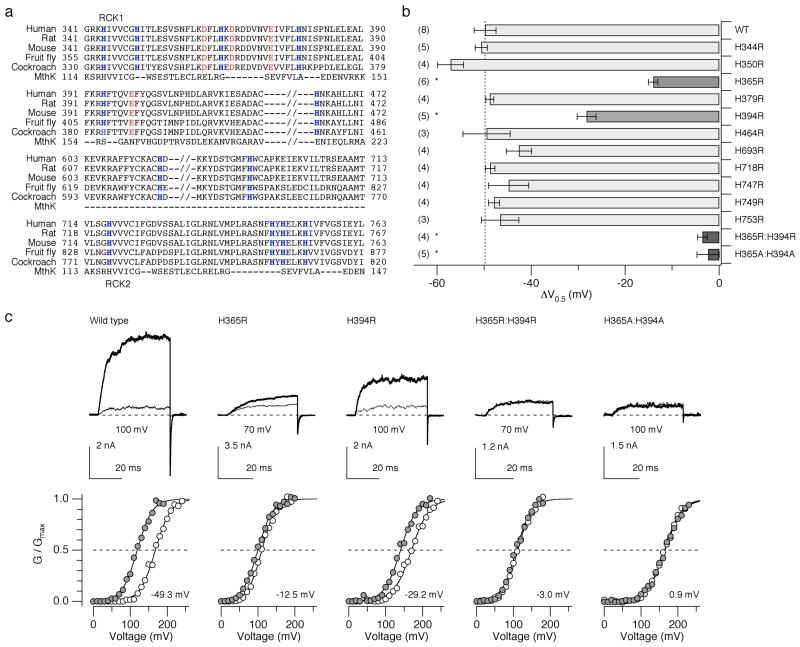
Activation of Slo1 channels from diverse species by low pHi (Fig. 1b) and the previous findings that the leftward G-V shift has an EC50 value of pHi=6.5 23 and that the shift is antagonized by the histidine modifier diethyl pyrocarbonate 23 collectively suggest that the stimulatory effect of low pHi is mediated by conserved histidine residues in Slo1 accessible from the cytoplasmic side. Each hSlo1 subunit contains 12 conserved histidine residues, most of which are located in the large cytoplasmic C-terminal domain. In particular, the putative RCK1 and RCK2 domains contain 10 histidines (Fig. 2a). Each of the conserved histidine residues was replaced with arginine and the resulting mutants were assayed for their pHi sensitivity using the shift of the G-V curve (ΔV0.5) observed when pHi was lowered from 7.2 to 6.2 (Fig. 2b). A decrease in pHi from 7.2 to 6.2 in the absence of Ca2+ in the intracellular solution causes a –50 mV shift in V0.5 for the wild-type channel (Fig. 2b). We found that among the single histidine-to-arginine mutations examined, mutation of His365 and His394 located in the RCK1 domain significantly diminished the pHi sensitivity (Fig. 2b, c). The ΔV0.5 value for the mutant H365R was –14.0 ± 1.0 mV and for the mutant H394R, it was –28.2 ± 2.0 mV (P < 0.01) or about 28% and 56% of the shift observed in the wild-type channel, respectively. When the two mutations were present concurrently (H365R:H394R), the V0.5 shift by lowering pHi from 7.2 to 6.2 was completely eliminated (P < 0.01). A greater decrease in pHi to 5.7 and an increase in pHi to 7.7 were without any effect (data not shown). Mutation of the two histidine residues to alanine (H365A:H394A) also disrupted the pHi sensitivity (P < 0.01). Moreover, in these double mutants, the pHi decrease failed to alter the kinetics of activation and deactivation (Fig. 2c; Supplementary Fig. 1).
Figure 2.
Mutation of two histidine residues located in the RCK1 domain of hSlo1 abolishes the sensitivity to low pHi. (a) Sequence alignment of the C-terminal intracellular domains of Slo1 from human (GI:507922), rat (rSlo1, GI:46396068), mouse (GI:18448948), the fruit fly Drosophila melanogaster (GI:115311626), and the American cockroach Periplaneta americana (GI:25991360). The MthK sequence is also shown aligned 34. Histidine residues conserved among Slo1 are shown in blue. (b) Changes in V0.5 caused by a decrease in pHi from 7.2 to 6.2 in the wild-type and mutant channels in the absence of Ca2+ (also see Supplementary Table 1). * P < 0.01. Error bars represent s.e.m. (c) Representative currents recorded at pHi=7.2 (thin sweeps) and 6.2 (thick sweeps) for the wild-type and select mutant Slo1 channels in the absence of Ca2+. The currents were elicited by pulses from 0 to the voltages indicated where G/Gmax is about 0.1 at pHi=7.2. Normalized G-V curves at pHi=7.2 (open circles) and 6.2 (filled circles) are shown below the current sweeps. The number in each graph represents ΔV0.5. The smooth curves are Boltzmann fits to the data.
Mutation of His616 located in the putative linker segment between the RCK1 and RCK2 domains (H616R) severely compromised the electrophysiological expression level and we could not obtain any macroscopic currents, even at 300 mV at high [Ca2+]i. However, single-channel measurements verified that the mutant H616R retained a pHi sensitivity indistinguishable from that of the wild-type channel (Supplementary Fig. 2).
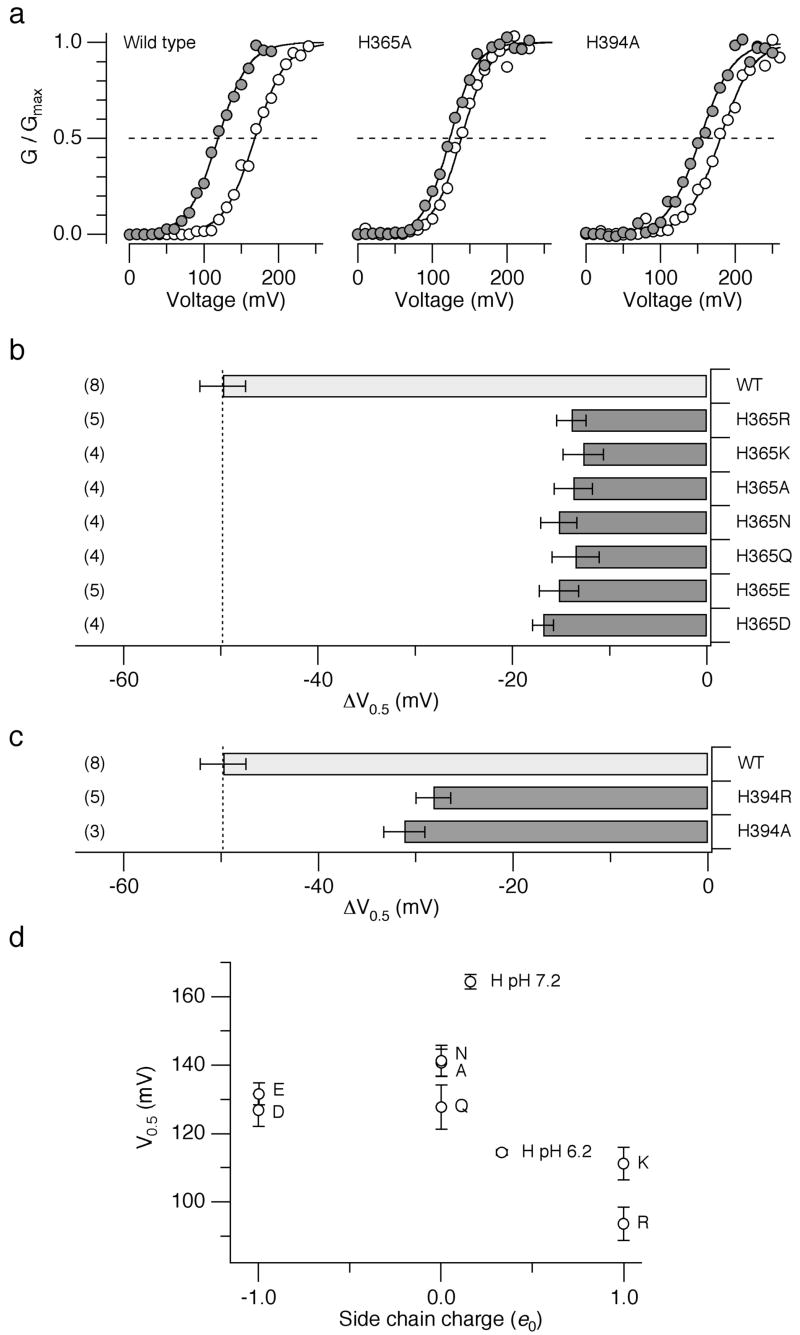
To infer about the physicochemical characteristics of His365 essential for the low pHi sensitivity of the Slo1 channel, the histidine was substituted with a variety of amino acids. None of the mutants (Arg, Lys, Ala, Asn, Gln, Glu, or Asp at position 365) showed the pHi sensitivity comparable to that of the wild-type channel; the ΔV0.5 values were about –15 mV, only 30% of the shift observed in the wild-type channel (Fig. 3a, b). Likewise, at position His394, substitution with Arg or Ala equally diminished the pHi sensitivity (Fig. 3a, c). The mutagenesis results therefore suggest that the full wild-type like pHi sensitivity requires histidine at positions 365 and 394.
Figure 3.
Mutation of His365 and His394. (a) Representative G-V curves from the wild-type, H365A and His394 channels at pHi=7.2 (open circles) and 6.2 (filled circles) in the absence of Ca2+. (b) Mean ΔV0.5 values caused by lowering pHi from 7.2 to 6.2 in the wild-type and the mutant channels with different amino acids at position 365. (c) Mean ΔV0.5 values caused by lowering pHi from 7.2 to 6.2 in the wild-type and the mutant channels with different amino acids at position 394. (d) Mean V0.5 values at pHi=7.2 in the absence of Ca2+ from the mutant channels with different amino acids at position 365 as a function of the side chain charge status. Arg and Lys are assumed to be fully positively charged and Asp and Glu were assumed to be fully negatively charged. Ala, Asn, and Gln were assumed to have no net charge. The values for the wild-type channels at pHi=6.2 and 7.2 are also shown assuming that the side chain pKa=6.5. Error bars represent s.e.m.
The pKa value of the imidazole side chain of histidine is often around 6 to 7 and lowering pHi from 7.2 to 6.2 is expected to render the side chain positively charged to a greater extent. It may be reasoned that it is the presence of a positive charge at position 365 that in part shifts the voltage dependence of channel activation to the negative direction. According to this idea, V0.5 of the channel with Lys and Arg at position 365 at pHi=7.2 should resemble that of the wild-type channel with histidine at pHi=6.2. This prediction is in part born out by comparison of the V0.5 values of the mutants with different amino acids at position 365 at pHi=7.2 (Fig. 3d). The V0.5 values in the mutants H365R and H365K were in fact more negative than those of other mutants and similar to that of the wild-type channel at pHi=6.2. The full pHi sensitivity of the Slo1 channel thus requires histidine at positions 365 and 394 and the charge status of the imidazole side chain modulated by pHi plays an essential role.
Electrostatic interactions are involved in the pHi sensitivity
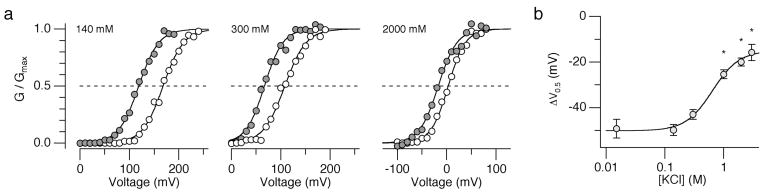
The mutagenesis results led us to hypothesize that an electrostatic mechanism underlies the pHi-induced activation of the BK channel; protonated His365 and His394 in Slo1 at low pHi electrostatically interact with nearby residues to facilitate channel activation. To test this idea, the pHi sensitivity of the wild-type Slo1 channel was measured using internal solutions with varying ionic strengths; high ionic strength solutions should diminish the negative shift in V0.5 normally observed with lowering pHi. Consistent with this prediction, the mean ΔV0.5 value observed on lowering pHi from 7.2 to 6.2 became progressively smaller with increasing ionic strengths (Fig. 4a, b).
Figure 4.
High ionic strength solutions diminish ΔV0.5 caused by lowering pHi from 7.2 to 6.2 in the absence of Ca2+. (a) Representative G-V curves from the wild-type hSlo1 channels in different concentrations of KCl (mM). (b) ΔV0.5 values at different [KCl]i. The curve represents the Hill equation fit. Error bars represent s.e.m. n = 3 to 8. * P < 0.01.
High ionic strength solutions may slightly increase the pKa value of the histidine side chain 25 so that the range of the pHi sensitivity of the channel is shifted higher. This possibility can be discounted because increasing pHi from 7.2 to 7.7 failed to alter the voltage dependence of the channel activation in both high and normal ionic solutions, indicating that any change in pKa of the imidazole side chain by the ionic strength manipulations made a negligible contribution. Addition of sucrose, a non-electrolyte, did not affect the pHi sensitivity (data not shown). Taken together, the above results suggest that electrostatic interactions involving His365 and His394 are critical in the pHi-mediated regulation of the Slo1 BK channel.
Asp367 in the RCK1 Ca2+ sensor is important for the pHi sensitivity
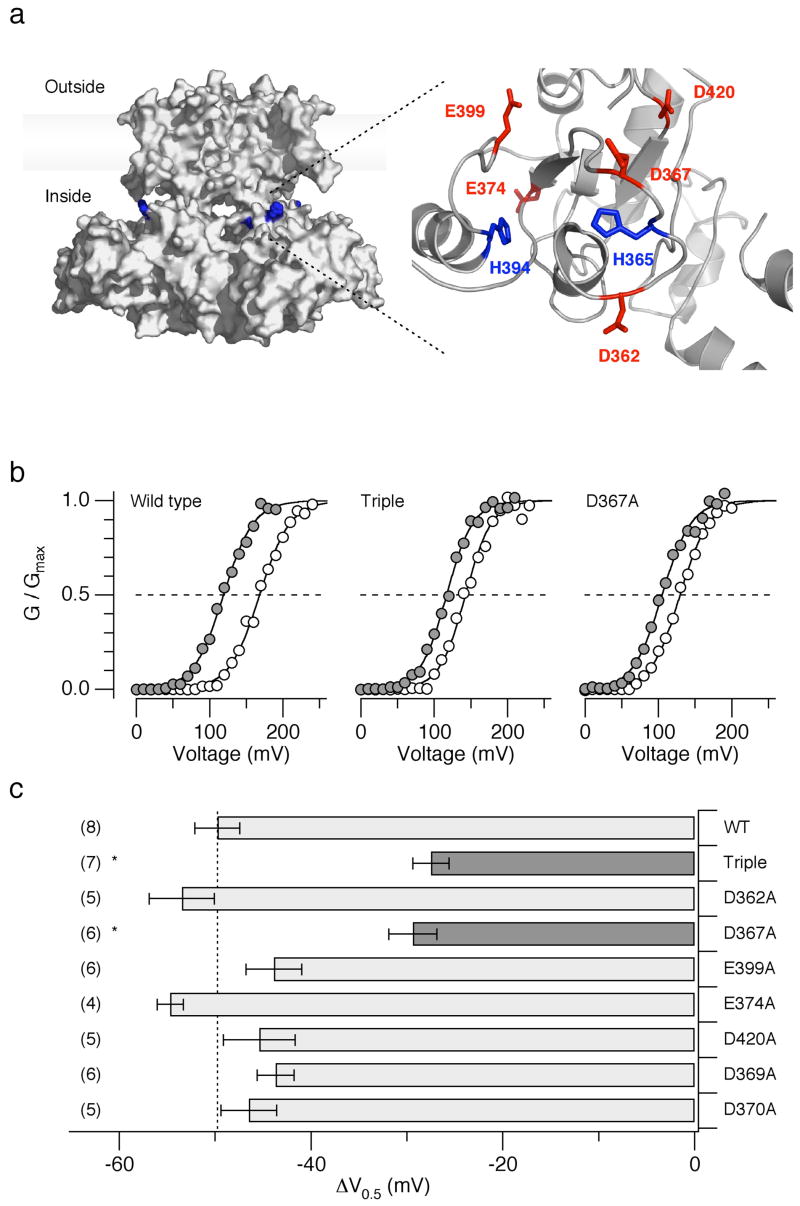
A high-resolution atomic structure of the Slo1 channel is not yet available but the amino-acid sequence in the RCK1 domain of Slo1 is relatively similar to that of the bacterial K+ channel MthK whose high-resolution structures are known 13,26. A homology model of the Slo1 RCK1 domain based on an MthK structure 7 suggests that the two histidine residues critical for the pHi sensitivity, His365 and His394, may be in close proximity of the negatively-charged residues Asp362, Asp367, Asp369, Asp370, Glu374, Glu399, and Asp420 (Fig. 5a). Some of these negatively-charged residues are important for the divalent cation sensitivity of the Slo1 channel, probably forming a high- and a low-affinity divalent cation sensors 27–29. The possible proximity of His365/His394 to the negatively-charged residues involved in the divalent cation sensitivity suggests that some of these negative charges might be the electrostatic interaction partners of protonated His365 and/or His394. If so, neutralization of the negatively-charged residues should also disrupt the pHi sensitivity of the channel. Neutralization of Glu374, Asp369, Asp370, or Asp420 failed to alter the ΔV0.5 caused by lowering pHi from 7.2 to 6.2 (Fig. 5c). In contrast, ΔV0.5 was significantly diminished by the triple mutation D362A:D367A:E399A (P < 0.01; Fig. 5b, c), which is known to interfere with the Ca2+ sensitivity mediated by the RCK1 domain of the channel 27,29. Among the three negatively charged residues, only Asp367 plays a critical role in the pHi sensitivity of the channel because, when mutated separately, neutralization of Asp367 but not Asp362 or Glu399 noticeably diminished the ΔV0.5 in response to a decreased in pHi (Fig. 4b, c). However, the mutation D367A does not completely eliminate the pHi sensitivity, leaving open the possibility that other structural components, including backbone dipoles, electrostatically interact with His365 and His394.
Figure 5.
Mutation of Asp367 diminishes the pHi sensitivity. (a) A speculative overall structure of hSlo1 illustrating the locations of His365 and His394 (left) and a homology model of the Slo1 RCK1 domain (right). The hSlo1 sequence was aligned with those of Kv1.2 (2A79) 50 and of MthK (1LNQ) and the overall structure (left) was inferred 51. The homology model of the RCK1 domain (right) was developed for mSlo1 by Latorre and Brauchi 7. The mSlo1 sequence is identical to that of hSlo1 in the RCK1 domain (see Fig. 2a). The images were prepared with MacPyMOL. (b) Representative G-V curves from the wild-type, D362A:D367A:E399A (“Triple”) and D367A channels at pHi=7.2 (open circles) and 6.2 (filled circles) in the absence of Ca2+. (c) Mean ΔV0.5 values in the wild-type and mutant channels caused by lowering pHi from 7.2 to 6.2 (also see Supplementary Table 1). Error bars represent s.e.m.
His365 and His394 are also involved in Ca2+-dependence of Slo1
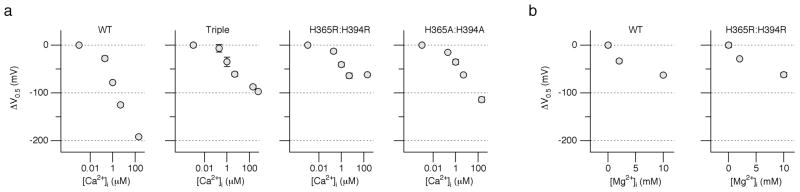
Our results show that the pHi sensitivity of the Slo1 channel requires His365 and His394 as well as Asp367, one of the residues in the RCK1 domain implicated for high-affinity Ca2+ sensing 27,29. The involvement of Asp367 in both the pHi and Ca2+ sensing of the channel suggest that His365 and His394 may in turn participate in the channel’s Ca2+ sensing. In the wild-type channel, increasing [Ca2+]i to 200 μM shifted V0.5 to the negative direction by ~200 mV (Fig. 6a). The triple mutation D362A:D367A:E399A, which disrupts the high-affinity Ca2+ sensor in the RCK1 domain 27, reduces the V0.5 shift caused by 200 μM [Ca2+]i by half to ~100 mV with the remaining Ca2+ sensitivity mediated by the Ca2+-bowl segment at the distal C-terminus 27,29. Comparison of the Δ V0.5 values at different [Ca2+]i (Fig. 6a) showed that the two histidine double mutants H365R:H394R and H365A:H394A, neither of which showed any appreciable pHi sensitivity, also had noticeably diminished sensitivity to [Ca2+]i; the V0.5 shift by 200 μM [Ca2+]i was about 100 mV or 50% of that found in the wild-type channel. The dependence of V0.5 on [Ca2+]i in the pHi-insensitive histidine mutants in fact closely resembled that of the triple mutant D362A:D367A:E399A with the disrupted RCK1 Ca2+ sensor.
Figure 6.
Mutation of His365 and His394 disrupts the Ca2+-dependent activation but fails to alter the Mg2+-dependent activation. (a) Ca2+-dependent activation measured by changes in V0.5 in the wild-type, D362:D367:E399, H365R:H394R, and H365A:H394A channels. For each channel type, the results were normalized to the mean V0.5 value in the virtual absence of Ca2+. n = 4 to 9. (b) Mg2+-dependent of activation measured by changes in V0.5 in the wild-type (n = 3) and H365R:H394R channels (n = 5). Error bars represent s.e.m.
In addition to Ca2+, intracellular Mg2+ also activates the Slo1 channel, and Glu374 and Glu399 in the RCK1 domain, potentially near His365 and His394 (Fig. 6b), form a low-affinity divalent cation-sensing site that transduces alterations in [Mg2+]i 27–29. Therefore, we examined whether His365 and His394 are involved in the channel’s low-affinity divalent cation ion sensitivity. The Mg2+-dependent activation of the double mutant H365R:H394R was indistinguishable from that of the wild-type channel (Fig. 6b). Similar results were also obtained with the double mutant H365A:H394A (data not shown).
DISCUSSION
Slo1 BK channels constitute an important element linking cellular metabolism and membrane excitability in part because of the synergic activation by intracellular Ca2+ and membrane depolarization. Recent results have shown that Slo1 BK channels are also activated by intracellular H+, a key regulator of cellular metabolism, thereby providing an additional coupling pathway between cell metabolism and membrane excitability. Here we have elucidated the mechanism of such signaling linkage provided by the Slo1 channel and intracellular H+. Two histidine residues, His365 and His394 located in the RCK1 domain are essential for the pHi sensitivity of the Slo1 BK channel. Protonated His365 and His394 electrostatically interact with Asp367 and potentially with other charged residues and/or dipoles to selectively transduce changes in [Ca2+]i and [H+]i to allosterically facilitate opening of the channel gate.
The mutagenesis results presented here suggest that histidine with the imidazole side chain at positions 365 and 394 is the only naturally occurring amino acid that supports the pHi-dependent activation of the Slo1 channel. Furthermore, substitution of His365 and His394 with arginine or lysine alters the voltage dependence of the channel at normal pHi to resemble that of the wild-type channel at low pHi at which His365 and His394 are expected to be protonated. These observations together show that His365 and His394 represent the primary H+ sensors of the channel required to facilitate its activation.
The H+ sensors His365 and His394, when protonated, electrostatically interact with Asp367 as evidenced by the results of the ionic strength manipulations and the charge-neutralization mutation of aspartic acid at position 367. The exact spatial arrangement of these interacting residues is not yet clear because of the lack of detailed structural information. However, the residues are likely to be arranged within ~10 to 15 Å of each other based on the typical effective range of long-distance electrostatic interactions 30. A homology model of the Slo1 RCK1 domain developed by Latorre and Brauchi 7 (Fig. 5a) suggests a distance of ~7 Å between the side chains of His365 and Asp367 and of ~20 Å between those of His394 and Asp367.
In addition to Asp367, other charged residues and/or dipoles, such as carbonyl oxgens, must electrostatically interact with His365 and His394 for neutralization of Asp367 alone eliminate only ~50% of the pHi sensitivity. Asp367 is part of the high-affinity divalent cation sensor in the RCK1 domain of the channel, which under physiological conditions, transduces [Ca2+]i 27,29. Therefore, we suggest that His365, His394 and Asp367 form a bi-functional ligand-sensing subdomain for intracellular H+ and Ca2+ such that mutation of the aforementioned residues alters the sensitivity to both H+ and Ca2+.
The electrostatic mechanism of the H+-dependent activation of the channel involving His365 and His394 provides unexpected insights into the properties of the high-affinity divalent cation sensor in the RCK1 domain. Mutation of Asp367 or Met513 in this domain interferes with the overall high-affinity divalent cation sensitivity of the channel 27,29,31; however, complete and detailed characteristics of the sensors have not been elucidated. Our study here shows that the two histidines residues are integral components of the high-affinity divalent cation sensor. The electrostatic interaction of the protonated side chains of His365 and His394 with Asp367 at low pHi functionally and partly mimics the interaction of Ca2+ with Asp367; H+ of the His365/His394 imidazole side chain acts as a Ca2+ mimic for Asp367. The contributions of His365 and His394 to the H+-dependent activation of the channel may be energetically independent of each other because the effects of the His365 and His394 mutations on the voltage dependence are additive. This conclusion must be considered somewhat tentative, however, because multiple gating transitions contribute to determination of the data description parameter V0.5.
While both H+ and Ca2+ shift the voltage dependence of the channel to the negative direction, thus providing a stimulatory influence on the channel, some differences in their actions on the channel do exist. The maximal shift in the voltage dependence induced by high concentrations of Ca2+, about –200 mV, is appreciably larger than that by H+. This difference occurs because the overall high-affinity Ca2+ sensitivity of Slo1 arises from two distinct but relatively equipotent sensors, the RCK1 sensor and the Ca2+ bowl 32, while the H+ sensitivity is mediated only by the RCK1 sensor. The Ca2+ bowl does not contribute to the H+ sensitivity, thus further illustrating functional differences between the two high-affinity Ca2+ sensors. Even within the RCK1 Ca2+/H+ sensor subdomain, clear functional specializations are observed. For example, His365, His394 and Asp367 transduce both Ca2+ and H+ but Asp362 near the high-affinity Ca2+ sensor in the RCK1 domain 27,29, does not contribute to the pHi sensitivity. The true binding affinity of the subdomain to H+ and Ca2+ is not known, but the EC50 values of the channel activation for H+ and Ca2+ based on ionic current measurements are about 0.35 μM 23 and ~10 μM 33, respectively, suggesting that the Slo1 channel may be considered more sensitive to H+ than to Ca2+.
The cytoplasmic gating ring domain of the Slo1 is considered to be structurally similar to that of the prokaryotic channel MthK 14,34. Yet, in clear contrast to the H+-stimulated gating of Slo1 described here, the MthK channel activity is profoundly inhibited by H+26. At low pHi (e.g., 6.2), the gating ring of MthK disassembles into four dimers 35 and the channel fails to open, even in the presence of 10 mM [Ca2+] 26. In the Slo1 channel, H+ essentially acts as a Ca2+ mimetic for the RCK1 Ca2+ sensor and it is highly unlikely that the Slo1 gating ring structure undergoes pH-dependent assembly and disassembly as observed in the MthK channel. The pHi-sensitivity of MthK has been suggested to require His193 located in its otherwise hydrophobic octamer assembly interface 26. His193 is poorly conserved among RCK domains 26 and it is equivalent to M442 in the Slo1 channel 34, distinct from His365 and His394 required for the H+-stimulated gating. Conversely, the residues equivalent to His365 and H394 do not exist in MthK (Fig. 2a). Thus, while MthK and Slo1 share a similar cytoplasmic structural organization, the two channels employ distinct mechanisms to transduce intracellular pH.
The high sensitivity of the Slo1 BK channel to changes in pHi renders this channel very well suited for coupling membrane excitability/neuronal transmission and cellular metabolism. The cellular metabolic state and pHi are intimately and reciprocally linked, and H+ may be viewed as a metabolic intracellular messenger 36,37. Accordingly, the H+ and Ca2+ sensitivity of the Slo1 BK channel conferred by His365, His394 and Asp367 is likely to have physiological implications. Fluctuations in pHi, under both physiological and pathophysiological conditions, are typically accompanied by changes in [Ca2+]i. For example, intense neuronal firing leads to a noticeable decrease in pHi 6,38. Pathophysiologically, malignant hyperthermia and hypercapnia decrease pHi 39,40. Furthermore, cerebral ischemia decreases pHi rapidly within a few minutes from by 0.5 to 1 unit 1, large enough to noticeably activate Slo1 BK channels. Concurrently with the increase in [H+]i, ischemia frequently causes a substantial increase in [Ca2+]i, in part through activation of Ca2+-permeant glutamate receptor channels, possibly leading to excitoxicity 41. Increases in both [H+]i and [Ca2+]i activate Slo1 BK channels, but the two ligand-dependent activation mechanisms may play differential roles in blunting the extent of hyperexcitability caused by Ca2+. It may be speculated that the H+-mediated activation of the Slo1 BK channel has a feed-forward anticipatory role while the Ca2+-dependent activation of the channel has a feedback role in regulation of the membrane excitability.
K+ channels are an exceptionally diverse family of ion channels. However, only a very small number of the channels are activated by intracellular H+. TREK-1, a two-pore domain voltage-independent leak K+ channels with four transmembrane segments, is one example 42. Among the voltage-dependent K+ channels, the Slo1 BK channel is unique in that intracellular H+ enhances its ionic current 22,23. Both the permeation and gating properties of the Slo1 channel are well suited to transduce changes in pHi. As shown in this study, low pHi prominently shifts the voltage dependence of activation to the negative direction to facilitate channel opening. Furthermore, unlike most other K+ channels, the single-channel conductance of the Slo1 channel, especially at physiological voltages, is resistant to pore blocking by intracellular H+43. In Shaker and other voltage-gated K+ channels, lowering pHi to 6.4 decreases the single-channel current size by about 50% without affecting their voltage dependence of activation 44. The unusual H+-activated gating based the electrostatic interactions encompassing histidine and aspartic acid residues in the RCK1 domain and the H+-resistant permeation characteristics of the Slo1 BK channel contribute to its role as an important coupling mechanism between the cell metabolic state and membrane excitability 8. To serve a variety of physiological needs, cells express a diverse complement of K+ channels, most of which are inhibited by H+. Inclusion of Slo1 BK channels activated by H+ and Ca2+ in the proteome permits fine-tuning of the membrane excitability according to their metabolic state.
METHODS
Channel expression and cell isolation
Human (KCNMA1; U11058), Drosophila (M96840) 45 and Periplaneta Slo1 (AF452164) 45 in the expression vectors pCI-neo (Promega), pcDNA3 (Invitrogen) and pcDNA3, respectively, were transiently expressed in HEK tsA cells using FuGENE 6 (Roche) as described 19. In some experiments, human Slo1 and β1 (KCNMB1; U38907) in pEGFP-N1 (Clontech) with 1:1 weight ratio were transfected together. We constructed the mutant channels using a PCR–based mutagenesis method (Stratagene). Other constructs used are described in the legends. Cultured rat aortic smooth muscle cells were prepared as described 46.
Electrophysiology and data analysis
Macroscopic and single–channel ionic currents were recorded from excised inside-out membrane patches at room temperature 19. Patch electrodes (Warner) had a typical resistance of 1.5 – 2.0 MΩ and the series resistance, 90% of the initial input resistance, was electronically compensated in macroscopic current measurements. Macroscopic capacitive and leak currents were subtracted using a P/6 protocol. The current signal was filtered at 10 kHz through the built-in filter of the patch-clamp amplifier (AxoPatch 200A; Axon) and digitized at 100 kHz using an ITC-16 AD/DA interface (Instrutech). The results were analyzed as previously described using IGOR Pro (Wavemetrics) 19. Statistical comparisons were performed using the unpaired or paired t test, as appropriate. Comparison of more than two groups was performed using ANOVA followed by a Tukey HSD test 47 as implemented in IGOR Pro. Statistical significance was assumed at P ≤ 0.05 and all data are presented as mean ± s.e.m.
Solutions
The extracellular solution for rat aortic smooth cells contained (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 Glucose, 10 HEPES, pH 7.4 with NaOH. The extracellular solution for HEK tsA cells contained (in mM): 140 KCl, 2 MgCl2, 10 HEPES, pH 7.2 with N-methyl-D-glucamine (NMDG). To compare Slo1 currents at different pHi in the virtual absence of Ca2+, the intracellular solution contained (in mM): 140 KCl, 11 EDTA and either 10 HEPES (NMDG) for pHi 7.2 or 10 MES (NMDG) for pHi 5.7, 6.2 and 6.7. These solutions were assumed to have [Ca2+]=10 nM 48. EDTA was selected because its chelating ability is less sensitive to changes in pH than EGTA. BAPTA was not used in the study as it may have a direct blocking action on the Slo1 channel 23. The concentrations of NMDG in the above internal solutions were from 3 to 20 mM and, at these concentrations, no effect on the Slo1 channel activity was observed. Free Ca2+ concentrations ([Ca2+]) were calculated using Patcher’s Power Tools for Electrophysiologists (http://www.mpibpc.gwdg.de/abteilungen/140/software/). The pH = 7.2 solutions with different [Ca2+] were prepared as descried 49 using EGTA or EDTA (for [Ca2+] = ~10 nM (no added Ca2+)), HEDTA ([Ca2+] = 200 nM – 5 μM) or without any chelator ([Ca2+= ]≥200 μM).
Supplementary Material
Acknowledgments
We thank Dr. M. L. Garcia for the original Slo1 construct and Drs. Latorre and Brauchi for the structural model. Supported in part by NIH and SFB 604 (TP A4).
Footnotes
AUTHOR CONTRIBUTIONS
Designed research: SH, SHH, TH; Performed research: SH, RX, TH; Analyzed data: SH, SHH, TH; Wrote the paper: SH, SHH, TH.
References
- 1.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 2.Kann O, Kovacs R. Mitochondria and neuronal activity. American journal of physiology. 2007;292:C641–57. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 3.Higo T, et al. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Austin C, Wray S. Interactions between Ca2+ and H+ and functional consequences in vascular smooth muscle. Circ Res. 2000;86:355–63. doi: 10.1161/01.res.86.3.355. [DOI] [PubMed] [Google Scholar]
- 5.Yao H, Haddad GG. Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium. 2004;36:247–55. doi: 10.1016/j.ceca.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 7.Latorre R, Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biological research. 2006;39:385–401. doi: 10.4067/s0716-97602006000300003. [DOI] [PubMed] [Google Scholar]
- 8.Toro L, Stefani E. Calcium-activated K+ channels: metabolic regulation. J Bioenerg Biomembr. 1991;23:561–76. doi: 10.1007/BF00785811. [DOI] [PubMed] [Google Scholar]
- 9.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–9. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 10.Xu W, et al. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–33. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 11.Gribkoff VK, et al. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat Med. 2001;7:471–7. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- 12.Tseng-Crank J, et al. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–30. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–22. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 15.Magleby KL. Gating mechanism of BK (Slo1) channels: so near, yet so far. J Gen Physiol. 2003;121:81–96. doi: 10.1085/jgp.20028721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu X, Qian X, Magleby KL. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron. 2004;42:745–56. doi: 10.1016/j.neuron.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang XD, Santarelli LC, Heinemann SH, Hoshi T. Metabolic regulation of potassium channels. Annu Rev Physiol. 2004;66:131–59. doi: 10.1146/annurev.physiol.66.041002.142720. [DOI] [PubMed] [Google Scholar]
- 19.Tang XD, et al. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–5. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- 20.Jaggar JH, et al. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–12. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–12. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 22.Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Effect of acidosis on Ca2+-activated K+ channels in cultured porcine coronary artery smooth muscle cells. Pflügers Arch. 1998;436:509–14. doi: 10.1007/s004240050665. [DOI] [PubMed] [Google Scholar]
- 23.Avdonin V, Tang XD, Hoshi T. Stimulatory action of internal protons on Slo1 BK channels. Biophys J. 2003;84:2969–80. doi: 10.1016/S0006-3495(03)70023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Church J, Baxter KA, McLarnon JG. pH modulation of Ca2+ responses and a Ca2+-dependent K+ channel in cultured rat hippocampal neurones. J Physiol (Lond) 1998;511:119–32. doi: 10.1111/j.1469-7793.1998.119bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KK, Fitch CA, Lecomte JT, Garcia-Moreno EB. Electrostatic effects in highly charged proteins: salt sensitivity of pKa values of histidines in staphylococcal nuclease. Biochemistry. 2002;41:5656–67. doi: 10.1021/bi0119417. [DOI] [PubMed] [Google Scholar]
- 26.Ye S, Li Y, Chen L, Jiang Y. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell. 2006;126:1161–73. doi: 10.1016/j.cell.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–4. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 28.Shi J, et al. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 2002;418:876–80. doi: 10.1038/nature00941. [DOI] [PubMed] [Google Scholar]
- 29.Zeng XH, Xia XM, Lingle CJ. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J Gen Physiol. 2005;125:273–86. doi: 10.1085/jgp.200409239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miksovska J, et al. Distant electrostatic interactions modulate the free energy level of QA- in the photosynthetic reaction center. Biochemistry. 1996;35:15411–7. doi: 10.1021/bi961299u. [DOI] [PubMed] [Google Scholar]
- 31.Bao L, Rapin AM, Holmstrand EC, Cox DH. Elimination of the BKCa channel’s high-affinity Ca2+ sensitivity. J Gen Physiol. 2002;120:173–89. doi: 10.1085/jgp.20028627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian X, Niu X, Magleby KL. Intra- and intersubunit cooperativity in activation of BK channels by Ca2+ J Gen Physiol. 2006;128:389–404. doi: 10.1085/jgp.200609486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox DH, Cui J, Aldrich RW. Allosteric gating of a large conductance Ca-activated K+ channel. J Gen Physiol. 1997;110:257–81. doi: 10.1085/jgp.110.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roosild TP, Miller S, Booth IR, Choe S. A mechanism of regulating transmembrane potassium flux through a ligand-mediated conformational switch. Cell. 2002;109:781–91. doi: 10.1016/s0092-8674(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 35.Dong J, Shi N, Berke I, Chen L, Jiang Y. Structures of the MthK RCK domain and the effect of Ca2+ on gating ring stability. J Biol Chem. 2005;280:41716–24. doi: 10.1074/jbc.M508144200. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi KI, Copenhagen DR. Modulation of neuronal function by intracellular pH. Neurosci Res. 1996;24:109–16. doi: 10.1016/0168-0102(95)00989-2. [DOI] [PubMed] [Google Scholar]
- 37.Kelly T, Church J. pH modulation of currents that contribute to the medium and slow afterhyperpolarizations in rat CA1 pyramidal neurones. J Physiol (Lond) 2004;554:449–66. doi: 10.1113/jphysiol.2003.051607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol (Lond) 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decanniere C, Van Hecke P, Vanstapel F, Ville H, Geers R. Metabolic response to halothane in piglets susceptible to malignant hyperthermia: an in vivo 31P-NMR study. J Appl Physiol. 1993;75:955–62. doi: 10.1152/jappl.1993.75.2.955. [DOI] [PubMed] [Google Scholar]
- 40.Denton JS, McCann FV, Leiter JC. CO2 chemosensitivity in Helix aspersa: three potassium currents mediate pH-sensitive neuronal spike timing. Am J Physiol Cell Physiol. 2007;292:C292–304. doi: 10.1152/ajpcell.00172.2006. [DOI] [PubMed] [Google Scholar]
- 41.Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor. Trends Neurosci. 1987;10:299–302. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- 42.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–6. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 43.Brelidze TI, Magleby KL. Protons block BK channels by competitive inhibition with K+ and contribute to the limits of unitary currents at high voltages. J Gen Physiol. 2004;123:305–19. doi: 10.1085/jgp.200308951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starkus JG, Varga Z, Schonherr R, Heinemann SH. Mechanisms of the inhibition of Shaker potassium channels by protons. Pflügers Arch. 2003;447:44–54. doi: 10.1007/s00424-003-1121-0. [DOI] [PubMed] [Google Scholar]
- 45.Derst C, et al. The large conductance Ca2+-activated potassium channel (pSlo) of the cockroach Periplaneta americana: structure, localization in neurons and electrophysiology. The European journal of neuroscience. 2003;17:1197–212. doi: 10.1046/j.1460-9568.2003.02550.x. [DOI] [PubMed] [Google Scholar]
- 46.Tammaro P, Smith AL, Hutchings SR, Smirnov SV. Pharmacological evidence for a key role of voltage-gated K+ channels in the function of rat aortic smooth muscle cells. Br J Pharmacol. 2004;143:303–17. doi: 10.1038/sj.bjp.0705957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zar JH. Biostatistical analysis. Prentice Hall; Upper Saddle River, N. J: 1999. [Google Scholar]
- 48.Tang XD, et al. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol. 2001;117:253–74. doi: 10.1085/jgp.117.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santarelli LC, Wassef R, Heinemann SH, Hoshi T. Three methionine residues located within the regulator of conductance for K+ (RCK) domains confer oxidative sensitivity to large-conductance Ca2+-activated K+ channels. J Physiol (Lond) 2006;571:329–48. doi: 10.1113/jphysiol.2005.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 51.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–23. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.